Abstract
Background
Racial differences in the epidemiology and outcomes of heart failure are well known. However, the association of race with the natural history of heart failure has not been previously studied in a propensity-matched population of chronic heart failure in which all measured baseline patient characteristics are well-balanced between the races.
Methods and Results
Of the 7788 patients with chronic systolic and diastolic heart failure in the Digitalis Investigation Group trial, 1128 were nonwhites. Propensity scores for being nonwhite were calculated for each patient and were used to match 1018 pairs of white and nonwhite patients. Matched Cox regression analyses were used to estimate associations of race with outcomes during 38 months of median follow-up. All-cause mortality occurred in 34% (rate, 1180/10000 person-years) of whites and 33% (rate, 1130/10000 person-years) of nonwhite patients (hazard ratio when nonwhite patients were compared with whites, 0.95, 95% confidence interval, 0.80–1.14; p=0.593). All-cause hospitalization occurred in 63% (rate, 3616/10000 person-years) of whites and 65% (rate, 3877/10000 person-years) of nonwhite patients (hazard ratio, 1.03, 95% confidence interval, 0.90–1.18; p =0.701). Respective hazard ratios (95% confidence intervals) for other outcomes were: 0.95 (0.75–1.12) for cardiovascular mortality, 0.82 (0.60–1.11) for heart failure mortality, 1.05 (0.91–1.22) for cardiovascular hospitalization and 1.17 (0.98–1.39) for heart failure hospitalization.
Conclusion
In a propensity-matched population of heart failure patients where whites and nonwhites were balanced in all measured baseline characteristics, there was no racial differences in major natural history end points.
Keywords: Heart failure, race, natural history, propensity scores
The natural history of heart failure has been reported to be worse among nonwhites, particularly among African Americans, compared to whites.1–6 Most of these observational studies used traditional regression-based risk adjustment models. However, propensity score matching has emerged as a more efficient and transparent method to establish causal associations in observational studies.7–15 As in randomized clinical trials, in propensity-matched studies, risk-adjusted balanced study populations can be assembled without access to outcomes data. Further, bias reduction using propensity matching can be objectively estimated and the covariate balance can be presented in a reader-friendly tabular format.12–17 Therefore, the objective of this study was to evaluate whether race had an independent effect on major natural history endpoints in a population of propensity-matched ambulatory chronic heart failure patients who were well-balanced in all measured baseline covariates.
Methods
This is a post-hoc propensity-matched study of the Digoxin Investigation Group (DIG) trial (1991–1993), conducted in the United States and Canada. The rationale, design and results of the DIG trial have been published previously.18, 19 Of the 7788 ambulatory patients with chronic heart failure and normal sinus rhythm, 6800 had ejection fraction ≤ 45%. Overall, 1128 (15.5%) patients were nonwhites, of whom 80% were African Americans and race was self-identified by patients.6 Details of racial and ethnic information for nonwhite patients was not available for this analysis. We focus our current analysis on a subset of 2788 propensity-matched patients. The primary outcomes were mortality and hospitalizations due to all causes, cardiovascular causes and worsening heart failure. Data on vital status were 99% complete.20
Statistical Analysis
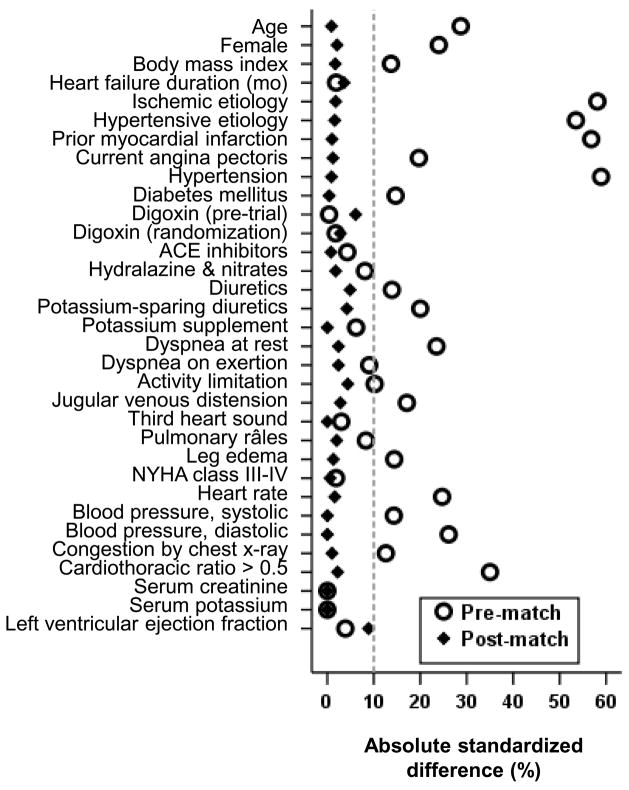
There were significant imbalances in baseline covariate distribution between nonwhite and white patients (Table 1). To account for this imbalance, we calculated propensity scores for being nonwhite for each patient, which was then used to match nonwhite and white patients using an SPSS macro.12–15, 21 Of the several propensity score methods, matching has several advantages. It allows estimation of bias before and after matching and assembly of a post-match cohort in which two groups are well-balanced in all measured baseline covariates. Further, it allows display of that balance in visually pleasant graphic or tabular formats. Finally, it provides a rather conservative estimate of an association. Absolute standardized differences were used to estimate bias before and after matching.12–17 Absolute standardized differences in propensity scores between white and nonwhite patients before and after matching were 84% and 0.1% respectively and those for all measured covariates were <5% (Figure 1). An absolute standardized difference of <10% is considered an acceptable reduction of bias.12–17
Table 1.
Baseline patient characteristics by race, before and after propensity score matching
| Before matching* | Nonwhites (N=1018) | After matching† | |||
|---|---|---|---|---|---|
| N (%) or mean (±SD) | Whites (N=1018) | P | P | Whites (N=1018) | |
| Age (years) | 64.5 (±10.2) | <0.0001 | 61.3 (±12.0) | 0.961 | 61.2 (±11.5) |
| Age ≥65 years | 533 (58.2%) | <0.0001 | 435 (42.7%) | 0.858 | 431(42.3%) |
| Female | 217 (21.3%) | <0.0001 | 324 (31.8%) | 0.633 | 314 (30.8%) |
| Body mass index, kg/square meter | 27.2 (±5.2) | 0.001 | 28.0 (±6.4) | 0.838 | 28.1 (±5.7) |
| Heart failure duration (mo) | 29.4 (±36.5) | 0.662 | 30.1 (±35.7) | 0.448 | 28.8 (±37.5) |
| Primary cause of heart failure | |||||
| Ischemic | 745 (73.2%) | <0.0001 | 466 (45.8%) | 0.915 | 475 (46.7%) |
| Hypertensive | 65 (6.4%) | 257 (25.2%) | 250 (24.6%) | ||
| Idiopathic | 133 (13.1%) | 187 (18.4%) | 179 (17.6%) | ||
| Others | 75 (7.4%) | 108 (10.6%) | 114 (11.2%) | ||
| Prior myocardial infarction | 696 (68.4%) | <0.0001 | 419 (41.2%) | 0.822 | 414 (40.7%) |
| Current angina pectoris | 293 (28.8%) | <0.0001 | 207 (20.3%) | 0.782 | 202 (19.8%) |
| Hypertension | 421 (41.4%) | <0.0001 | 707 (69.4%) | 0.847 | 711 (69.8%) |
| Diabetes mellitus | 277 (27.2%) | 0.001 | 346 (34.0%) | 0.925 | 348 (34.2%) |
| Medications | |||||
| Pre-trial digoxin use | 447 (43.9%) | 0.929 | 449 (44.1%) | 0.168 | 480 (47.2%) |
| Trial use of digoxin | 515 (50.6%) | 0.690 | 506 (49.7%) | 0.535 | 492 (48.3%) |
| ACE inhibitors | 944 (92.7%) | 0.331 | 955 (93.8%) | 0.855 | 953 (93.6%) |
| Hydralazine & nitrates | 16 (1.6%) | 0.067 | 28 (2.8%) | 0.692 | 31 (3.0%) |
| Diuretics | 792 (77.8%) | 0.002 | 848 (83.3%) | 0.269 | 829 (81.4%) |
| Potassium-sparing diuretics | 95 (9.3%) | <0.0001 | 44 (4.3%) | 0.349 | 53 (5.2%) |
| Potassium supplement | 295 (29.0%) | 0.162 | 324 (31.8%) | 1.000 | 324 (31.8%) |
| Symptoms and signs of heart failure | |||||
| Dyspnea at rest | 196 (19.3%) | <0.0001 | 298 (29.3%) | 0.594 | 309 (30.4%) |
| Dyspnea on exertion | 759 (74.6%) | 0.042 | 718 (70.5%) | 0.595 | 707 (69.4%) |
| Activity limitation | 767 (75.3%) | 0.022 | 721 (70.8%) | 0.325 | 741 (72.8%) |
| Jugular venous distension | 116 (11.4%) | <0.0001 | 177 (17.4%) | 0.525 | 188 (18.5%) |
| Third heart sound | 250 (24.6%) | 0.499 | 237 (23.3%) | 1.000 | 237 (23.3%) |
| Pulmonary râles | 161 (15.8%) | 0.061 | 193 (19.0%) | 0.654 | 201 (19.7%) |
| Lower extremity edema | 208 (20.4%) | 0.001 | 270 (26.5%) | 0.762 | 264 (25.9%) |
| NYHA functional class | |||||
| I | 162 (15.9%) | 0.937 | 159 (15.6%) | 0.998 | 160 (15.7%) |
| II | 539 (52.9%) | 551 (54.1%) | 547 (53.7%) | ||
| III | 292 (28.7%) | 286 (28.1%) | 289 (28.4%) | ||
| IV | 25 (2.5%) | 22 (2.2%) | 22 (2.2%) | ||
| Heart rate (/minute), | 77.7 (±12.4) | <0.0001 | 80.8 (±12.7) | 0.690 | 81.0 (±13.1) |
| Blood pressure (mm Hg) | |||||
| Systolic | 127 (±20) | <0.0001 | 130 (±22) | 0.768 | 130 (±22) |
| Diastolic | 75 (±11) | <0.0001 | 78 (±12) | 0.741 | 78 (±12) |
| Chest radiograph findings | |||||
| Pulmonary congestion | 141 (13.9%) | 0.005 | 188 (18.5%) | 0.820 | 192 (18.9%) |
| Cardiothoracic ratio >0.5 | 592 (58.2%) | <0.0001 | 758 (74.5%) | 0.614 | 748 (73.5%) |
| Serum potassium (mEq/L) | 4.35 (±0.42) | 0.001 | 4.28 (±0.46) | 0.717 | 4.27 (±0.49) |
| Serum creatinine (mg/dL) | 1.3 (±0.4) | 0.686 | 1.3 (±0.4) | 0.914 | 1.3 (±0.4) |
| Ejection fraction (%) | 32.0 (±12.3) | 0.381 | 31.5 (±13.2) | 0.317 | 30.4 (±11.6) |
ACE, Angiotensin-converting enzyme; NYHA, New York Heart Association.
Of the 6660 white patients, a random subset of 1018 patients were selected to provide equal sample sizes for prematch and post match comparisons and to avoid significant P values in the prematch comparisons from a larger sample size.
Of the 1128 nonwhite patients, 1018 (90%) were matched with 1018 white patients with similar propensity scores
Figure 1.
Absolute standardized differences before and after propensity score matching comparing covariate values for white and nonwhite patients
Baseline characteristics of nonwhite and white patients were compared using Pearson’s chi-square and Wilcoxon’s rank-sum tests and are displayed in Table 1. To avoid inflation of significance in the larger pre-match sample (n=7788), we assembled a pre-match sample size (n=2036) that was similar to the post-match group. This was done by selecting a random subset of 1018 white patients from the 6660 pre-match white patient population and then merged them with 1018 matched nonwhite patients. Kaplan-Meier and matched Cox regression analyses were used to determine the association of race with various outcomes. All statistical tests were done using SPSS for windows version 15 (SPSS Inc. Chicago, IL), with 2 tailed 95% confidence levels; a p value <0.05 was required to reject the null hypothesis.
Results
Patients had a mean age of 61 years and 31 % were women. Significant imbalance in several baseline characteristics before matching and the balance achieved after matching are displayed in Table 1 and Figure 1. During the median follow-up period of 38 months, 681 (33%) patients died from all causes, 521 due to cardiovascular causes and 230 due to progressive heart failure, and 1304 patients were hospitalized for all causes, 1048 from cardiovascular causes and 665 due to worsening heart failure.
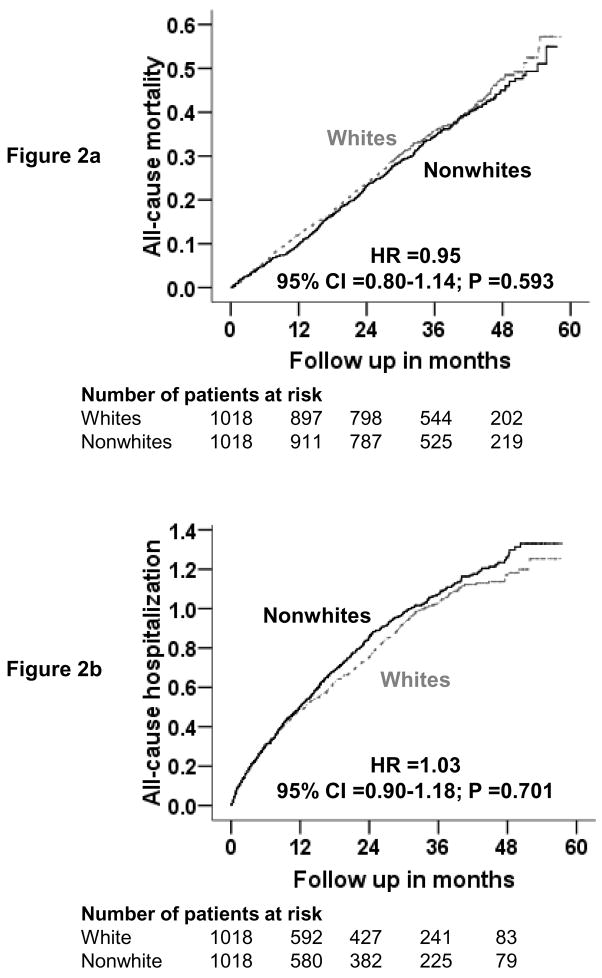
Kaplan-Meier plots for all-cause mortality are displayed in Figure 2a. All-cause mortality occurred among 347 white patients (rate, 1180/10000 person-years) during 2941 person-years of follow-up and 334 nonwhite patients (rate, 1130/10000 person years) during 2955 person-years of follow-up (hazard ratio {HR} when nonwhite patients were compared with whites, 0.95, 95% confidence interval {CI}, 0.80–1.14, p =0.593; Table 2). Race was not associated with cause-specific mortalities (Table 2).
Figure 2.
Kaplan-Meier plots for all-causes mortality (2a) and all-causes hospitalizations (2b).
Table 2.
Cause-specific mortalities in heart failure patients by race
| Rate/10,000 person-years follow-up (Deaths/follow-up in years)* | Rate difference** (/10,000 person-years) | Matched hazard ratio† (95% confidence interval) | P value | ||
|---|---|---|---|---|---|
| White (N=1,018) | Nonwhite (N=1,018) | ||||
| All-cause | 1180 (347/2,941) | 1130 (334/2,955) | − 50 | 0.95 (0.80–1.14) | 0.593 |
| Cardiovascular | 911 (268/2,941) | 856 (253/2,955) | − 55 | 0.95 (0.75–1.12) | 0.388 |
| Worsening heart failure‡ | 411 (121/2,941) | 369 (109/2,955) | − 42 | 0.82 (0.60–1.11) | 0.192 |
| Other cardio-vascular§ | 500 (147/2,941) | 487 (144/2,955) | − 13 | 1.00 (0.77–1.30) | 1.000 |
| Non-cardio-vascular | 190 (56/2,941) | 193 (57/2,955) | + 3 | 1.02 (0.67–1.58) | 0.913 |
| Unknown | 78 (23/2,941) | 81 (24/2,955) | + 3 | 1.27 (0.64–2.49) | 0.494 |
Total follow up period is same for all cause-specific mortalities as for all-cause mortality
Absolute rate differences were calculated by subtracting the rates of death in the white group from the rates of death in the nonwhite group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from matched Cox proportional-hazards models.
This category includes patients who died from worsening heart failure, even if the final event was an arrhythmia.
This category include cardiac deaths presumed to result from arrhythmia without evidence of worsening heart failure and deaths due to atherosclerotic coronary disease, bradyarrhythmias, low-output states, and cardiac surgery and vascular deaths due to stroke, embolism, peripheral vascular disease, vascular surgery, and carotid endarterectomy.
Kaplan Meier plots for all-cause hospitalization are displayed in Figure 2b. All-cause hospitalization occurred in 642 white patients (rate, 3616/10000 person-years) during 1776 person-years of follow-up and 662 nonwhite patients (rate, 3877/10000 person-years) during 1708 person-years of follow-up (HR when nonwhite patients were compared with whites, 1.03, 95% CI, 0.90–1.18, p =0.701; Table 3). There was a trend toward increased heart failure hospitalization among nonwhites (HR when nonwhite patients were compared with whites, 1.17, 95% CI, 0.98–1.39, p =0.093; Table 3). In the pre-match cohort (n=2036), there was a significant association between race and heart failure hospitalization (unadjusted HR, 1.44, 95% CI, 1.23–1.69, p <0.0001), which remained significant after adjustment for propensity scores (adjusted HR, 1.34, 95% CI, 1.12–1.59, p <0.0001). Post-match associations of race and other cause-specific hospitalizations are displayed in Table 3.
Table 3.
Cause-specific hospitalizations in heart failure patients by race
| Cause for hospitalization* | Rate/10,000 person-years follow up (Hospitalizations/follow-up in years) | Rate Difference (/10,000 person-years)† | Matched hazard ratio (95% confidence interval)‡ | P value | |
|---|---|---|---|---|---|
| White (N=1,018) | Nonwhite (N=1,018) | ||||
| All-cause | 3616 (642/1776) | 3877 (662/1708) | + 261 | 1.03 (0.90–1.18) | 0.701 |
| Cardiovascular | 2418 (500/2068) | 2762 (548/1984) | + 344 | 1.05 (0.91–1.22) | 0.498 |
| Worsening heart failure | 1213 (301/2481) | 1543 (364/2,359) | + 330 | 1.17 (0.98–1.39) | 0.093 |
| Ventricular arrhythmia, cardiac arrest | 97 (28/2899) | 107 (31/2909) | + 10 | 1.13 (0.65–1.95) | 0.675 |
| SV arrhythmias§ | 157 (22/2867) | 128 (24/2901) | – 29 | 0.90 (0.54–1.51) | 0.696 |
| AV block, bradyarrhythmia | 7 (2/2935) | 3 (1/2954) | – 4 | 0.50 (0.05–5.51) | 0.571 |
| Suspected digoxin toxicity | 69 (20/2906) | 82 (24/2912) | + 13 | 1.18 (0.62–2.25) | 0.622 |
| Myocardial infarction | 159 (46/2893) | 155 (45/2908) | – 4 | 1.06 (0.66–1.69) | 0.811 |
| Unstable angina | 344 (95/2760) | 386 (106/2747) | + 42 | 1.07 (0.78–1.46) | 0.690 |
| Stroke | 192 (55/2861) | 205 (59/2874) | + 13 | 1.02 (0.68–1.53) | 0.917 |
| Coronary revascularization¶ | 69 (20/2908) | 38 (11/2924) | – 31 | 0.83 (0.36–1.93) | 0.670 |
| Cardiac transplantation | 41 (12/2924) | 7 (2/2949) | – 34 | 0.29 (0.06–1.38) | 0.118 |
| Other cardiovascular** | 506 (137/2707) | 398 (111/2786) | – 108 | 0.85 (0.64–1.12) | 0.249 |
| Respiratory infection | 268 (76/2834) | 259 (73/2821) | – 9 | 1.15 (0.80–1.65) | 0.458 |
| Other non-cardiovascular | 1426 (339/2378) | 1346 (326/2422) | – 80 | 0.85 (0.71–1.02) | 0.074 |
| Unspecified | 20 (6/2935) | 17 (5/2952) | – 3 | 0.33 (0.07–1.65) | 0.178 |
Data shown include the first hospitalization of each patient due to each cause.
Absolute differences were calculated by subtracting the percentage of patients hospitalized in the white group from the percentage of patients hospitalized in the nonwhite group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from a Cox proportional-hazards models that used the first hospitalization of each patient for each reason.
Supraventricular (SV) arrhythmias include Atrioventricular (AV) block and bradyarrhythmias
This category includes coronary-artery bypass grafting and percutaneous transluminal coronary angioplasty
This category includes embolism, venous thrombosis, peripheral vascular disease, hypertension, other vascular surgery, cardiac catheterization, other types of catheterization, pacemaker implantation, installation of automatic implantable cardiac defibrillator, electrophysiologic testing, transplant-related evaluation, nonspecific chest pain, atherosclerotic heart disease, hypotension, orthostatic hypotension, and valve operation.
Discussion
The findings from this propensity-matched study, in which patients were balanced in all measured baseline covariates, suggest that race per se was not associated with any major natural history endpoints including mortality and hospitalization due to all causes and cardiovascular causes. This is the first report of a propensity-matched study of the effect of race on outcomes in chronic heart failure. However, there was a trend toward increased risk of hospitalization due to worsening heart failure among nonwhites.
These results are not surprising as the pathophysiological basis of heart failure and response to pharmacotherapy among white and nonwhite heart failure patients seem rather similar than dissimilar.2, 22 African Americans heart failure patients seem to respond to angiotensin-converting enzyme (ACE) inhibitors and beta-blockers in a way similar to white patients.2, 22 Data from the African-American Heart Failure Trial indicated a unique survival benefit from a combination of isosorbide dinitrate and hydralazine among African American heart failure patients.23 However, the mechanism of such benefit has not yet been established and there is no evidence to suggest that combined vasodilator therapy would not be beneficial among some whites or other ethnic groups.24–26
Our finding of an increased risk of hospitalization due to worsening heart failure among nonwhite heart failure patients is consistent with similar finding by other investigators.4, 6, 27 This is unlikely to be explained by differences in measured baseline characteristics as patients were well balanced in all measured baseline characteristics. However, it is possible that there were imbalances in unmeasured covariates such as patient and/or family preferences, and changes in baseline covariates during follow up. For example, data from general population and hospitalized heart failure patients suggest that African Americans with chronic kidney disease are more likely to advance to kidney failure requiring dialysis.28, 29
We observed that before matching there were significant racial differences in prognostically important baseline characteristics. However, after propensity matching, all measured baseline characteristics were well-balanced between white and nonwhite patients, and race per se was not significantly associated with any major natural history end points. However, pre-match differences in baseline characteristics indicate that race may be a marker of other prognostic covariates. For example, nonwhite heart failure patients were much younger than their white counterparts. While this may suggest an apparently favorable prognostic association, these data also imply that nonwhite patients may have developed their heart failure at an earlier age. Compared to white patients, nonwhites were also more likely to be women, have a history of hypertension and diabetes, and have cardiomegaly. These underscore the need and the importance of optimum management of hypertension and diabetes, two of the most common causes of heart failure. Suboptimal treatment of these conditions in nonwhite patients is well documented in the literature.30–32
Several limitations of our study must be acknowledged. This is not a population-based study, which limits its generalizability. In clinical trials patients receive additional care from experienced research personnel (for example over 90% were receiving ACE inhibitors) and are followed up more rigorously than in routine clinical practice. Further, patients in this study were younger, predominantly male, had normal sinus rhythm, and are from the pre-beta-blocker era of heart failure therapy, which may further limit generalizability. Finally, like any non-randomized study, propensity matching cannot account for unmeasured covariates, which may explain away our key findings. However, for any hidden covariate to function as a confounder, it must be strongly associated with both race and outcomes, and not strongly related to any of the covariates displayed in Table 1.
In conclusion, in a propensity-matched population of heart failure patients in which whites and nonwhites were well balanced in all measured baseline covariates, despite a trend for increased heart failure hospitalization among nonwhites, race by itself was not associated with all-cause and cardiovascular mortality or hospitalization. However, the higher prevalence of hypertension, diabetes and cardiomegaly, and the development of heart failure at a younger age among nonwhites highlight the need for early detection and optimal treatment of hypertension and diabetes and primary prevention of heart failure at an earlier age in these patients.
Acknowledgments
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Yancy CW. Heart failure in blacks: etiologic and epidemiologic differences. Curr Cardiol Rep. 2001;3:191–197. doi: 10.1007/s11886-001-0022-0. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW. The role of race in heart failure therapy. Curr Cardiol Rep. 2002;4:218–225. doi: 10.1007/s11886-002-0054-0. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96:3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J. 2006;152:348–354. doi: 10.1016/j.ahj.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 5.East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J. 2004;148:151–156. doi: 10.1016/j.ahj.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Mathew J, Wittes J, McSherry F, et al. Racial differences in outcome and treatment effect in congestive heart failure. Am Heart J. 2005;150:968–976. doi: 10.1016/j.ahj.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 8.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Asso. 1984;79:516–524. [Google Scholar]
- 9.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 10.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 11.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–857. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed A, Zannad F, Love TE, et al. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–1343. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99:549–553. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 18.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 19.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 20.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Levesque R. Macro. In: Levesque R, editor. SPSS® Programming and Data Management, 2nd Edition. A Guide for SPSS® and SAS® Users. 2. Chicago, IL: SPSS Inc; [Last access date: June 4, 2005]. Available online at: http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 22.Dries DJ, Yancy CW, Strong MA, Drazner MH. Racial response to angiotensin-converting enzyme therapy in systolic heart failure. Congest Heart Fail. 2004;10:30–33. doi: 10.1111/j.1527-5299.2004.02022.x. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 24.Sankar P, Kahn J. BiDil: race medicine or race marketing? Health Aff (Millwood) 2005;Suppl Web Exclusives:W5-455–463. doi: 10.1377/hlthaff.w5.455. [DOI] [PubMed] [Google Scholar]
- 25.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 26.Bibbins-Domingo K, Fernandez A. BiDil for heart failure in black patients: implications of the U.S. Food and Drug Administration approval. Ann Intern Med. 2007;146:52–56. doi: 10.7326/0003-4819-146-1-200701020-00009. [DOI] [PubMed] [Google Scholar]
- 27.Brown DW, Haldeman GA, Croft JB, Giles WH, Mensah GA. Racial or ethnic differences in hospitalization for heart failure among elderly adults: Medicare, 1990 to 2000. Am Heart J. 2005;150:448–454. doi: 10.1016/j.ahj.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 29.McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17:1710–1715. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 30.Sonel AF, Good CB, Mulgund J, et al. Racial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: insights from CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?) Circulation. 2005;111:1225–1232. doi: 10.1161/01.CIR.0000157732.03358.64. [DOI] [PubMed] [Google Scholar]
- 31.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Relationship between quality of care and racial disparities in Medicare health plans. Jama. 2006;296:1998–2004. doi: 10.1001/jama.296.16.1998. [DOI] [PubMed] [Google Scholar]
- 32.Wendel CS, Shah JH, Duckworth WC, Hoffman RM, Mohler MJ, Murata GH. Racial and ethnic disparities in the control of cardiovascular disease risk factors in Southwest American veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study. BMC Health Serv Res. 2006;6:58. doi: 10.1186/1472-6963-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]