Abstract
Depression, anxiety and conduct disorders are common in children and adolescents and selective serotonin reuptake inhibitors (SSRIs) are often used to treat these conditions. Fluoxetine (Prozac) is the first approved SSRI for the treatment of depression in this population. Although it is believed that overall, fluoxetine is effective in child and adolescent psychiatry, there have been reports of specific adverse drug effects; most prominently suicidality and psychiatric symptoms such as agitation, worsening of depression and anxiety. Chronic fluoxetine substantially increases brain extracellular 5-HT concentrations and the juvenile developing brain may respond to supraphysiological 5-HT levels with specific adverse effects not seen or less prominent in adult brain. Using novelty induced hypophagia (NIH), as well as open field (OF) and elevated plus maze (EPM) tests, we show that both Swiss Webster (SW) and C57Bl/6 (B6) mice, receiving fluoxetine in a clinically relevant dose and during their juvenile age corresponding to child-adolescent period in human, exhibit a paradoxical anxiogenic response. The adverse effects of juvenile fluoxetine disappeared upon drug discontinuation and no long term behavioral consequences were apparent. No adverse effect to chronic fluoxetine was seen in adult mice and a dose dependent anxiolytic effect developed. These data show that the age of the mice, independently of the strains and tests used in this study, is the determining factor of whether the response to chronic fluoxetine is anxiolytic or anxiogenic. Taken together, the response of the juvenile and adult brain to fluoxetine could be fundamentally different and the juvenile fluoxetine administration mouse model described here may help to identify the mechanism underlying this difference.
Keywords: anxiety, fluoxetine, development, mice, behavior, novelty induced hypophagia
Introduction
In the United States depression affects up to 2.5 percent of children and 8.3 percent of adolescents (Birmaher et al, 1996; Silverstone, 2004; Wang et al, 2003). The selective serotonin reuptake inhibitor (SSRI) fluoxetine (Prozac) is approved by the Food and Drug Administration for child and adolescent depression (http://www.fda.gov/bbs/topics/ANSWERS/2003/ANS01187.html). SSRIs are also used in anxiety and conduct disorders such as separation anxiety and aggression in these populations. Although it is believed that overall, fluoxetine is effective in child and adolescent psychiatry (March et al, 2004), there have been reports on adverse drug effects in these populations; most prominently suicidality (Hammad et al, 2006) and psychiatric effects such as agitation, worsening of depression and anxiety (March et al, 2004).
Previous pharmacological and genetic studies indicate that increased 5-HT levels during development result in long-term behavioral and morphological changes in the brain (Ansorge et al, 2008; Ansorge et al, 2004; Cases et al, 1995; Cases et al, 1996; Maciag et al, 2006; Popa et al, 2008). For example, the pharmacological blockade of the 5-HT transporter (5-HTT) by SSRIs, starting at neonatal or early postnatal life, results in life-long anxiety and depression-like behavioral abnormalities (Ansorge et al, 2004; Maciag et al, 2006; Popa et al, 2008). Since rodents are born less mature compared to humans (Carlson & Willott, 1998; Rauschecker, 1999)(Supplementary Fig. 1), these pharmacological studies may be relevant to the clinical use of SSRIs during pregnancy.
Since SSRIs are often prescribed for children and adolescents, it is important to know their possible short- and long-term side effects during the child and adolescent periods. Here we show that administration of fluoxetine to juvenile mice on two genetic backgrounds, at a dose that produces clinically relevant plasma drug levels, results in an anxiogenic, instead of the expected anxiolytic effect. However, these adverse effects were reversed upon discontinuation of the drug. Interestingly, the paradoxical anxiogenic effect returned on re-exposure to fluoxetine in adulthood in one of the two strains studied. This suggests that although fluoxetine, when administered during the juvenile period, does not cause permanent behavioral changes, it can lead to an abnormal drug response on re-exposure later in life.
Materials and Methods
Animals
Timed pregnant Swiss Webster (SW) and C57Bl/6 (B6) females, approximately 8 days before delivery, were purchased from Taconic (Germantown, NY) and Charles River (Wilmington, MA), respectively. Animals were single-housed with a 12h light/dark cycle and with food and water available ad libitum. Male pups were implanted at 2 weeks of age with osmotic minipump Model 1007D (Alzet, Cupertino, CA) providing continuous drug delivery for 7 days. Mice were anesthetized using isoflurane. Minipumps were inserted subcutaneously through midscapular incisions which were then closed by wound glue. Minipumps were filled with 0.9% saline solution containing fluoxetine HCl (Toronto Research, Chemical, North York, ON, Canada) in concentrations delivering 2, 3 and 4 mg/kg/day drug in a volume of 12 µl/day. Controls were implanted with minipumps filled with saline solution. Pumps were removed under anesthesia at 3 weeks of age. Pump implantation/removal did not alter overall behavior in the novelty induced hypophagia (NIH) test as novel cage latencies to drink between implanted and non-implanted adult mice (16 weeks of age) were not significantly different (Supplementary Fig. 2). Following the removal of minipumps at weaning (at 3 weeks of age), delivery of fluoxetine was continued via the drinking water. The concentration of fluoxetine in the drinking water corresponding to 1.5 and 3 mg/kg/day doses in juvenile mice was 0.015 and 0.03 mg/ml, respectively. Fluoxetine was also administered to adult 8 week old mice. Drug concentration in the drinking water was 0.03, 0.12 and 0.18 mg/ml for delivering ∼3, 12 and 18 mg/kg/day drug doses. All animal procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College, and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Measurements of fluoxetine and norfluoxetine in plasma and brain
Fluoxetine and norfluoxetine were extracted from plasma and brain and their levels were determined by HPLC (Millipore Waters 600E with Waters 717 plus Autosampler) as described previously (Alvarez et al, 1998) using protriptyline as an internal standard (Sigma, St. Louis, MO). Blood and brain samples were collected at the middle of the light phase. Standards were made by adding fluoxetine and norfluoxetine (Sigma) to plasma and brain samples from control animals to yield the following concentrations: 0, 31.25, 62.5, 125, 250, 500, 1000 ng/ml. A 1.0 ml volume of 0.6 M sodium carbonate-sodium bicarbonate buffer (pH 9.0) containing the internal standard protriptyline (100 ng/ml) was added to either 0.1 ml plasma or weighed brain samples. Brain samples were homogenized using pellet pestles and a motor (Vineland, NJ). After the addition of 7 ml of a mixture of ethyl acetate and n-heptane (20:80, v/v), the vials were capped and vigorously mixed for 1.5 min, then centrifuged at 3000g for 10 min. The organic layer was transferred to another tube containing 0.2 ml of acidic phosphate buffer (0.025 M potassium dihydrogen phosphate adjusted to pH 2.3 with 85% phosphoric acid) then mixed for 1 min and centrifuged at 3000g for 10 min. The organic layer was discarded, and a 150 µl aliquot of the aqueous phase was injected for chromatographic separation. Purospher ® STAR RP-8 endcapped (5µm) column was purchased from Merck (Darmstadt, Germany). The mobile phase was a mixture of an acidic aqueous solution (containing 0.1 ml of perchloric acid and 1.5g of tetramethyl-ammonium perchlorate per liter) and acetonitrile (58:42, v/v). The filtered mobile phase was used at a flow-rate of 1 ml/min. The column effluent was monitored at 228 nm by using a Waters 474 Scanning Fluorscence Detector. Quantification was performed by calculating the peak-height ratios of each compound to the internal standard.
Behavioral testing
Fluoxetine treated and control (saline pump and no drug in drinking water) juvenile mice were tested in a battery of behavioral tests starting at 5.5 weeks of age. Fluoxetine was administered through the end of the testing period (6.5 week). Tests were conducted in the following order separated by 1–2 days of rest: (i) elevated plus maze (EPM), (ii) open field (OF), (iii) NIH test and (iv) forced swim test (FST). Other control and fluoxetine groups, treated during the juvenile period between 2 and 6 weeks of age, were tested at 12 weeks of age, after a 6 week drug free period to assess the long-term effect of fluoxetine on anxiety. In a parallel experiment, fluoxetine was administered to adult 8 week old mice and then tested for anxiety (at 12 weeks of age). Different doses were tested with independent groups of animals. Finally, groups of mice, pre-exposed to fluoxetine during the juvenile period were re-exposed to the drug at 12 weeks of age for 4 weeks and tested between 15 and 16 weeks of age. Some of the SW animals were pre-tested in the NIH test at 12 weeks (before fluoxetine administration) while others were not. Pretesting had no apparent effect because home and novel cage latencies at 12 weeks (first test) and at 16 weeks (second test) were not different (Supplementary Fig. 3). Since pre-testing at 12 weeks of age in the NIH test did not alter latency at 16 weeks of age, the pretested and non-pretested groups were combined.
All testing was performed between 11 A.M. and 4 P.M. On test days, animals were transported to the dimly illuminated behavioral laboratory and left undisturbed for at least 1 hr before testing.
NIH test
Mice were single-housed for 3 days before training began. Then, for 3 consecutive days mice were presented with diluted (1:3; milk:water) sweetened condensed milk (Carnation). Milk was presented in LM Animal Farms Quick Quench Universal Water Bottle (150ml, Petco). Bottles were positioned through wire cage lids. Home cage testing occurred in the dark on day 4. Each mouse was tested for 10 min and the latency to drink was recorded. Novel cage testing was on day 5 under bright lighting and by placing the mice into new clean cages of the same dimensions as the home cage but without bedding. Latency to drink was again recorded.
FST
In the FST, mice were placed in a clear, 21°C water-filled cylinder (diameter, 20 cm; depth, 13 cm) for 6 min. In this test, immobility of the mice is measured between 0 and 6 min.
EPM
EPM was performed using a cross maze with 30 × 5 cm arms at low light conditions (60 W bulb at 3 m height at 25% intensity). Animals were introduced to the middle portion of the maze facing an open arm. Entries into and time spent in the open and closed arms were measured by a video-tracking system (Noldus Information Technology, Wageningen, The Netherlands).
OF
The OF test was performed in a 24 × 40 cm black box, divided into 12 even-sized (8 × 10 inch) rectangles. The total number of crosses in the open field was recorded at normal light conditions (60 W bulb at 3 m height at 100% intensity) for 10 min to measure locomotor activity. The time spent in and the number of entries into the two rectangles at the center of the field were recorded by the video-tracking system to evaluate anxiety.
Proliferation in the dentate gyrus
Six week old SW animals received a single injection of 100 mg/kg BrdU intraperitoneally (Sigma, St. Louis, MO). Animals were transcardially perfused under deep anesthesia with 4% paraformaldehyde and sections were processed for immunohistochemistry essentially as described earlier (Tatapudy et al, 2008). Cells, pulse labeled at the S phase, were counted by computer assisted stereology, a method validated by conventional stereology, as described in our earlier report (Tatapudy et al, 2008).
Statistical analysis
One way ANOVAs with LSD posthoc tests were used in both the behavioral and proliferation studies.
Results
Experimental design
The regional development of the rodent brain proceeds on a timeline of days and weeks versus to months and years in humans (Supplementary Fig. 1). Overall, the mouse brain is relatively less mature than the human at birth as eye opening and the onset of hearing occurs only during the second postnatal week in mice (Carlson & Willott, 1998; Rauschecker, 1999). Mouse reaches maturity around 8 weeks of age. To capture the time frame and developmental events corresponding to the approximate child and adolescent period in human, we administered fluoxetine to mice from 2 to 6 weeks of age (referred to as juvenile age throughout this paper) and then assessed anxiety-related behavior. Fluoxetine was administered throughout the testing period.
Continuous administration of fluoxetine provides clinically relevant steady state plasma drug levels in juvenile mice
The half life of fluoxetine and its metabolite norfluoxetine in mice is shorter than in human (t1/2: 8 and 16h, respectively); therefore the drug was administered continuously from 2 weeks of age until weaning age (3 weeks of age) by osmotic minipumps followed by the delivery of the drug via the drinking water. First we determined the fluoxetine dose that provides a clinically relevant plasma drug and metabolite level in SW mice (Fig. 1). Because there are significant individual differences in the metabolism of fluoxetine in human adults, drug levels show a broad range from a low 50–60 to a high 400–500 ng/ml; though the levels can be even higher in some individuals (Alvarez et al, 1998; Amsterdam et al, 1997; Lundmark et al, 2001; Orsulak et al, 1988). Steady state levels in children and adolescents are similar to adult values (Wilens et al, 2002). Since during the first week of treatment relatively high norfluoxetine concentrations were associated with relatively low fluoxetine levels (norfluoxetine/fluoxetine ratio ≈7; Fig. 1A and supplementary Fig. 4), 1.5–3 mg/kg/day drug provided low fluoxetine and median norfluoxetine plasma levels, respectively (Fig. 1A–B). Once mice were weaned and the drug was delivered via the drinking water, 3 mg/kg/day dose provided low to intermediate fluoxetine and norfluoxetine plasma levels (Fig. 1C). Also, the norfluoxetine/fluoxetine ratio was 2–3, closer to the 1.3–1.5 value measured in human (Lundmark et al, 2001) (Supplementary Fig. 4).
Figure 1.
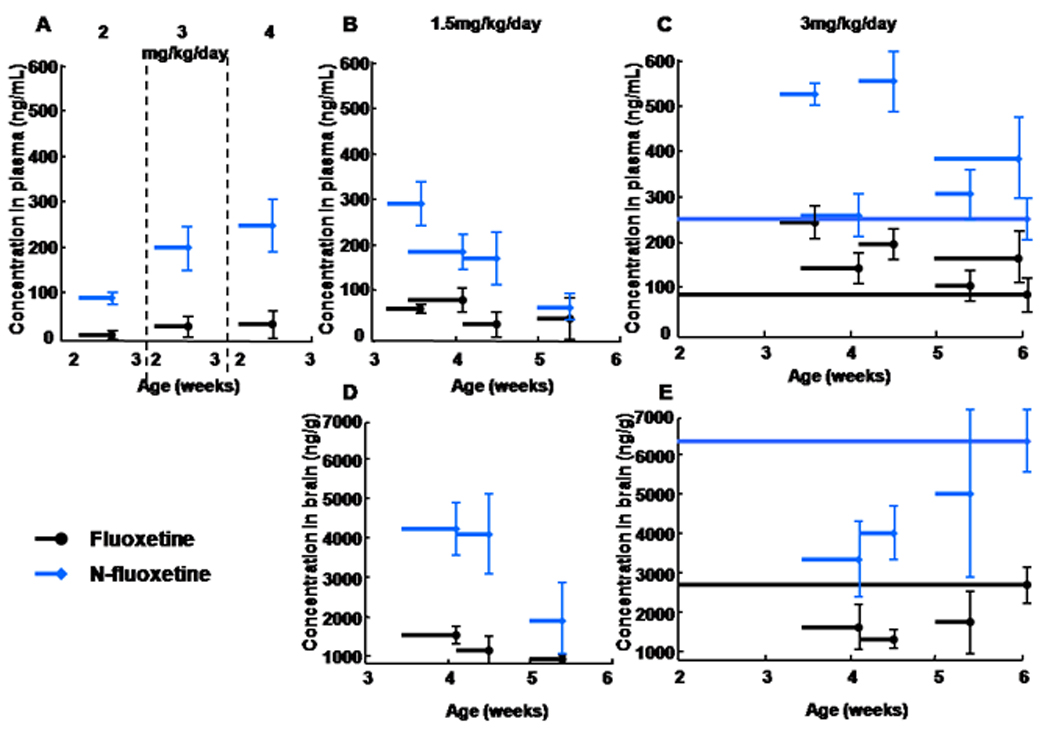
Plasma and brain levels of fluoxetine and its metabolite norfluoxetine following administration via osmotic minipumps between 2 and 3 weeks of age and via the drinking water from weaning at 3 weeks to 6 weeks of age. In contrast to human, neither fluoxetine nor norfluoxetine shows an appreciable day to day accumulation in mice (due to the relatively short half life, see results); therefore, we first measured levels following short, 3–4 day administration periods distributed throughout the juvenile period, then measured levels following administration throughout the entire 2–6 week period. Horizontal lines with error bars representing SE indicate the length of the administration and the mean drug levels (3–4 animals per time point). (A) The 3 mg/kg/day dose provided low to medium clinical plasma levels of total fluoxetine and norfluoxetine (∼230 ng/ml total). (C) Once mice were weaned and the drug was delivered via the drinking water for 3–4 days, the same 3 mg/kg/day dose provided 100–250 ng/ml and 250–570 ng/ml fluoxetine and norfluoxetine plasma levels, respectively. When fluoxetine was continuously administered for 4 weeks, the endpoint values (at 6 weeks of age) were ∼100 ng/ml for the parent drug and ∼250 ng/ml for the metabolite, both at midrange clinically relevant levels. (B) A lower 1.5 mg/kg/day dose was also tested but the corresponding plasma drug levels, although appropriate initially, became low towards the 6th postnatal week, which made the use of this dose less desirable. (D) and (E) Brain levels are approximately 25 times higher than plasma levels.
The levels of both fluoxetine and norfluoxetine were approximately 25 times higher in the brain than in the plasma in SW mice indicating a substantial drug accumulation in the CNS (Fig. 1D–E). This is consistent with a previous study that measured ≈20 times higher fluoxetine and norfluoxetine levels in brain as compared to serum (Henry et al, 2005). High brain drug levels are likely due to the lipophilicity of fluoxetine and norfluoxetine (Bolo et al, 2000; Strauss et al, 2002).
The plasma and brain fluoxetine levels, measured in SW mice at the 3 mg/kg/day dose, are adequate for the development of therapeutic effects in humans and correspond to 76–85% 5-HT transporter (5-HTT) occupancy (in various brain regions) (Meyer et al, 2004). Mice have a similar relationship between fluoxetine plasma levels and 5-HTT occupancy/5-HTT binding activity (Hirano et al, 2005).
Juvenile mice respond to fluoxetine with a paradoxical anxiogenic effect
SSRIs are effective at alleviating both anxiety and depression symptoms present in comorbid anxiety-depression disorders. Yet it has been a challenge to reproduce these anxiolytic and especially the antidepressant effects of SSRIs in animals. One particular problem is the time course of the response to the drug treatment: antidepressants require chronic administration in human but most of the animal tests detect the effect of acute treatments. Also, there is no current measure of rodent “mood” and therefore the antidepressant effect of SSRIs is difficult to assess. Some recent data indicate however, that the anxiolytic effect of chronic SSRIs, including fluoxetine, may be detectable in rodents (Dulawa & Hen, 2005; Dulawa et al, 2004; Rygula et al, 2006; Zazpe et al, 2007). Indeed, the novelty induced hypophagia (NIH) test is sensitive to the chronic but not the acute administration of various antidepressants including fluoxetine in adult mice (Dulawa & Hen, 2005; Dulawa et al, 2004). The NIH test measures avoidance to approach and consume palatable food (sweetened milk) in a stressful environment. Higher approach latency indicates greater avoidance, which reflects a higher anxiety level. Chronic SSRI treatments reduce this approach latency.
Surprisingly, 3 mg/kg/day continuous administration of fluoxetine during the juvenile period resulted in a significant increase in the latency to drink in a novel cage at 6 weeks of age in SW mice (Treatment: F1,58=9.60, P=0.0029; N=31 and 29) indicating an anxiogenic instead of the expected anxiolytic effect (Fig. 2A). Latency to drink in the home cage was not different between control (saline/water) and fluoxetine treated SW animals (F1,58=0.94, P=0.336;, Fig. 2A) indicating no baseline difference between the groups and that the increased latency of fluoxetine treated mice in the novel cage was not the result of decreased motivation or increased anhedonia. Another measure of anxiety, the difference in approach latency between the novel cage and the home cage, was also significantly greater in the fluoxetine-treated group compared with saline-treated controls (Control 254.80±43.79 sec vs. Fluoxetine: 389.27±45.27 sec, mean±SD; F1,58=4.55, P=0.037).
Figure 2.
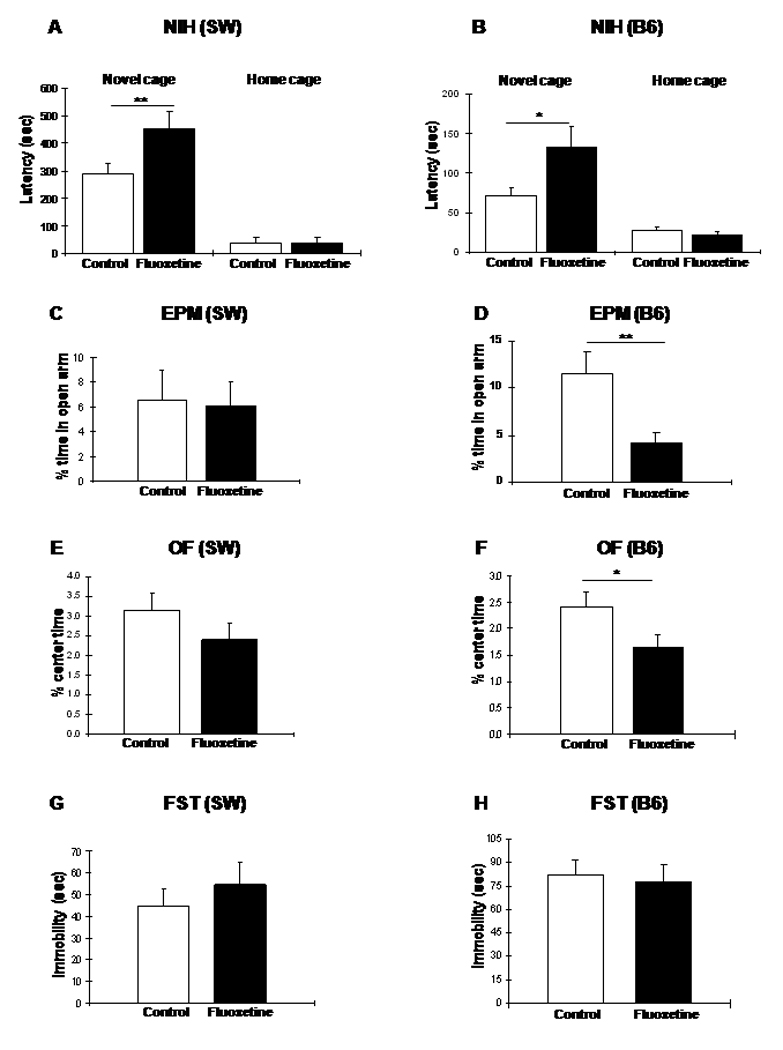
Paradoxical anxiogenic effect of fluoxetine in 6 week old SW (A, C, E, G) and B6 (B, D, F, H) mice treated with 3 mg/kg/day for ∼4 weeks during the juvenile period as measured in the NIH (A and B), EPM (C and D), OF (E and F), and FST (G and H) tests at the end of the treatment (ANOVA with LSD posthoc test: *p<0.05; **p<0.01).
To confirm the surprising paradoxical effect produced by juvenile fluoxetine administration in SW mice, we repeated this experiment with the relatively less “anxious” B6 mice in NIH (latency to drink in novel environment at 6 weeks of age: SW 290.06±26.57 sec.; B6 71.64±25.37 sec.; F1,63=35.33, P<0.0000001; Fig. 2A and B). Similar to SW mice, juvenile B6 mice showed an increase in novel cage latency to 3 mg/kg/day fluoxetine (Treatment: F1,68=4.35, P=0.041; N=34 and 36)(Fig. 2B). Like in the SW groups, no difference was seen in home cage latencies between the control and fluoxetine treated B6 animals (F1,68=0.80, P=0.373;, Fig. 2B).
Two other behavioral tests, the elevated plus maze (EPM) and open field (OF) are usually insensitive to chronic fluoxetine administered in adulthood (Borsini et al, 2002; Prut & Belzung, 2003) but the B6 strain exhibits an anxiolytic response in the OF test to chronic fluoxetine treatment (Chen et al, 2006). A strain difference was also reflected in of how SW and B6 mice respond to juvenile fluoxetine treatment. While administration of 3 mg/kg/day fluoxetine in juvenile SW mice produced no behavioral changes in either test (Fig. 2C and E), B6 mice exhibited a paradoxical reduction in time spent in the open arm of EPM (F1,58=7.87, P=0.007; Fig. 2D) and in the center of the OF (F1,58=4.48, P=0.038; Fig. 2F). Total activity of fluoxetine treated B6 mice was not different from that of saline treated animals in the EPM (F1,58=0.22, P=0.637). However, total locomotor activity of these mice was reduced in OF (saline: 3102±85 cm, N=34; fluoxetine: 2656±83 cm N=36; mean±SD; F1,68=13.94, P=0.0004). Reduced activity in OF can also be interpreted as increased anxiety because suppression of general locomotor activity represented one of the dimensions of anxiety in a large mouse QTL study (Henderson et al, 2004). Also, 5-HT1A receptor knockout mice exhibit not only anxiety in EPM and OF but also reduced locomotor activity in OF (Gross et al, 2002).
The forced swim test (FST) is generally used to detect the antidepressant-like effect of fluoxetine and other SSRIs following acute administration. All major groups of antidepressants reduce immobility time in this test. However, FST is not a good predictor of antidepressant effect when clinically more relevant chronic administration conditions are used. Indeed, only one (BALB/c) of the four tested (B6, 129SvEv, DBA/2) mouse strains responded to a three week long drug treatment (Dulawa et al, 2004). When juvenile SW and B6 mice following chronic fluoxetine administration were tested in FST, we saw no significant behavioral changes (SW: F1,56=0.05, P=0.822; B6: F1,50=0.09, P=0.758; Fig. 2G and H).
Juvenile fluoxetine treatment has no effect on neuronal proliferation in the dentate gyrus
Fluoxetine and other antidepressants increase neuronal proliferation in the adult dentate gyrus (Malberg et al, 2000) while anxiety induced by stress and depression-like conditions in animal models are associated with reduced dentate proliferation (Czeh et al, 2001; Gould et al, 2000). Some studies however dispute these links (Bessa et al, 2008; Holick et al, 2008; Reif et al, 2006; Vollmayr et al, 2003). The increased anxiety of juvenile fluoxetine treated mice was not associated with a significant reduction in proliferation at 6 weeks of age as measured by the incorporation of BrdU to cellular DNA with 2 h survival time (BrdU positive cell number per section: control 7.72±0.98 and fluoxetine 7.06±0.98; t=0.47, p=0.64, 8–10 sections per animal, 6 animals per group).
Juvenile fluoxetine treatment has no lasting effect on anxiety-related behavior
Previous data showed that in utero and early postnatal exposure to fluoxetine results in lasting behavioral abnormalities including increased anxiety (Ansorge et al, 2004; Noorlander et al, 2008; Popa et al, 2008). This raised the possibility that the increased anxiety of juvenile mice at 6 weeks of age in our experiments may not be an adverse drug effect but rather the manifestation of a permanent anxiety phenotype. To differentiate between these scenarios, SW and B6 mice were exposed to fluoxetine in their juvenile age (2–6 weeks of age) and then withdrawn from the drug and tested 6 weeks later (at 12 weeks of age) in NIH test, EPM, OF and FST. None of the tests indicated anxiety or behavioral change (Fig. 3) suggesting that juvenile administration of fluoxetine does not elicit permanent anxiety-like behaviors in SW or B6 mice and that the increased anxiety response of juvenile mice of both strains in the NIH test, as well as of the B6 in the EPM and OF tests, was likely an adverse drug response.
Figure 3.
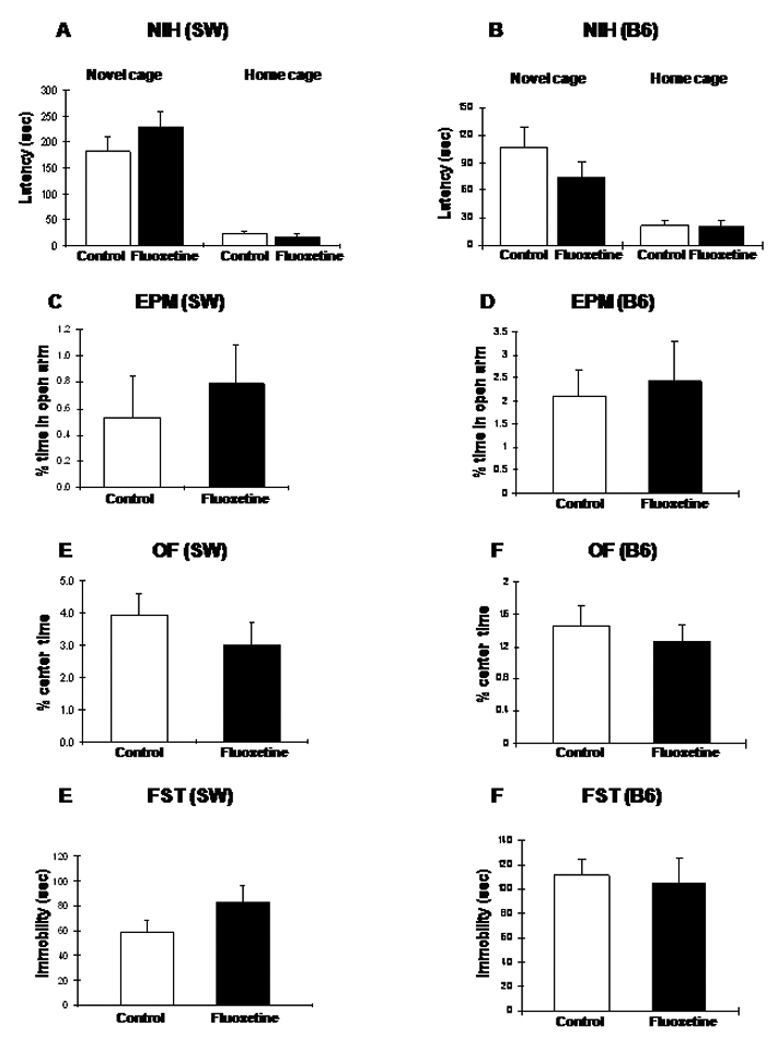
Behavior of 12 week old SW (A, C, E, G) and B6 (B, D, F, H) mice following juvenile (between 2 and 6 weeks of age) fluoxetine treatment (3 mg/kg/day) and a 6 week drug free period. (A and B) The anxiogenic effect of fluoxetine seen at 6 weeks of age in SW and B6 mice in the NIH test is no longer detectable at 12 weeks of age. (C-H) Other behavioral parameters are also normal in adult mice exposed to fluoxetine during the juvenile period.
Adult mice respond to fluoxetine with anxiolytic effects
Since the effect of fluoxetine administration in SW and B6 juvenile mice was anxiogenic, we assessed if the drug elicits the expected anxiolytic effect in adults. Higher than the 3 mg/kg/day doses were also tested because to achieve an anxiolytic/antidepressant effect in adult mice, fluoxetine in the 10–25 mg/kg/day range has been used (Dulawa & Hen, 2005). As shown in Fig. 4, 18 mg/kg/day fluoxetine administered for 3–4 weeks starting at 8 weeks of age resulted in an anxiolytic effect in SW mice. On the other hand, the 3 mg/kg/day (that produced anxiety in juvenile mice), as well as an intermediate 12 mg/kg/day dose, resulted in no detectable effect in adult SW mice indicating that once the brain fully develops, these lower drug doses have no adverse effects in this strain (Fig. 4). As reported earlier (Chen et al, 2006), C57Bl/6 mice also exhibit an anxiolytic response in the NIH test following 3 weeks of 18 mg/kg/day fluoxetine administration starting at 8–10 weeks of age (the current and Chen et al. studies were performed by using the same method and equipment; see Material and Methods).
Figure 4.
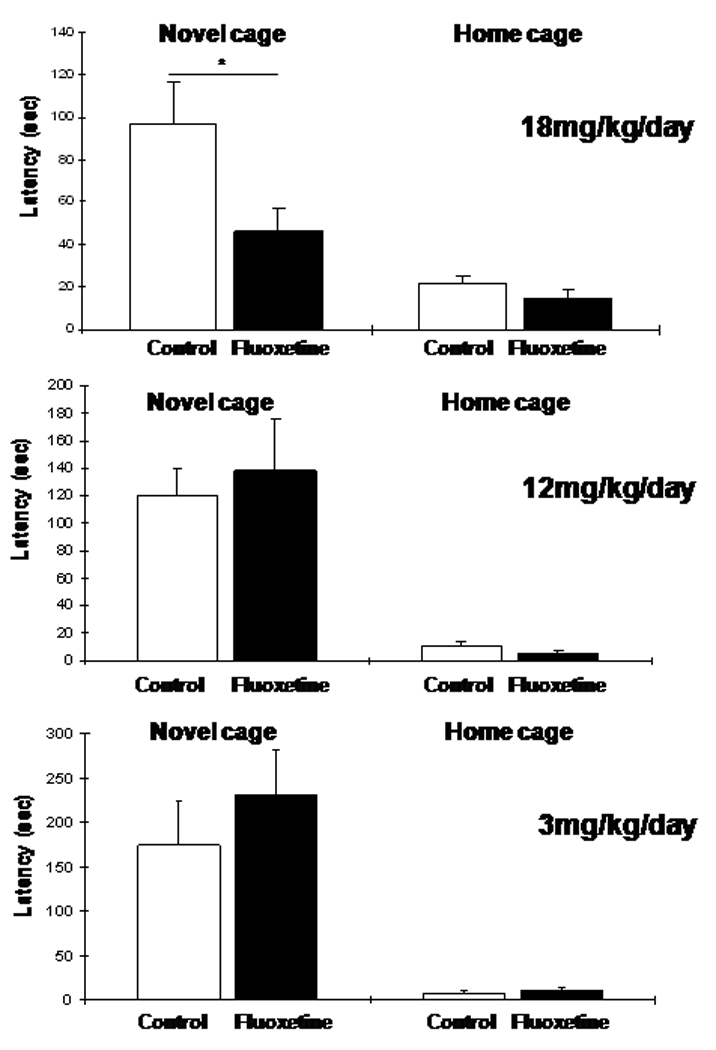
Four weeks of chronic fluoxetine administration produces an anxiolytic response in adult SW mice in the NIH test. However, only the relatively high 18 mg/kg/day dose was effective, while the 3 and 12 mg/kg/day doses resulted in no change in novel cage latencies (ANOVA with LSD posthoc test: *p<0.05).
Plasma drug levels in juvenile and adult SW mice following the 3 mg/kg/day dose were comparable (juvenile: Figs. 1C; adult: fluoxetine 73.2±6.3, norfluoxetine 230.5±21.3 ng/ml, mean±SD; N=5 per group). The higher 12 mg/kg/day (fluoxetine 840.4±116.2, norfluoxetine 1458.7±268.2 ng/ml, mean±SD; N=5 per group) and 18 mg/kg/day doses produce plasma concentrations at the highest range measured in patients undergoing treatment. Similarly high levels were detected at 18 mg/kg in B6 mice as well (fluoxetine: 958.8±118.7; norfluoxetine, 2050.9±233.5 ng/ml; N=5 per group). These data indicate that the juvenile but not the adult brain is sensitive to clinically relevant drug concentrations in mice and that only very high drug levels elicit anxiolytic effects in adult mice in the NIH test. Since a much lower drug level was sufficient to elicit the paradoxical anxiogenic response, the anxiogenic and anxiolytic effects of fluoxetine in juvenile and adult mice are probably mediated by different mechanisms.
In the EPM and OF tests, adult SW mice did not show an anxiolytic effect to 18 mg/kg/day fluoxetine (Fig. S5). In contrast, B6 mice exhibit reduced anxiety to 18 mg/kg/day fluoxetine in the OF test (Chen et al, 2006). This strain dependent pattern of adult response mirrors the juvenile pattern; i.e. B6 responds though the juvenile and adult responses are opposite while SW does not respond in either age (Fig. 2 and Fig. 4, summarized in Table 1).
Table 1.
Anxiolytic vs. anxiogenic response to chronic fluoxetine is age dependent across two strains and various behavioral tests
 reduced and
reduced and  increased anxiety-like behavior
increased anxiety-like behavior  no behavioral change
no behavioral change
The paradoxical anxiogenic response of juvenile mice to fluoxetine is recapitulated on re-exposure in adulthood in SW but not in B6 mice
Although juvenile exposure to fluoxetine had no long lasting effects on baseline anxiety and depression-like behavior in adult mice, it could have altered drug response permanently. To test this possibility, SW and B6 mice treated during the juvenile period (2–6 weeks) were re-exposed as adults (after a 6 week drug free period) to 3 or 12 mg/kg/day fluoxetine for 4 weeks (12–16 week) (Fig. 5). While 3 mg/kg/day fluoxetine had no effect (Fig. S6), re-exposure to 12 mg/kg/day resulted in an increase in novel cage latency in SW mice (One way ANOVA: F3,30=3.17, P=0.038; posthoc analysis: Fluoxetinejuvenile→Fluoxetineadult vs. Controljuvenile→Controladult, P=0.0356 and F→F vs. C→F, P=0.006, 8–10 animals per group; Fig. 5A). No change was seen in F→C as compared to C→C mice, in agreement with previous data (Fig. 3A) showing that juvenile fluoxetine treatment has no long lasting effect on behavior in the NIH test in the SW mice. Latencies in home cage were not significantly changed in any group compared to the C→C group (One way ANOVA, F3,30=1.38, P=0.267) indicating that home cage behavior is not altered by the treatment. In contrast to SW mice, re-exposure of B6 mice to 12 mg/kg/day fluoxetine resulted in no behavioral effect in NIH test (Fig. 5B). These B6 mice showed no behavioral changes in EPM and OF either or in FST (Supplementary Fig. 7)(SW mice responded to neither juvenile nor adult administration of fluoxetine in EPM and OF, see Fig. 2 and Fig. 3; thus, there was no behavior to recapitulate and the effect of fluoxetine re-exposure was not assessed in SW mice in these tests). Taken together, these data suggest that the juvenile low fluoxetine exposure led to permanent changes in the antidepressant drug response in the NIH test in SW but not in B6 mice.
Figure 5.
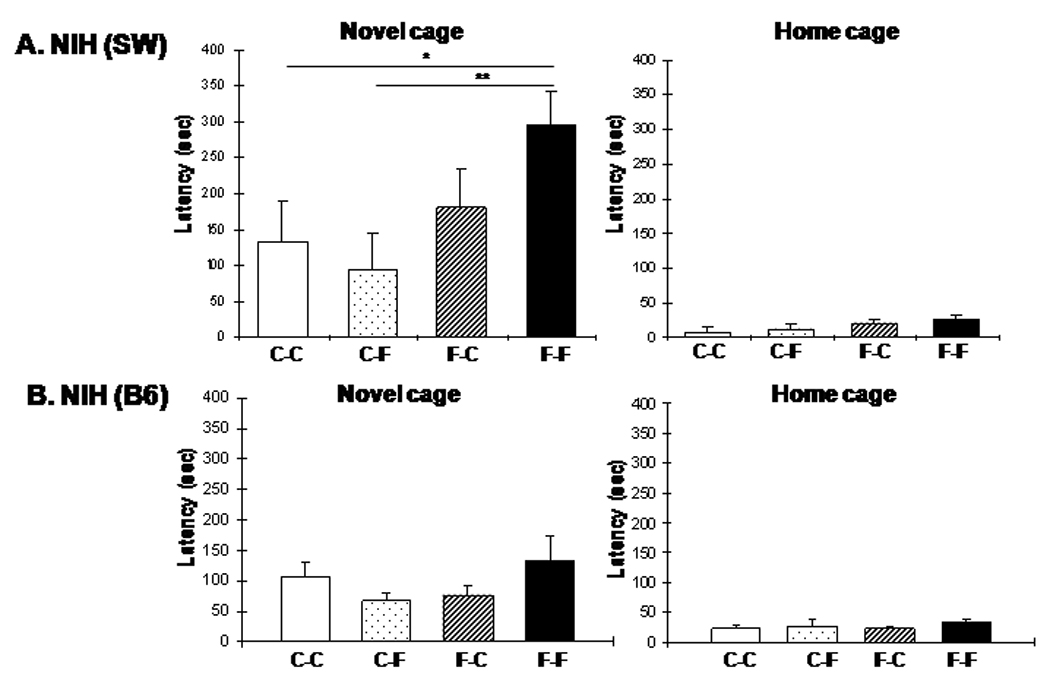
The anxiogenic effect of fluoxetine produced in juvenile animals is recapitulated on re-exposure to the drug in adulthood in the NIH test in SW, but not B6 mice. (A) Re-administration of 12 mg/kg/day dose for 4 weeks (12–16 weeks of age) in the SW mice resulted in increased approach latencies in the novel cage in F→F compared to C→C and C→F treatment groups (ANOVA with LSD posthoc test: *p<0.05; **p<0.01). No differences were observed in the FST test between the groups. (B) Re-exposure to 12 mg/kg/day fluoxetine produced no change in B6 mice in the NIH test. (C-D) No fluoxetine effect in EPM and OF in B6 mice either. CC: Controljuvenile→Controladult, CF: Controljuvenile→ Fluoxetineadult, FC: Fluoxetinejuvenile →Controladult, FF: Fluoxetinejuvenile→Fluoxetineadult.
Discussion
Both genetic (inactivation of MAO-A and 5-HTT) and pharmacological (administration of SSRIs) experiments indicate that excessive levels of 5-HT during prenatal and early postnatal life in rodents have long term morphological and behavioral consequences (Ansorge et al, 2008; Ansorge et al, 2004; Cases et al, 1995; Cases et al, 1996; Maciag et al, 2006; Popa et al, 2008). Indeed, fluoxetine administration between E8 and E18 as well as from P4 to P20 resulted in persistent anxiety and depression-like behaviors (Ansorge et al, 2004; Noorlander et al, 2008). Due to the early onset of the 5-HT effect in these experiments and because rodents are born less mature compared to human (e.g. eye opening and hearing onset are on the second postnatal week in rodents) (Carlson & Willott, 1998; Rauschecker, 1999), these pharmacological and genetic studies may not answer the question of whether SSRIs have short- or long-term adverse effects in children and adolescents. To match the developmental time-frame corresponding to the child and adolescent period more precisely in mice, fluoxetine in our experiments was administered between 2 and 6 weeks of age in mice.
The main finding of our study is that chronic fluoxetine when administered to juvenile mice elicits an anxiogenic effect while in adult mice the drug results in the expected anxiolytic effect. Indeed, both SW and B6 mice at juvenile age exhibit increased anxiety to chronic fluoxetine in the NIH test while as adults they respond to the drug with an anxiolytic effect (Table 1). Although response to chronic fluoxetine in the EPM and OF is strain specific, when there is an anxiolytic effect in adults, there is invariably an anxiogenic response in juvenile mice (Table 1). Therefore, the age of the mice, independently of the strain and test used in our experiments, is the primary determining factor of whether the response to chronic fluoxetine is anxiolytic or anxiogenic.
Adverse effects including anxiety and depressed mood to fluoxetine have been also observed in children and adolescents (March et al, 2004). Although extrapolating our results to the pediatric/adolescent use of fluoxetine may not be straightforward, the abnormal response of juvenile mice to fluoxetine may suggest that the antidepressant effect of the drug may be counteracted by anxiety promoting adverse effects which could reduce the overall therapeutic efficacy of the drug. Taken together, administration of fluoxetine between 2 and 6 weeks of age in mice reproduces some of the adverse effects of the drug described in human studies and the juvenile fluoxetine administration model may be suitable to study the origin and nature of adverse fluoxetine effects specific for the child and adolescent period.
While the anxiogenic effect of chronic fluoxetine in juvenile mice was apparent at 3 mg/kg/day in both SW and B6, the anxiolytic effect of the drug was detectable only at 18 mg/kg/day dose in these strains. The 3 mg/kg/day dose, whether in juvenile or adult mice, resulted in therapeutically relevant plasma drug levels (100–300 ng/ml fluoxetine-norfluoxetine), indicating that the developing brain may be particularly sensitive to the adverse effects of fluoxetine. Data show that 20 mg/day chronic fluoxetine administration corresponds to about 100 ng/ml drug plasma level and 76–85% 5-HTT occupancy in human. This occupancy level is sufficient for the development of a therapeutic effect (Meyer et al, 2004). Mice have a similar relationship between fluoxetine plasma levels and 5-HTT occupancy/5-HTT binding activity (Hirano et al, 2005). The sensitivity of juvenile mouse brain to the adverse effects of fluoxetine is consistent with the notion that children and adolescents have a different adverse effect profile to SSRIs than adults. The 18 mg/kg/day dose that required for the development of the anxiolytic activity in adult mice corresponds to very high human plasma levels that are well over the normal therapeutic level. This may indicate a species difference in the anxiolytic effect of fluoxetine in adults.
Anxiety has been associated with reduced proliferation in the dentate gyrus of adult rodents (Gould et al, 2000). There was no change in neuronal proliferation in fluoxetine treated juvenile mice exhibiting anxiety in our experiments. Although it is not clear if this was due to the low albeit therapeutically relevant fluoxetine level, the age of animals or some other factors, our data show that the anxiety phenotype of fluoxetine treated juvenile mice may not be linked to a neuronal proliferation defect in the dentate gyrus.
In contrast to the studies that targeted an earlier developmental period (Ansorge et al, 2004; Noorlander et al, 2008), our studies with juvenile mice showed no permanent anxiety or depression-like phenotypes in either B6 or SW mice. A similar study with fluoxetine administration between P21 and P49 showed no long-term behavioral effects either (Norcross et al, 2008). The anxiety phenotype of fluoxetine treated mice at 6 weeks of age that disappeared after a 6 week drug free period is also consistent with the reversibility of adverse drug events to dose reduction or treatment discontinuation in children and adolescents. In summary, our study indicates that in contrast to previous assertions, fluoxetine administration during postnatal development in mice, which corresponds to the child and adolescent period, is not likely to cause permanent behavioral alterations.
An unexpected finding in our study is that re-exposure to fluoxetine of adult mice, previously treated with fluoxetine during the juvenile period, recapitulated the paradoxical anxiogenic effect of the drug. However, this effect was detected only in SW mice indicating that this phenomenon is strain dependent. Nevertheless, the “recall” of the anxiogenic response upon re-exposure could indicate permanent changes in the antidepressant drug response in this strain. If similar effects occur in the human population, our data would suggest that exposure of susceptible individuals to fluoxetine in adolescence may render them less responsive to future drug treatment.
Supplementary Material
Acknowledgements
We thank Sandra Bruening and Charles Inturrisi for their help in the fluoxetine measurements and pharmacokinetic studies, respectively. We thank Noel Yan Ki Chan for her help with the NIH tests. We also thank for Drs. Kevin Bath and Francis Lee for providing us plasma from B6 mice chronically treated with fluoxetine. Finally we thank Sonali Tatapudy for counting BrdU positive cells. The work was supported by NIMH 1R21MH072820 and 5R01MH058669 to M.T.
Footnotes
Disclosure. The authors have no conflict of interest to declare.
References
- Alvarez JC, Bothua D, Collignon I, Advenier C, Spreux-Varoquaux O. Determination of fluoxetine and its metabolite norfluoxetine in serum and brain areas using high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 1998;707:175–180. doi: 10.1016/s0378-4347(97)00588-4. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Michelson D, Hornig-Rohan M, Beasley CM. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J Psychiatry. 1997;154:963–969. doi: 10.1176/ajp.154.7.963. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Perel J, Nelson B. Childhood and adolescent depression: a review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Bolo NR, Hode Y, Nedelec JF, Laine E, Wagner G, Macher JP. Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy. Neuropsychopharmacology. 2000;23:428–438. doi: 10.1016/S0893-133X(00)00116-0. [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Carlson S, Willott JF. Caudal pontine reticular formation of C57BL/6J mice: responses to startle stimuli, inhibition by tones, and plasticity. J Neurophysiol. 1998;79:2603–2614. doi: 10.1152/jn.1998.79.5.2603. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav Genet. 2004;34:267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- Henry ME, Schmidt ME, Hennen J, Villafuerte RA, Butman ML, Tran P, Kerner LT, Cohen B, Renshaw PF. A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: A 19-F MRS study. Neuropsychopharmacology. 2005;30:1576–1583. doi: 10.1038/sj.npp.1300749. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, Hashimoto H, Yamada S. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol. 2005;144:695–702. doi: 10.1038/sj.bjp.0706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Lundmark J, Reis M, Bengtsson F. Serum concentrations of fluoxetine in the clinical treatment setting. Ther Drug Monit. 2001;23:139–147. doi: 10.1097/00007691-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. Jama. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, Ginovart N, Spencer EP, Cheok A, Houle S. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Noorlander CW, Ververs FF, Nikkels PG, van Echteld CJ, Visser GH, Smidt MP. Modulation of serotonin transporter function during fetal development causes dilated heart cardiomyopathy and lifelong behavioral abnormalities. PLoS ONE. 2008;3:e2782. doi: 10.1371/journal.pone.0002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross M, Poonam M, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, Harvey-White J, Holmes A. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 2008;200:413–424. doi: 10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulak PJ, Kenney JT, Debus JR, Crowley G, Wittman PD. Determination of the antidepressant fluoxetine and its metabolite norfluoxetine in serum by reversed-phase HPLC with ultraviolet detection. Clin Chem. 1988;34:1875–1878. [PubMed] [Google Scholar]
- Popa D, Lena C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006;174:188–192. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Silverstone PH. Qualitative review of SNRIs in anxiety. J Clin Psychiatry. 2004;65 Suppl 17:19–28. [PubMed] [Google Scholar]
- Strauss WL, Unis AS, Cowan C, Dawson G, Dager SR. Fluorine magnetic resonance spectroscopy measurement of brain fluvoxamine and fluoxetine in pediatric patients treated for pervasive developmental disorders. Am J Psychiatry. 2002;159:755–760. doi: 10.1176/appi.ajp.159.5.755. [DOI] [PubMed] [Google Scholar]
- Tatapudy S, Bruening S, Gleason G, Toth M. Validation and use of a computer-assisted counting procedure to quantify BrdU-labeled proliferating cells in the early postnatal mouse hippocampus. J Neurosci Methods. 2008;172:173–177. doi: 10.1016/j.jneumeth.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- Wang PS, Simon G, Kessler RC. The economic burden of depression and the cost-effectiveness of treatment. Int J Methods Psychiatr Res. 2003;12:22–33. doi: 10.1002/mpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Cohen L, Biederman J, Abrams A, Neft D, Faird N, Sinha V. Fluoxetine pharmacokinetics in pediatric patients. J Clin Psychopharmacol. 2002;22:568–575. doi: 10.1097/00004714-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Zazpe A, Artaiz I, Labeaga L, Lucero ML, Orjales A. Reversal of learned helplessness by selective serotonin reuptake inhibitors in rats is not dependent on 5-HT availability. Neuropharmacology. 2007;52:975–984. doi: 10.1016/j.neuropharm.2006.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


