Abstract
The ability to recall contextual details associated with an event begins to develop in the first year of life, yet adult levels of recall are not reached until early adolescence. Dual-process models of memory suggest that the distinct retrieval process that supports the recall of such contextual information is recollection. In the present investigation, we used both behavioral and electrophysiological measures to assess the development of memory for contextual details, as indexed by memory for temporal order, in early childhood. Results revealed age-related improvements in memory for temporal order despite similar levels of memory for the individual items themselves. Furthermore, this pattern of recall was associated with specific components in the electrophysiological response. Consistent with electrophysiological research in adults, distributed, positive-going activity late in the waveform was associated with increases in recall of contextual details and the development of recollective processes.
Introduction
Memory for individual items and memory for contextual details surrounding the items are thought to be the result of separable mnemonic subsystems (e.g. Schacter & Tulving, 1994; Schacter, Wagner & Buckner, 2000; Yonelinas, 2002). Research utilizing behavioral and functional neuroimaging techniques (i.e. event-related potentials and functional magnetic resonance imaging) in neurotypical adults and adults with localized lesions supports this claim with findings that item memory and memory for item-context relations rely on (1) different mnemonic processes and (2) separable neural systems in both the medial temporal lobe and prefrontal cortex (Aggleton & Brown, 1999; Eichenbaum & Cohen, 2001; Yonelinas, Otten, Shaw & Rugg, 2005).
Familiarity has been defined as the global assessment of the strength of a memory trace, whereas recollection refers to retrieval of qualitative information surrounding an event (Yonelinas, 2002). Recollection involves retrieval of contextual information specific to the study episode whereas familiarity does not. Although memory for an individual item may involve recollective processes, it can be subserved by familiarity processes alone. In contrast, memory for contextual details, such as the temporal order of items, likely requires recollective processes. Evidence for this dissociation comes from neuroimaging literature in adults, which indicates that familiarity and recollection rely on different networks of brain regions. Familiarity processes (typically engaged when memory for individual items is evaluated) have been associated with regions in the lateral prefrontal cortex, superior parietal cortices, and precuneus. In comparison, recollective processes (engaged when memory for details surrounding individual items is probed) have been shown to activate the anterior medial prefrontal cortex, lateral parietal/temporal regions and the hippocampus (see Yonelinas et al., 2005, for details).
A developmental dissociation between recollection and familiarity has also been documented in behavioral research during middle childhood and adolescence (Cycowicz, 2000; Cycowicz, Friedman, Snodgrass & Duff, 2001; Czernochowsk, 2005; Sluzenski, Newcombe & Kovacs, 2006; see Nelson, Thomas & de Haan, 2007, for review). For example, in one investigation, 7- to 8-year-old children and adults were asked to recall pictures of individual items and their color (a distinct contextual detail). Although an age-related increase was observed for memory for individual items, a statistically independent increase was also observed for memory of the color of the items (Cycowicz et al., 2001). Taken together, studies from the developmental literature suggest that familiarity processes appear to become relatively stable during early childhood, whereas recollective processes continue to develop over the course of childhood and into adolescence. Although some attempts have been made to localize the cortical source of these developmental changes in memory processes (with the frontal cortex being a likely candidate; see Cycowicz et al., 2001), little is currently known about the development of the different networks, of brain regions underlying these processes and how they relate to individual differences in performance during childhood (see Nelson et al., 2007, for discussion). Constraints of the fMRI environment, coupled with the high cognitive demands of paradigms typically used to dissociate these processes in adults, have pushed researchers to seek novel paradigms to investigate this question.
Event-related potentials (ERPs), which represent the activity of large populations of neurons that have been synchronously activated in response to a discrete stimulus, can be recorded even in very young children and may be the most useful tool to address this question early in development (see de Haan, 2007, for review of ERP studies in development). When electrophysiological responses elicited by a certain class of stimuli are averaged together (e.g. all responses to novel items versus responses to previously learned items), differences in the spatial-temporal properties of the resulting waveforms allow for the inference of differences in neural processing related to cognition. For example, studies of memory retrieval in both adults and children have demonstrated differences in the amplitude and latency of certain components between familiar or previously learned items and novel items (e.g. Marshall, Drummey, Fox & Newcombe, 2002). During infancy, measures of both the amplitude of the middle latency component (referred to as ‘Nc’) and slow wave activity to familiar and novel items have been shown to reliably distinguish between infants who subsequently recall the items after a 1-month delay versus those who do not (e.g. Bauer, Wiebe, Carver, Waters & Nelson, 2003; Carver, Bauer & Nelson, 2000). In the single study to date in 4-year-old children, measures of slow wave activity have been shown to distinguish correctly recognized familiar items from correctly rejected novel items after a 5-minute delay (i.e. an old–new effect; Marshall et al., 2002). Differences such as these have been termed ‘the episodic memory (EM) effect’ in the adult literature (Friedman & Johnson, 2000).
The extent to which differences in electrophysiological responses to stimuli are the result of familiarity versus recollective processes (or the cognitive ‘source’ of the EM effect) has become the focus of much interest. Previous research with adults has shown that different temporo-spatial patterns of brain activity underlie the familiarity and recollection processes posited in dual process models of recognition memory (e.g. Duarte, Ranganath, Winward, Hayward & Knight, 2004). Specifically, these studies have shown that enhanced positivity at frontopolar scalp sites, especially over left medial prefrontal sites, is associated with familiarity processes and begin about 100 ms earlier than later memory components, which consist of positive modulations over bilateral frontal and left parietal sites and vary in a manner consistent with recollective processes (see Friedman & Johnson, 2000, for a review). Thus, results from ERP studies in adults suggest that both familiarity and recollective processes can be detected in the electrophysiological response and have different temporal and spatial distributions across the scalp. Familiarity processes are reflected in early components, whereas recollective processes are reflected in later, more distributed brain activity.
Similar patterns regarding differences in the temporal distribution of components reflecting familiarity and recollective processes have been observed in middle childhood and adolescence (Cycowicz, Friedman & Duff, 2003; Czernochowski, 2005; Czernochowski, Mecklinger, Johansson & Brinkmann, 2005). In these paradigms, children are asked to recall both individual items and details of their study context (e.g. the original color of the stimulus, or when it occurred within a study session). As in adults, retrieval of study context information produces differences in distributed activity late in the electrophysiological response, thought to reflect recollective processing (although this dissociation is observed only when performance levels are high; see Czernochowski et al., 2005). Because these paradigms are similar to those used with adults and require a specific, overt behavioral response indicating the source of the information, performance levels of children are consistently below those of adults and rarely reach an acceptable threshold (e.g. greater than 75% correct). Even 10- to 12-year-old children have greater difficulty retrieving context compared to adults. Thus, there is a lower bound to the ages that can be tested with these paradigms, and the contribution of familiarity and recollection to these effects in infants and young children remains unclear.
In the present investigation we examined age-related changes in memory for items versus memory for contextual details in 3- and 4-year-old children with a behavioral recall paradigm adapted from infant memory literature. A main goal of the current study was to begin to assess, during a passive viewing paradigm, which components of the electrophysiological response are related to memory for individual items (and thus likely reflect familiarity processes) versus memory for context as indicated by temporal order memory (and thus likely reflect recollection processes) in early childhood.
Method
Participants
Forty-eight 3- and 4-year-old typically developing children were tested: 22 children were 3 years of age (14 female, mean age at testing = 3 years ±18 days), and 26 children were 4 years of age (15 female, mean age at testing = 4 years ±11 days). Participants’ mothers were recruited before giving birth for participation in an ongoing longitudinal research project examining cognitive development (see Nelson, 2007, for a recent summary of previous reports on the sample). In accordance with the American Psychological Association’s guidelines for ethical treatment of human participants, parents provided informed consent for their children to participate and the University’s Institutional Review Board approved all procedures prior to the start of the investigation.
In analyses with repeated measures, listwise deletion procedures were used; therefore, sample sizes vary slightly between tasks, and degrees of freedom for each analysis were adjusted accordingly. When the assumption of sphericity was violated, Greenhouse-Geisser corrections were used. Behavioral analyses include data from 31 children (17 3-year-olds and 14 4-year-olds) and electrophysiological analyses include data from 38 participants (14 3-year-old and 22 4-year-old children). Behavioral data were excluded due to: incomplete data due to refusal to complete a particular event sequence (n = 5), missed appointment (n = 1), deviations from standardized protocol (n = 4), or video equipment failure (n = 7). Electrophysiological data were excluded as a result of: refusal to wear the cap (n = 5), excessive movement artifact resulting in an insufficient number of trials (n = 2), incomplete data due to missed appointment (n = 1), and equipment failure (n = 2).
Materials
Event sequences
Children were each shown three different nine-item event sequences. The event sequences contained multiple props or objects that could be used to produce nine different individual actions within a common theme (e.g. going camping, see Figure 1). Thus, within each sequence, memory for both the individual items (e.g. baiting a hook or catching a fish or setting up the tent) and the order of these items (i.e. baiting the hook before catching the fish and then setting up the tent) could be assessed. To induce variability in recall of the temporal order of the items we manipulated the internal organization of the event sequences by altering the number of enabling connections between items in the event sequences (see Bauer, Hertsgaard, Dropik & Daly, 1998). Enabling relations exist when it is necessary to complete the individual items in the correct temporal order in order to achieve the desired end state (for example, baiting a hook before catching a fish). However, each individual item can be completed independent of the others (i.e. in our task one could attempt to catch the fish without bait, but would not be successful). Event sequences with more enabling relations have greater internal organization, which leads to a more organized representation. Ultimately, sequences with more enabling relations are easier to recall because each item in the event serves as a reminder of subsequent items. The differential effects of reminders may be associated with the strength of the organization of the event representation rather than with the event type per se. This argument implies that if an event representation is sufficiently well organized to support reliable ordered recall, then there should exist the potential for recollection to facilitate memory for the event (see Bauer et al., 1998, for elaboration).
Figure 1.
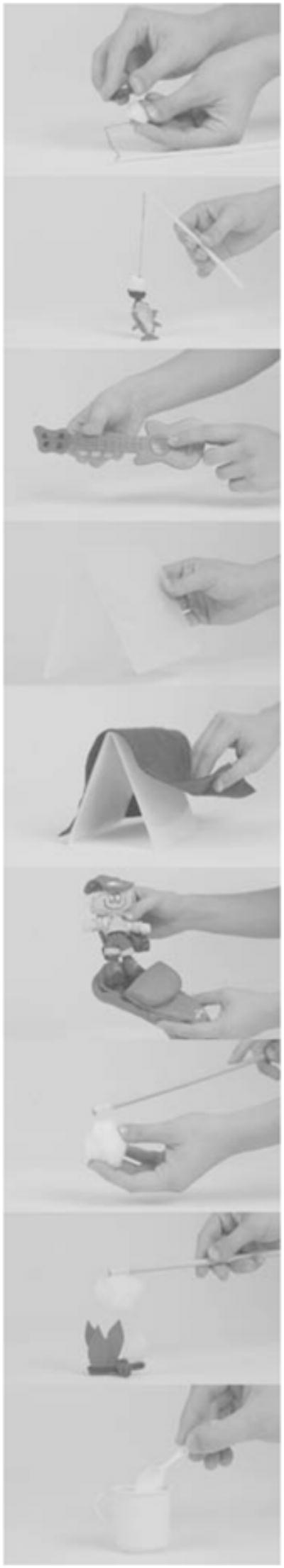
Example of ‘Camping’ themed event sequence containing multiple props that could be used to produce nine different individual actions in a specified temporal order (in order from top to bottom: bait the hook, catch the fish, play the guitar, set up the tent, put the cover on the tent, get in the sleeping bag, put the marshmallow on the stick, roast the marshmallow, drink hot cocoa).
Because there were nine items in each of the event sequences, all sequences contained eight possible relations between items. In our manipulation, event sequences with ‘high’ connectivity between the items contained six enabling relations, sequences with ‘medium’ connectivity contained three enabling relations, and sequences with ‘low’ connectivity contained no enabling relations (i.e. all relations between items were arbitrary). Three event sequences were randomly selected from a pool of 15 total sequences (five of each type) for each child, one of each connectivity level (6, 3, 0 enabling relations).
ERP stimuli
For ERP testing, stimuli consisted of digital photographs of a woman’s hand completing each individual item of the three event sequences observed in behavioral testing, and three novel event sequences matched for the number of enabling relations (randomly selected from the remaining pool of sequences). In addition, pictures of the entire group of props of each sequence were presented, resulting in 10 unique stimuli per event. Pictures of the old and new sequences were randomly presented blocked by sequence type; thus repetition of pictures occurred within blocks. The order of the blocks was counterbalanced between participants and each picture was presented twice for a total of 120 trials (40 per sequence type).
Procedure
Children visited the lab twice. The first session consisted of orientation to the task, baseline assessment of children’s spontaneous behaviors with the event sequences, modeling of the sequences, and a measure of immediate recall. Orientation emphasized that remembering both the items and the order of items was important. Baseline measures were used as a control for problem-solving abilities and/or fortuitous production of the items. Modeling of the event sequences was done twice in succession with verbal narration by an experienced experimenter. Immediate recall of the individual items and temporal order was assessed to ensure learning.
One week later (M = 7 days, SD = 1 day), children returned to the laboratory and electrophysiological and behavioral measures of memory were obtained. In the former, children were seated in a dimly lit room while they viewed pictures of the three familiar and three novel sequences while event-related potentials (ERPs) were recorded from 32 sites on the scalp. Experienced observers monitored children’s gaze during recording and trials were repeated if the child moved or looked away during stimulus presentation. In accordance with previous studies, EEG was recorded from 32 channels sampled at 100 Hz, referenced to Cz and rereferenced off-line to mathematically linked mastoids (see DeBoer, Scott & Nelson, 2007, for review). Electro-ocular activity was recorded from a transverse position above and below the eye to allow for detection and deletion of blink artifacts. Impedances at all leads were kept below 10 kΩ. Immediately following the ERP session, children were asked to behaviorally recall the event sequences.
Data reduction and analysis
Both immediate and 1-week delayed behavioral recall sessions were videotaped and scored off-line by experienced observers. Production of both individual items (maximum = 9 per sequence) and pairs of items in the correct temporal order (maximum = 8 per sequence) were assessed for each sequence that each individual completed at baseline, immediate recall, and 1-week delayed recall. As is standard practice in studies of elicited imitation (Bauer, Wenner, Dropik & Wewerka, 2000), only the first production of each individual item was recorded to reduce the possibility of successful recall of order information due to trial and error. Twenty-five percent of the tapes were re-coded by a second experienced observer to ensure reliability. Inter-rater reliability for behavioral coding was 92.89% for 3-year-olds and 93.50% for 4-year-olds.
Electrophysiological data were excluded if the EEG signal exceeded ±150 μV in any 100 ms window. Individual averages were created for each condition (familiar, novel) for sequence type (6, 3, or 0 enabling relations) with the constraint that an equal number of trials were included for each of the six conditions (M = 16 trials, SD = 3 per condition). Two components of the ERP relevant to our data have been described previously. First, the presence of a well-defined, negative amplitude, middle latency component (occurring 400–600 ms after stimulus onset) that has been related to attentional processes (e.g. Courchesne, Granz & Norcia, 1981; Nelson & Collins, 1991) and is also modulated by memory (e.g. de Haan & Nelson, 1997; Carver et al., 2000). To compare this middle latency component between conditions and age groups, the maximum negative amplitude and latency to peak amplitude in the time interval including the component were calculated and used as the dependent measures. The second component occurred later in the waveform (approximately 900 ms after stimulus onset), was positive in amplitude, and was relatively more distributed over a 500–600 ms window, and has been referred to as positive slow wave activity or PSW (e.g. Nelson, 1994). PSW is typically thought to be invoked by stimuli that have been seen previously and that have been partially encoded (i.e. stimuli that are being updated in memory). To compare PSW between conditions and age groups, area under the curve during the time window that included the activity was calculated and used as the dependent measure.
Results
Behavioral recall
To determine if there were differences between the age groups in encoding or 1-week delayed behavioral recall of the event sequences, a 2 (age: 3 year, 4 year) × 3 (phase: baseline, immediate, delay) × 3 (sequence type: 6 enabling relations, 3 enabling relations, arbitrary) mixed analysis of variance was conducted for both the mean number of individual items and the mean number of pairs of items produced in the target order with repeated measures on phase and sequence type. Means are provided in Table 1. The results clearly indicate that the children learned the event sequences in the laboratory and remembered both the items and the order of the items across the 1-week delay. Production of both individual items and items in the correct temporal order was significantly greater at immediate and delayed recall compared to baseline performance (main effect of phase, F(2, 58) = 399.69 and 92.51, respectively, ps < .001). The 3-and 4-year-old children recalled the individual items with equal proficiency and item memory was equal across the three sequence types (i.e. no effects of age or sequence type). In contrast, recall of the temporal order of items in the sequences varied as a function of age, F(1, 29) = 8.60, p < .01, with 4-year-old children recalling more temporal order information than 3-year-old children. In addition, order memory varied parametrically as a function of the number of enabling relations in the event sequence, F(2, 58) = 60.18, p < .001. Ordered recall was greatest in sequences with six enabling relations, followed by sequences with three enabling relations, and lastly by arbitrary sequences with no enabling relations.
Table 1.
Mean recall of individual items and pairs of items produced in the correct temporal order for 3- and 4-year-old children
| 3-year-old children |
4-year-old children |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Individual items | ||||
| Overall | ||||
| Baseline | 2.38 | 0.39 | 2.75 | 0.41 |
| Immediate | 7.81 | 0.36 | 8.12 | 0.45 |
| Delay | 7.91 | 0.28 | 8.57 | 0.18 |
| Six enabling relations | ||||
| Baseline | 1.80 | 0.33 | 2.82 | 0.38 |
| Immediate | 7.67 | 0.40 | 8.41 | 0.26 |
| Delay | 7.83 | 0.26 | 8.72 | 0.18 |
| Three enabling relations | ||||
| Baseline | 2.00 | 0.37 | 2.24 | 0.40 |
| Immediate | 8.10 | 0.37 | 7.82 | 0.54 |
| Delay | 8.06 | 0.26 | 8.53 | 0.15 |
| Arbitrary | ||||
| Baseline | 3.35 | 0.47 | 3.19 | 0.47 |
| Immediate | 7.67 | 0.31 | 8.13 | 0.56 |
| Delay | 7.83 | 0.33 | 8.44 | 0.20 |
| Pairs of items in correct temporal order | ||||
| Overall | ||||
| Baseline | 0.40 | 0.15 | 0.58 | 0.20 |
| Immediate | 3.10 | 0.39 | 3.74 | 0.43 |
| Delay | 2.43 | 0.27 | 3.59 | 0.44 |
| Six enabling relations | ||||
| Baseline | 0.40 | 0.15 | 0.76 | 0.28 |
| Immediate | 4.48 | 0.33 | 6.00 | 0.44 |
| Delay | 3.72 | 0.28 | 5.78 | 0.49 |
| Three enabling relations | ||||
| Baseline | 0.30 | 0.15 | 0.47 | 0.15 |
| Immediate | 3.43 | 0.41 | 3.41 | 0.37 |
| Delay | 2.89 | 0.33 | 3.76 | 0.50 |
| Arbitrary | ||||
| Baseline | 0.50 | 0.15 | 0.50 | 0.18 |
| Immediate | 1.38 | 0.44 | 1.81 | 0.48 |
| Delay | 0.67 | 0.21 | 1.22 | 0.32 |
Electrophysiological measures
To determine the effects of age and sequence type on 1-week delayed recognition memory as indexed by ERPs, a 2 (age: 3 year, 4 year) × 3 (sequence type: 6 enabling relations, 3 enabling relations, arbitrary) × 2 (condition: familiar, novel) mixed analysis of variance with repeated measures was conducted on sequence type and condition for each of three dependent variables: peak amplitude of the middle latency component, latency to the peak amplitude of the middle latency component, and area under the curve for slow wave activity at the midline leads.1 Results from the midline leads suggested a frontocentral distribution of the components of interest; thus parallel analyses were also computed for four groups of lateral leads within this region (Left frontal: F7, F3, Fc5, Fc1; Right frontal: F8, F4, Fc6, Fc2; Left central: T3, C3, Cp5, Cp1; and Right central: T4, C4, Cp6, Cp2; see Figure 2).
Figure 2.
Groupings of lateral leads for analysis of electrophysiological data.
Overall, the results indicated differences in processing of the stimuli between the two age groups. Both latency to peak of the middle latency component and positive slow wave activity differed as a function of age. Four-year-old children displayed faster processing (as evidenced by shorter latencies) and greater efficiency in processing (as evidenced by smaller amplitudes/decreased slow wave activity) than the 3-year-old children. However, both groups allocated similar amounts of ‘obligatory attention’ to the stimuli (as evidenced by a lack of reliable difference in amplitude of the middle latency component).
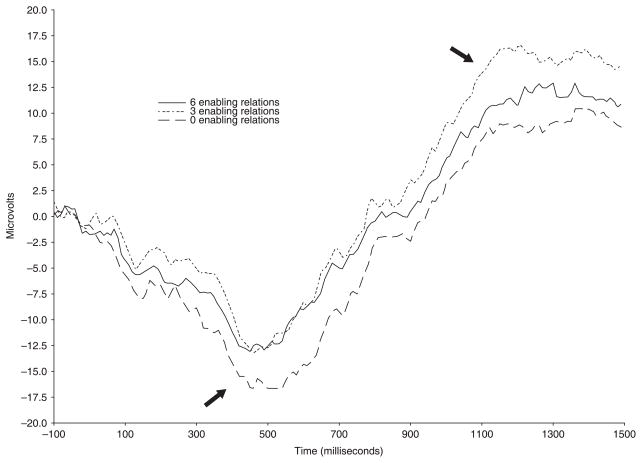
Across the midline leads, there was a main effect of lead and a marginal effect of sequence type on peak amplitude of middle latency component, F(2, 66) = 67.24, p < .001, and F(2, 66) = 2.9, p = .06, respectively.2 Similar to previous reports (see DeBoer et al., 2007, for review), the amplitude of the middle latency component was maximal across frontal (M = −26.71 μV) and central (M = −26.32 μV) midline leads compared to posterior leads (M = −15.83 μV). Additionally, amplitude of the middle latency component varied as a function of sequence type, with greatest negative amplitude produced in response to the arbitrary event sequences (M = −25.08 μV) compared to the sequences with six or three enabling relations (M = −22.02 and −21.76 μV, respectively; see Figure 3).
Figure 3.
Grand average ERP waveforms illustrating differences in the middle latency component and positive slow wave activity as a function of sequence type for both 3- and 4-year- old age groups at lead F4.
Analysis of the lateral regions further clarified the distribution of the middle latency component, as there was an interaction between coronal plane and hemisphere, F(1, 34), 11.88, p < .01. Amplitude was greater at frontal regions (M = −26.33 μV) compared to central regions (M = −21.83 μV). Within the frontal regions, amplitude was greater over the right (M = −28.85 μV) versus left (M = −23.81 μV) hemisphere.
Analysis of latency to peak amplitude at frontal and central midline leads revealed differential processing of the stimuli as a function of age, condition, and lead, F(1, 33) = 4.49, p < .05. Follow-up analyses indicated that at the vertex (Cz) 4-year-old children had a marginally shorter latency to peak to novel (M = 470.24 ms) compared to familiar (M = 490.24 ms) stimuli, F(1, 21) = 3.74, p = .07. However, 3-year-old children did not show this difference, as latency to peak for both conditions was similar across leads and conditions (M = 491 ms). Examination of latency to peak in the lateral leads revealed no main effect of age or condition, or an age by condition interaction.
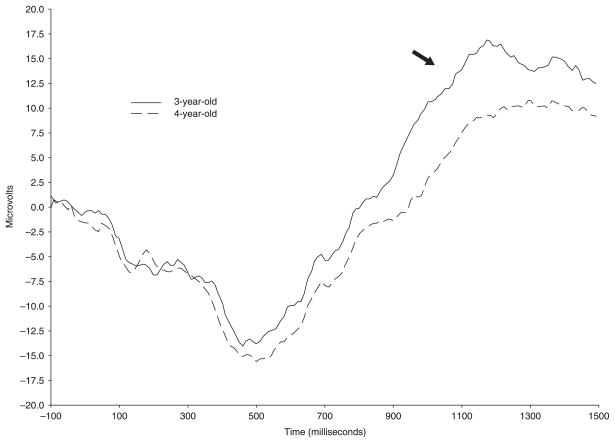
Slow wave activity at the midline leads was maximal at the vertex, F(1, 33) 7.49, p = .01, and differed as a function of sequence type as illustrated in Figure 3, F(2, 66) 3.62, p < .05. Greater slow wave activity was elicited by sequences with three enabling relations (M = 4725.17) compared to sequences with six enabling relations (M = 6917.87). However, arbitrary sequences (M = 5696.80) did not differ from either sequence that contained enabling relations. This same pattern of the effect of sequence type on positive slow wave activity emerged in analysis of lateral leads, F(2, 68) 7.42, p < .01. In addition, at lateral leads slow wave activity differed as a function of age. Specifically, 3-year-old children showed greater positive slow wave activity (M = 6854.90) than 4-year-old children (M = 4540.06; see Figure 4).
Figure 4.
Grand average ERP waveforms depicting greater positive slow wave activity in the 3-year-old age group (solid line) compared to the 4-year-old age group (dotted line) at lead F7.
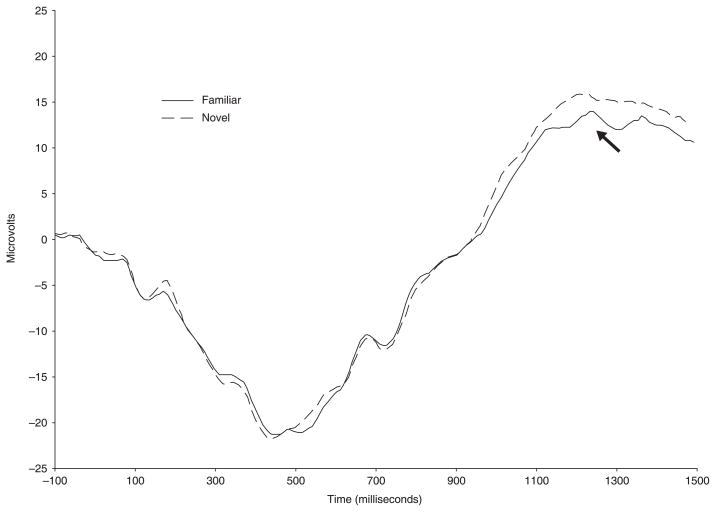
Although there was no main effect of condition in the omnibus ANOVA, we conducted paired t-tests on mean amplitude in the 900–1500 ms time window at individual leads due to hypotheses of old–new effects based on previous research (Marshall et al., 2002). Consistent with the results of the paired t-test analyses reported in the Marshall paper, we observed a significant difference between old and new stimuli in the right hemisphere, at T4, t(37) = 2.15, p = .04. A marginal old–new effect was also observed at electrode sites Cz and F3, t(37) = 1.87, p = .07, t(37) = 1.78, p = .08, respectively. Although the magnitude of the effect was similar to the Marshall et al. study (2002), the direction of the effect differed. In our investigation, at all three electrode sites amplitude was greater to the new in comparison to the old stimuli. For example, at Cz mean amplitude (standard deviation) to old/familiar stimuli was 9.32 μV (±8.14 μV) and mean amplitude to new/novel stimuli was 11.34 μV (±7.28 μV); illustration of this effect can be found in Figure 5. These findings suggest that differences between responses to old and new items were indeed present in the data, but to a lesser degree and in the opposite direction than the previous study with this age group.
Figure 5.
Example of old–new effect in positive slow wave activity at Cz collapsed across age groups and sequence types.
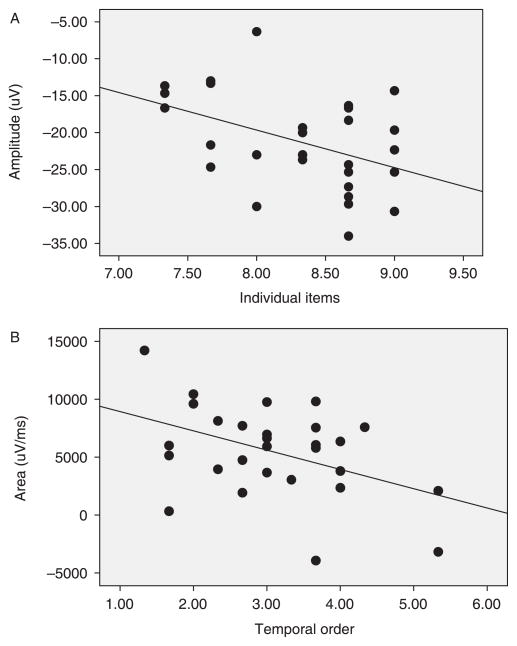
To determine how differences in processing at the neural level related to recall of memory sequences we ran correlational analyses collapsing across age and sequence type for both behavioral and electrophysiological measures. Recall of individual items in the event sequences was related to peak amplitude of the middle latency component to novel stimuli at both frontal (Fz) and central (Cz) midline leads, r(28) −.39, p < .04, and r(29) = −.43, p < .05, respectively (see Figure 6). Although this finding might seem counterintuitive at first (i.e. that ERP responses to novel stimuli contributed to this effect), previous research has suggested that this middle latency component is modulated by shifts in attention (as it is highly correlated with decreases in heart rate); greater amplitude of this component is associated with increased attention elicited by novel stimuli (Richards, 2003). If the children correctly remember and identify previously experienced stimuli (i.e. familiar items) then it is likely they would be directing more attentional resources toward the new stimuli, which would result in a larger amplitude of the middle latency component.
Figure 6.
Correlations between amplitude of middle latency component to novel stimuli across midline leads and the number of individual items recalled (A), and positive slow wave activity across the midline leads to pictures of familiar stimuli and number of pairs of actions in the correct temporal order recalled (B) after the 1-week delay.
In contrast, recall of pairs of items in the correct temporal order was related, as expected, to positive slow wave activity elicited in response to familiar stimuli at both frontal and central midline leads, r(28) −.36, p = .06, and r(29) = −.49, p < .05, respectively. Finally, there was a double dissociation within the correlational findings, as amplitude of the middle latency component (to either the familiar or novel stimuli) was not related to successful production of pairs of actions in the correct temporal order (ps > .18), and positive slow wave activity (to either novel or familiar stimuli) was not related to recall of individual items (ps > .70).
Discussion
This investigation reports an age-related improvement in the ability to recall the temporal order of events, despite similar levels of recall for individual items within an event. Moreover, this improvement during early childhood is correlated with changes in distributed positive slow wave activity that occurs late in the electrophysiological response. In contrast, memory for individual items is correlated with the middle latency component observed in the electrophysiological response. This result is similar to findings in the adult ERP literature that report a temporal dissociation between early components reflecting item memory effects and later components reflecting memory for contextual details (Duarte et al., 2004; Friedman & Johnson, 2000).
Although it could be the case that memory for individual items involves recollective processes, memory for these items can be subserved by familiarity processes alone. In contrast, memory for contextual details, such as the temporal order of items, requires recollective processes. In the present report, recollective processes differed both as a function of age and sequence type. This difference was apparent both in behavioral and electrophysiological measures. Four-year-olds recalled more temporal order information and evidenced a decrease in PSW compared to 3-year-olds. Sequences with greater internal organization (i.e. more enabling relations) were remembered more accurately across both age groups (despite similar levels of recall for individual items) and elicited the least amount of slow wave activity. We suggest that these differences in slow wave activity may reflect differential engagement of recollective processes and, ultimately, the extent to which contextual details surrounding the event are recalled. Although previous electrophysiological investigations of memory in infants and young children have identified differences within the ERP response (between familiar and novel items), limited progress has been made to date regarding the cognitive source of these differences. We interpret the differences reported in the current study to be the result of variation in the efficiency of recollective processing and reflective of the underlying organization of the memory representation, including both individual items and the surrounding context. In short, results from the present investigation reveal increases in behavioral ordered recall and decreases in PSW activity both in older children and for event sequences with high connectivity, suggesting that differential engagement of recollective processes was required to recall these events and their context.
Measures of slow wave activity have previously been shown to distinguish correctly recognized familiar items from correctly rejected novel items after a 5-minute delay in 4-year-old children (i.e. an old–new effect; Marshall et al., 2002). This difference was also observed in the present report as revealed by the paired t-test analyses. One possible explanation for why this difference was not detected in the omnibus ANOVA may be the additional factors of interest we added to the repeated measures design. Although we report similar variance in the electrophysiological data, the present investigation included an additional age group that consisted of younger children (and was accompanied by a corresponding increase in sample size), three separate memory conditions of varying difficulty level (i.e. sequence types), and an increased number of electrodes. Parameters within the paradigm itself may have also contributed to the reported pattern of results. In the present investigation the individual stimuli were well encoded (immediate recall was quite accurate as the average number of items recalled immediately was 8.24 out of 9, or 91.5%), the delay between exposure and test was long (1 week), allowing for consolidation of the memory trace for the items, and the children passively viewed many ‘novel’ items (60). This procedure varies markedly from the investigation by Marshall and colleagues (Marshall et al., 2002). Specifically, there are differences in the level of encoding (i.e. the opportunity to recall the items before the delay was imposed), the duration of the delay over which the items were to be remembered (5 minutes vs. 1 week), instructions and requirements during the ERP recording (requiring a verbal response vs. passive viewing), and novel items presented during ERP (trial unique versus repeating novel items). In the present research, the high levels of encoding may have allowed more processing resources to be devoted to new stimuli, and because there were two repetitions of the ‘novel’ stimuli they may have become less novel over the course of the experiment (see Snyder, Webb & Nelson, 2002; Wiebe, Cheatham, Lukowski, Haight, Muehluck & Bauer, 2006, for elaboration).3 This effect may have been exacerbated by the fact that the children were not told to explicitly determine whether the items were old or new during recording of the electrophysiological data. Future investigations are required to tease apart the independent contribution of each of these manipulations.
Further research is also necessary to determine the significance of the differences in the direction of the old–new effects. Although the magnitude of the amplitude difference between old and new stimuli reported in the present investigation was comparable to the previous study with a similar age group (see Figure 4 in Marshall et al., 2002), the pattern of results was different. Based on evidence from infant ERP literature, the direction of the old–new effect has been shown to differ across development, even within the same participants. For example, in one longitudinal study by Bauer and colleagues, amplitude of the middle latency component to new/novel stimuli was greater than the amplitude to old/familiar stimuli when infants were 9 months of age (a result that is similar to the pattern reported here). However, when the same infants were assessed at 10 months of age, on the same paradigm, the direction of the differentiation changed: amplitude of the middle latency component was greater to old/familiar compared to new/novel stimuli (a result that is similar to the Marshall et al., 2002, study; for additional details and discussion see Bauer, Wiebe, Carver, Lukowski, Haight, Waters & Nelson, 2006).
At present, the link between recollection and positive slow wave activity in early childhood remains tentative. There may be differences between this association and how recollection is typically measured and defined in adults. Recollection ERP studies in adults require contextual memories to be verified. Paradigms are designed to require confirmation (either verbally or behaviorally) of the recollection for each individual item and its associated context. In contrast, recollection in the present investigation was inferred based on an overall increase in behavioral recall of the temporal order of items in an event sequence relative to baseline. Thus, it was not possible to sort the electrophysiological data after collection to include only old items that were correctly recollected and new items that were correctly rejected for comparison. The ERP results reported above are truly conservative as they include both successful and failed recollection retrieval attempts. Given the differences in the paradigm used in the present report, a challenge for future investigations is development of memory paradigms that can be used with young children that not only capture both recollection and familiarity processes but also allow for separation of correctly recollected old items and correctly rejected new items.
The electrophysiological components in our investigation were maximal across frontocentral recording sites. This finding is consistent with previous reports (Cycowicz et al., 2003) which suggest that development of frontal lobe structures may underlie the development of improvements in memory for contextual details and recollection (Cycowicz et al., 2001). Although we cannot draw definitive conclusions about the source of the neural network generating the observed ERP components, consideration of the course of development of memory for contextual details and general knowledge of brain development can shed some light on the source of the age-related changes. The ability to recall contextual details (the hallmark of recollection) has a protracted developmental time course, with significant improvements seen in preschool and elementary school years, continuing into adolescence (e.g. Drummey & Newcombe, 2000; Nelson et al., 2007). This developmental trajectory coincides with the well-documented period of rapid development in the prefrontal cortex, including synaptic elimination and myelination. This observation lends support to the hypothesis that the age-related increases in memory for contextual detail are the result of maturation of frontal lobe structures and, equally importantly, in connectivity between the prefrontal cortex and medial temporal lobe (Menon, Boyett-Anderson & Reiss, 2005; Nelson et al., 2007). However, developments in medial temporal lobe components and reciprocal connections with other regions (including the frontal lobe) may contribute to this effect in some as yet unspecified manner. In short, because electrical activity was recorded at the surface of the scalp, the definitive ‘source’ of the neural network generating these ERP components remains unidentified.4 It is our hope that future investigations utilizing fMRI and/or diffusor tensor imaging (DTI) will be used to shed further light on these underlying developments in the brain.
In the present investigation, results from the behavioral recall measures indicated that memory for temporal order of the items varied as a function of age and event type, despite equal measures of item recall. Electrophysiological indices suggested that differences in item recall were associated with amplitude of a middle latency component, whereas differences in memory for temporal order varied as a function of positive slow wave activity (PSW). Together these results suggest that familiarity and recollective processes are present in early childhood and are differentially mediating memory for items and their context. Using electrophysiological methods in combination with behavioral paradigms such as the one used in the present study, it may be possible to tease apart the differential contribution of these two processes to successful mnemonic performance early in life.
Acknowledgments
The authors would like to thank Jennifer Haight, Lindsay Lewis, and the members of the Cognition in Transition Laboratory for their outstanding attention to detail in coding the elicited imitation data. We would also like to thank the members of the Center for Neurobehavioral Development for their assistance with ERP data collection, Sandi Wewerka, and Christina Phill for assistance with study design, and the children and families who participated in the study. This research was supported by grants from NIH to Charles A. Nelson (NS34458) and Michael K. Georgieff (HD29421), a grant from the NICHD to Patricia J. Bauer (HD28425), and a grant from the NIH National Center for Research Resources (RR00400). Portions of these data were presented at the meeting of the Society for Research Child Development in Boston, MA, March 2007.
Footnotes
Results of analyses using the mean amplitude of the middle latency component were largely similar to results with peak amplitude; thus, only the latter are reported.
Due to equipment failure, data at Fz from one 3-year-old child were excluded from midline analyses.
Given that the counterbalancing scheme of the current investigation was designed to minimize order effects in the ERP as a function of sequence type, this possibility cannot be empirically investigated with the current dataset. Some children observed sequences with six enabling relations first, followed by sequences with three enabling relations, followed by arbitrary sequences. In this scenario, any gradual decrease in the amplitude difference to novel and familiar stimuli could have been attributed to either differences in strength of encoding or habituation to the ERP recording environment. Since there were six possible counterbalancing schemes, none contained enough power to statistically investigate this possibility.
Due to differences between paradigms, reference site for ERP recording, and ERP data processing, further studies are necessary to determine the significance of the lateralized nature of the reported effects.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavior and Brain Sciences. 1999;22:425–489. [PubMed] [Google Scholar]
- Bauer PJ, Hertsgaard LA, Dropik P, Daly BP. When even arbitrary order becomes important: developments in reliable temporal sequencing of arbitrarily ordered events. Memory. 1998;6:165–198. doi: 10.1080/741942074. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Wenner JA, Dropik P, Wewerka SS. Parameters of remembering and forgetting in the transition from infancy to early childhood. Monographs of the Society for Research in Child Development. 2000;65(4) Serial No. 263. [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Lukowski AF, Haight JC, Waters JM, Nelson CA. Electrophysiological indexes of encoding and behavioral indexes of recall: examining relations and developmental change late in the first year of life. Developmental Neuropsychology. 2006;29 (2):293–320. doi: 10.1207/s15326942dn2902_2. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Waters JM, Nelson CA. Developments in long-term explicit memory in the first year of life: behavioral and electrophysiological indices. Psychological Science. 2003;14:629–635. doi: 10.1046/j.0956-7976.2003.psci_1476.x. [DOI] [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Developmental Science. 2000;3:234–246. [Google Scholar]
- Courchesne E, Granz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- Cycowicz YM. Memory development and event-related brain potentials in children. Biological Psychiatry. 2000;54:145–174. doi: 10.1016/s0301-0511(00)00055-7. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Duff M. Pictures and their colors: what do children remember? Journal of Cognitive Neuroscience. 2003;15 (5):759–768. doi: 10.1162/089892903322307465. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Snodgrass JG, Duff M. Recognition and source memory for pictures in children and adults. Neuropsychologia. 2001;39:255–267. doi: 10.1016/s0028-3932(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Czernochowski D. Age differences in familiarity and recollection: ERP evidence for the development of recognition memory in childhood. Saarbrucken: Universitat des Saarlandes; 2005. [DOI] [PubMed] [Google Scholar]
- Czernochowski D, Mecklinger A, Johansson M, Brinkmann M. Age-related differences in familiarity and recollection: ERP evidence from a recognition memory study in children and young adults. Cognitive, Affective, and Behavioral Neuroscience. 2005;5 (4):417–433. doi: 10.3758/cabn.5.4.417. [DOI] [PubMed] [Google Scholar]
- DeBoer T, Scott LS, Nelson CA. Methods for acquiring and analyzing infant event-related potentials. In: de Haan M, editor. Infant EEG and event-related potentials. New York: Psychology Press; 2007. pp. 5–37. [Google Scholar]
- de Haan M. Infant EEG and event-related potentials. New York: Psychology Press; 2007. [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: a neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- Drummey AB, Newcombe NS. Developmental changes in source memory. Developmental Science. 2002;5:502–513. [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cognitive Brain Research. 2004;18:255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- Friedman D, Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microscopy Research and Technique. 2000;51 (6):6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Marshall DH, Drummey AB, Fox NA, Newcombe NS. An event-related potential study of item recognition memory in children and adults. Journal of Cognition and Development. 2002;3 (2):201–224. [Google Scholar]
- Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Cognitive Brain Research. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Neural correlates of recognition memory in the first postnatal year of life. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York: Guilford Press; 1994. pp. 269–313. [Google Scholar]
- Nelson CA. A developmental cognitive neuroscience approach to the study of atypical development: a model system involving infants of diabetic mothers. In: Dawson G, Fischer K, Coch D, editors. Human behavior and the developing brain. 2. New York: Guilford Press; 2007. pp. 1–27. [Google Scholar]
- Nelson CA, Collins PF. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: implications for visual recognition memory. Developmental Psychology. 1991;27 (1):50–58. [Google Scholar]
- Nelson CA, Thomas KM, de Haan M. Neural bases of cognitive development. In: Mussen P, editor. Handbook of child psychology. New York: John Wiley & Sons; 2007. pp. 3–57. [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Developmental Science. 2003;6 (3):312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Tulving E. What are the memory systems of 1994? In: Schacter DL, Tulving E, editors. Memory systems of 1999. Cambridge, MA: The MIT Press; 1994. pp. 1–38. [Google Scholar]
- Schacter DL, Wagner AD, Buckner RL. Memory systems of 1999. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. New York: Oxford University Press; 2000. pp. 627–643. [Google Scholar]
- Sluzenski J, Newcombe NS, Kovacs S. Binding, relational memory and recall of naturalistic events: a developmental perspective. Journal of Experimental Psychology: Learning, Memory and Cognition. 2006;32:89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Snyder K, Webb SJ, Nelson CA. Theoretical and methodological implications of variability in infant brain response during a recognition memory paradigm. Infant Behavior and Development. 2002;25:466–494. [Google Scholar]
- Wiebe SA, Cheatham CL, Lukowski AF, Haight JC, Muehluck AJ, Bauer PJ. Infants’ ERP responses to novel and familiar stimuli change over time: implications for novelty detection and memory. Infancy. 2006;9:21–44. [Google Scholar]
- Yonelinas A. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas A, Otten L, Shaw K, Rugg M. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25 (11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]