Abstract
Background
Serum creatinine and related estimating equations predict cardiovascular events and mortality among persons with coronary heart disease (CHD). Cystatin C is a novel and sensitive endogenous marker of kidney function. Whether cystatin C concentrations are associated with adverse events among ambulatory persons with CHD is unknown.
Methods and Results
Nine hundred ninety ambulatory persons with CHD were categorized into quartiles of serum cystatin C at inception, with ≤0.91 mg/L constituting the lowest quartile (I) and ≥1.30 mg/L constituting the highest (IV). Cox proportional hazards models evaluated time to all-cause mortality, cardiovascular events (composite of CHD death, myocardial infarction, and stroke), and incident heart failure. After a median follow-up of 37 months, 132 participants (13%) died, 101 (10%) had cardiovascular events, and 57 (7%) had incident heart failure. Compared with participants in the lowest cystatin C quartile, those in the highest quartile were at increased risk of all-cause mortality (hazard ratio, 3.6; 95% CI, 1.8 to 7.0), cardiovascular events (hazard ratio, 2.0; 95% CI, 1.0 to 3.8), and incident heart failure (hazard ratio, 2.6; 95% CI, 1.0 to 6.9) in analyses adjusted for traditional cardiovascular risk factors. Cystatin C in the highest quartile predicted similar risk for these outcomes among participants with lower (≤60 mL/min per 1.73 m2) or higher estimated glomerular filtration rate and among participants with or without microalbuminuria.
Conclusions
High cystatin C concentrations predict substantial increased risks of all-cause mortality, cardiovascular events, and incident heart failure among ambulatory persons with CHD. This risk is not completely captured by measures of kidney function routinely used in clinical practice.
Keywords: coronary disease, cystatin C, heart failure, kidney, mortality, myocardial infarction, stroke
Mild to moderate chronic kidney disease is common in the US population1 and is an independent risk factor for cardiovascular disease events and all-cause mortality.2 Among persons with established coronary heart disease (CHD), concomitant chronic kidney disease predicts cardiovascular events as strongly as other established risk factors such as diabetes mellitus and hypertension.3 Serum creatinine concentrations or creatinine-based estimating equations have served as the primary tool for evaluation of kidney function in clinical practice and in epidemiological research studies. However, creatinine concentrations are affected by several nonkidney factors including age, body weight, nutritional status, ethnicity, and sex4,5 and are insensitive to modest decreases in kidney function; for instance, kidney function may decline ≥50% before serum creatinine exceeds the normal range.4,6 Although the Modification of Diet and Renal Disease study formula is less affected by nonkidney determinants, it has been shown to overestimate kidney function among persons with glomerular filtration rates (GFR) >60 mL/min per 1.73 m2.7
Cystatin C is a novel endogenous marker of kidney function that may be more sensitive for detecting mild to moderate decrements in GFR.8–10 Most,8–13 but not all,14 studies suggest that it is not affected by age, sex, or muscle mass. Several recent publications have demonstrated that cystatin C is superior to serum creatinine or creatinine-based estimating equations for prediction of all-cause mortality,15–17 cardiovascular events,16 and incident congestive heart failure (CHF)18 in elderly community-based cohorts that were predominantly free of cardiovascular disease at inception. Whether elevated cystatin C concentrations are associated with adverse clinical outcomes among persons with prevalent CHD has not been studied extensively. Because persons with CHD represent a group at high risk for cardiovascular events and death, risk stratification may be particularly useful in this population. Furthermore, previous studies evaluating the predictive value of cystatin C for cardiovascular morbidity or mortality have not determined whether the observed associations and outcomes are robust among persons with and without microalbuminuria.
We evaluated whether serum cystatin C concentrations were associated with all-cause mortality, cardiovascular events, and incident CHF among ambulatory persons with CHD enrolled in the Heart and Soul Study. In addition, we evaluate whether the risk associated with elevated cystatin C differs among persons with or without low estimated GFR or microalbuminuria.
Methods
Participants
The Heart and Soul Study is a prospective cohort study designed to investigate the influence of psychosocial factors on CHD progression. Methods have been described previously.19,20 Briefly, participants were recruited from several outpatient clinics in the San Francisco Bay Area if they met 1 of the following inclusion criteria: (1) history of myocardial infarction; (2) angiographic evidence of >50% stenosis in 1 or more coronary vessels; (3) evidence of exercise-induced ischemia by treadmill or nuclear testing; or (4) history of coronary revascularization. Participants were excluded if they had had a myocardial infarction within the prior 6 months, were not able to walk 1 block, or were likely to move out of the area within 3 years. There was no exclusion on the basis of kidney disease. Because the study recruited heavily from a Veterans Affairs facility, the resulting cohort was 82% male. The study protocol was approved by the appropriate institutional review boards, and all participants provided written informed consent.
Between September 2000 and December 2002, a total of 1024 individuals enrolled and underwent a day-long baseline study appointment that included a medical history interview, a physical examination, and a comprehensive health status questionnaire. Fasting (12-hour) venous samples were drawn, and serum was frozen at −70°C. Subjects for whom frozen serum was not available (n=34) were excluded, resulting in a sample size of 990 participants for this analysis.
Measurements
Kidney Function
Serum cystatin C was measured from frozen samples collected at the baseline study visit with the use of a BNII nephelometer (Dade Behring, Inc, Deerfield, Ill) with a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring, Inc).21 Monoclonal antibodies to cystatin C were coated on polystyrene particles that agglutinate to increase the intensity of scattered light in proportion to the concentration of cystatin C. The assay range is 0.195 to 7.330 mg/L; the reference range for young healthy persons ranges from 0.53 to 0.95 mg/L. The intra-assay coefficient of variation ranges from 2.0% to 2.8%, and the interassay coefficient of variation ranges from 2.3% to 3.1%.
Serum creatinine was measured by the rate Jaffe method. The intraindividual coefficient of variation was ≈2%. Estimated GFR was calculated by the abbreviated (4-variable) Modification of Diet and Renal Disease Study formula, as follows: estimated GFR=186×(serum creatinine−1.154)×(age−0.203)×(0.742 if female)×(1.21 if black).5 We defined low estimated GFR as ≤60 mL/min per 1.73 m2, consistent with stage III or greater severity of chronic kidney disease.22
Urinary albumin and creatinine were measured by nephelometry and the rate Jaffe method, respectively. Urinary albumin-to-creati-nine ratios (mg albumin/g creatinine) were calculated, and microalbuminuria was defined as ≥30 mg/g.23
Other Measurements
Baseline demographics and medical history were determined by questionnaire. Participants underwent a complete physical examination that included blood pressure determination by trained study personnel using calibrated sphygmomanometers. Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications. High-sensitivity C-reactive protein was measured with the use of the Roche Integra assay and the Beckman Extended Range assay as previously described.24,25 Fasting serum samples were used to measure total cholesterol, high-density lipoprotein cholesterol, and triglyceride concentrations. Low-density lipoprotein cholesterol concentrations were calculated by the Friedewald equation.26
Outcomes
Annual telephone interviews were conducted with participants or their proxies to ask about interval death or hospitalization. For any reported event, medical records, electrocardiograms, death certificates, autopsy, and coroner’s reports were obtained and reviewed by 2 independent and blinded adjudicators. If the adjudicators agreed on the outcome classification, their classification was binding. If they disagreed, they conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator as necessary to obtain consensus.
Cardiovascular events were defined as CHD death, nonfatal myocardial infarction, or stroke. CHD death was defined as (1) death during the same hospitalization in which an acute myocardial infarction was documented or (2) death not explained by other causes and that occurred within 1 hour of the onset of terminal symptoms. Nonfatal myocardial infarction was defined by the American Heart Association diagnostic cretiria.27 Stroke was defined as a new neurological deficit not known to be secondary to brain trauma, tumor, infection, or other cause.28
CHF was defined as hospitalization for a clinical syndrome involving at least 2 of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, third heart sound, and cardiomegaly or pulmonary edema on chest radiography.29 These clinical signs and symptoms must have represented a clear change from the normal clinical status of the participant and must have been accompanied by either decreased cardiac output as determined by peripheral hypoperfusion (in the absence of other causes such as sepsis or dehydration) or peripheral or pulmonary edema requiring intravenous diuretics, inotropic agents, or vasodilator therapy. Supportive documentation of reduced cardiac output, elevated pulmonary capillary wedge pressure, decreased oxygen saturation, and end-organ hypoperfusion was assessed, when available.
All-cause mortality was determined by review of death certificates.
Statistical Analysis
Because age-, sex-, and race-specific normal ranges for serum cystatin C have not been established, we categorized cystatin C into quartile groups. Differences in baseline characteristics were compared with the use of ANOVA or the Kruskal-Wallis test for continuous variables and the χ2 test or Fisher exact test for categorical variables, as appropriate. Cox proportional hazards models evaluated whether cystatin C quartile groups were associated with time to each of the 3 outcome measures. Covariates for adjustment included important demographics (age, sex, race), traditional cardiovascular risk factors (age, sex, diabetes mellitus, hypertension, history of prior myocardial infarction, tobacco use, body mass index, high-density lipoprotein, and C-reactive protein), and variables previously associated with elevated cystatin C concentrations (age, sex, body mass index, tobacco use, and C-reactive protein).14 For incident CHF, we excluded participants with a self-reported history of heart failure at inception (n=174), resulting in a sample size of 816 participants for this outcome. Multiplicative interaction terms were created to evaluate the effect modification of the association of cystatin C with each outcome by the presence or absence of microalbuminuria and low estimated GFR. Stratified analyses were conducted to determine whether the association of cystatin with cardiovascular outcomes differed by microalbuminuria or estimated GFR categories.
Proportional hazards assumptions were assessed by visually inspecting log-minus-log plots and plots of Schoenfeld residuals versus survival time for the association of cystatin C with each outcome variable. We found no evidence that the proportionality assumption was violated. Two-tailed probability values <0.05 were considered statistically significant. Analyses were performed with the use of Stata statistical software, version 9 (College Station, Tex).
The authors had full access to the data and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
The mean age of the 990 study participants was 67 years, 82% were male, 61% were white, 51% had a history of myocardial infarction, 24% had diabetes, and 69% had hypertension. The mean cystatin C concentration was 1.20±0.56 mg/L, and mean estimated GFR was 77±23 mL/min per 1.73 m2. Associations of baseline characteristics by cystatin C quartiles are shown in Table 1. Participants with higher cystatin C were older and more likely to be male, to be white, and to have comorbid medical conditions. Persons with higher cystatin C also had higher serum triglycerides and lower high-density lipoprotein cholesterol, whereas low-density lipoprotein cholesterol concentrations were not significantly different across quartiles. Persons with higher cystatin C also had higher C-reactive protein concentrations.
TABLE 1.
Baseline Characteristics of Participants by Cystatin C Quartiles
| Cystatin C Quartiles |
|||||
|---|---|---|---|---|---|
| I | II | III | IV | P | |
| Cut points, mg/L | ≤0.91 | 0.92 to 1.05 | 1.06 to 1.29 | ≥1.30 | … |
| N | 239 | 248 | 262 | 241 | … |
| Demographics | |||||
| Age, y, mean±SD | 61±10 | 65±9 | 69±10 | 71±11 | <0.001 |
| Male sex | 171 (72) | 203 (82) | 226 (86) | 206 (85) | <0.001 |
| Race | … | … | … | … | 0.01 |
| White | 121 (51) | 149 (60) | 174 (66) | 153 (63) | … |
| Black | 53 (22) | 43 (17) | 30 (11) | 34 (14) | … |
| Other | 65 (27) | 54 (22) | 58 (22) | 54 (22) | … |
| Medical history | |||||
| Diabetes mellitus | 56 (24) | 62 (25) | 54 (21) | 88 (37) | <0.001 |
| Hypertension | 154 (65) | 165 (67) | 186 (72) | 190 (79) | 0.004 |
| Myocardial infarction | 115 (49) | 132 (54) | 133 (51) | 149 (62) | 0.02 |
| Coronary revascularization | 111 (47) | 147 (61) | 158 (61) | 156 (65) | <0.001 |
| CHF | 28 (12) | 37 (15) | 43 (17) | 66 (27) | <0.001 |
| Stroke | 27 (11) | 34 (14) | 33 (13) | 44 (18) | 0.15 |
| Tobacco use | 49 (21) | 45 (18) | 58 (22) | 44 (18) | 0.62 |
| Measurements | |||||
| Body mass index, kg/m2, mean±SD | 28±5 | 28±5 | 28±5 | 28±5 | 0.83 |
| LDL cholesterol, mg/dL, mean±SD | 107±34 | 103±32 | 106±32 | 101±37 | 0.21 |
| HDL cholesterol, mg/dL, mean±SD | 49±15 | 46±14 | 45±13 | 43±13 | <0.001 |
| Triglycerides, mg/dL* | 103 (69, 154) | 100 (68, 150) | 122 (79, 169) | 114 (78, 178) | 0.002 |
| C-reactive protein, mg/dL* | 1.6 (0.6, 3.8) | 1.7 (0.7, 3.6) | 2.4 (1.1, 5.3) | 3.2 (1.5, 7.4) | <0.001 |
Values are number (%) unless indicated otherwise. LDL indicates low-density lipoprotein; HDL, high-density lipoprotein.
Median (quartile 1, quartile 3).
Among the 990 study participants, there were 132 deaths (13%) after 40 530 patient-months of follow-up (median, 37 months). Six participants (0.6%) were lost to follow-up. Among the high cystatin C quartile (IV), there was a 9.4% annual mortality rate compared with 1.5% in the lowest quartile (I) (Figure 1). After multivariable adjustment, participants in quartile IV had a >3-fold mortality hazard compared with quartile I. The mortality hazards for quartiles II and III were similar to those for quartile I (Table 2).
Figure 1.
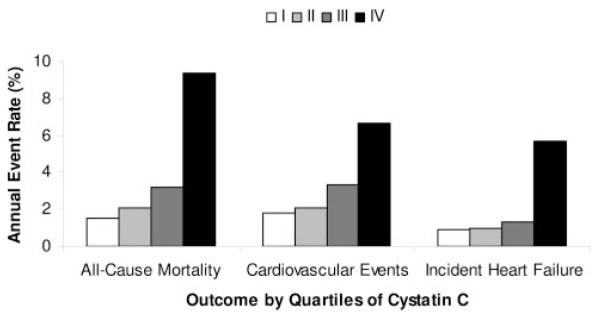
Probability value for trend <0.001 for each outcome.
TABLE 2.
Association of Cystatin C Quartiles With All-Cause Mortality, Cardiovascular Events, and Incident Heart Failure
| Cystatin C Quartiles |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I |
II |
III |
IV |
||||||||||
| No. of Events | HR | 95% CI | No. of Events | HR | 95% CI | No. of Events | HR | 95% CI | No. of Events | HR | 95% CI | P for Trend | |
| All-cause mortality | 12 | … | … | 18 | … | … | 29 | … | … | 73 | … | … | |
| Unadjusted | … | 1.0 | Reference | … | 1.3 | 0.6 to 2.8 | … | 1.9 | 1.0 to 3.7 | … | 5.7 | 3.1 to 10.5 | <0.001 |
| Adjusted* | … | 1.0 | Reference | … | 1.2 | 0.6 to 2.5 | … | 1.4 | 0.7 to 2.9 | … | 3.6 | 1.8 to 7.0 | <0.001 |
| Cardiovascular events | 14 | … | … | 17 | … | 23 | … | … | 47 | … | … | ||
| Unadjusted | … | 1.0 | Reference | … | 1.2 | 0.6 to 2.4 | … | 1.5 | 0.8 to 2.9 | … | 3.8 | 2.1 to 6.9 | <0.001 |
| Adjusted* | … | 1.0 | Reference | … | 1.0 | 0.5 to 2.0 | … | 1.1 | 0.5 to 2.2 | … | 2.0 | 1.0 to 3.8 | 0.04 |
| Incident heart failure | 6 | … | … | 7 | … | 9 | … | … | 35 | … | … | ||
| Unadjusted | … | 1.0 | Reference | … | 1.1 | 0.4 to 3.1 | … | 1.4 | 0.5 to 4.0 | … | 6.1 | 2.5 to 14.5 | 0.001 |
| Adjusted* | … | 1.0 | Reference | … | 0.7 | 0.2 to 2.2 | … | 0.8 | 0.3 to 2.4 | … | 2.6 | 1.0 to 6.9 | 0.05 |
HR indicates hazard ratio.
Adjusted for age, sex, black race, diabetes, hypertension, prior myocardial infarction, tobacco use, body mass index, high-density lipoprotein, and C-reactive protein.
Among the study cohort, 101 cardiovascular events occurred. Of these, 13 were CHD deaths, 70 were nonfatal myocardial infarctions, and 18 were strokes. The high cystatin C quartile experienced a 7% annual cardiovascular event rate compared with 2% among the low cystatin C quartile (Figure 1). After multivariable adjustment, participants in quartile IV had a 2-fold higher cardiovascular event hazard compared with quartile I, but no significant differences were observed among the lower 3 quartiles (Table 2).
When CHD death, nonfatal myocardial infarction, and stroke were evaluated as individual outcome measures, the relative hazards for participants in quartile IV compared with quartile I were 5.8 (95% CI, 0.6 to 51.3) for CHD death, 2.0 (95% CI, 0.9 to 4.3) for nonfatal myocardial infarction, and 1.1 (95% CI, 0.4 to 3.6) for stroke.
Among the 816 participants without a prior history of CHF, there were 57 hospitalizations for incident CHF during 32 078 patient-months of follow-up (median, 36 months). Participants in quartile IV experienced a 6% annual incidence rate of CHF compared with 1% in quartile I (Figure 1). After multivariable adjustment, participants in the highest cystatin C quartile had a >2-fold higher incident CHF hazard compared with the lowest quartile. There was no statistically significant difference in CHF hazards among the lowest 3 quartiles (Table 2).
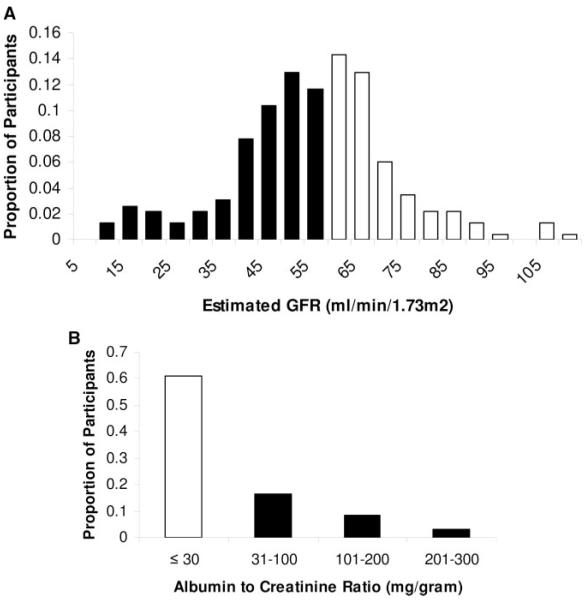
Serum cystatin C concentrations were highly correlated with estimated GFR and, to a lesser degree, with albuminuria (Spearman rank correlation r=0.74 and 0.23, respectively). There was substantial overlap, however, such that among participants with serum cystatin C concentrations in the highest quartile (≥1.30 mg/L), 25% had estimated GFR >60 mL/min per 1.73 m2, and 67% did not have microalbuminuria (Figure 2). We evaluated whether the event risks associated with elevated cystatin C differed among participants with low versus higher estimated GFR and present versus absent microalbuminuria. Because the highest quartile of cystatin C was associated with substantially higher risk for all outcomes, we dichotomized participants at the highest cystatin C quartile (serum cystatin C ≥1.3 versus <1.3 mg/L) to enhance power for subgroup analyses.
Figure 2.
Distribution of estimated GFR (A) and albumin-to-creatinine ratio (B) among the 241 study participants with cystatin C concentrations within the highest quartile of the study sample (≥1.3 mg/L). Light bars represent participants with estimated GFR >60 mL/min per 1.73 m2 (A) or without microalbuminuria (B) despite cystatin C concentrations among the highest quartile of the study sample.
Risk of each adverse clinical outcome associated with a cystatin C concentration ≥1.3 mg/L was similar among persons with or without a low estimated GFR and with or without microalbuminuria (Table 3). Probability values for interaction were all >0.35 with the exception of the association of high cystatin C with cardiovascular events, in which substantially greater risk was observed in the subgroup with low estimated GFR (P for interaction=0.04).
TABLE 3.
Adjusted Associations of High Cystatin* With Mortality, Cardiovascular Events, and Heart Failure by Strata of Estimated GFR and Microalbuminuria
| Outcome | High Cystatin, n (%) |
Hazard Ratio (95% CI) |
P for Interaction |
|---|---|---|---|
| All-cause mortality† | |||
| Low estimated GFR‡ | 183 (69) | 3.6 (1.5 to 8.7) | 0.36 |
| Higher estimated GFR | 58 (8) | 2.5 (1.4 to 4.4) | … |
| Microalbuminuria§ | 79 (54) | 3.0 (1.1 to 8.0) | 0.74 |
| No microalbuminuria | 126 (19) | 2.4 (1.5 to 3.9) | … |
| Cardiovascular events† | |||
| Low estimated GFR‡ | 183 (69) | 4.8 (1.4 to 15.9) | 0.04 |
| Higher estimated GFR | 58 (8) | 1.1 (0.5 to 2.4) | … |
| Microalbuminuria§ | 79 (54) | 1.1 (0.5 to 2.6) | 0.81 |
| No microalbuminuria | 126 (19) | 1.5 (0.8 to 2.8) | … |
| Incident heart failure† | |||
| Low estimated GFR‡ | 150 (74) | 3.2 (0.9 to 11.3) | 0.95 |
| Higher estimated GFR | 51 (8) | 1.7 (0.6 to 4.6) | … |
| Microalbuminuria§ | 57 (54) | 3.2 (0.9 to 11.0) | 0.36 |
| No microalbuminuria | 114 (21) | 1.0 (0.4 to 2.6) | … |
Compares highest quartile (≥1.3 mg/L) with lower 3 quartiles.
Adjusted for age, sex, black race, diabetes, hypertension, prior myocardial infarction, tobacco use, body mass index, high-density lipoprotein, and C-reactive protein.
Estimated GFR ≤60 mL/min per 1.73 m2.
Albumin-to-creatinine ratio ≥30 mg/g.
Discussion
In the present study, we demonstrate that higher serum cystatin C concentrations predict all-cause mortality, cardiovascular events, and incident heart failure independent of traditional cardiovascular risk factors among ambulatory persons with CHD. Moreover, we found that a substantial proportion of persons with cystatin C concentrations in the highest quartile of our study sample did not have an estimated GFR ≤60 mL/min per 1.73 m2 or microalbuminuria. High cystatin C level had similar predictive value for adverse clinical outcomes among persons with or without low estimated GFR or microalbuminuria.
Observational studies have previously demonstrated that mild to moderate kidney disease independently predicts all-cause mortality and cardiovascular events among persons with prevalent CHD,3,30,31 and the increased risk may be observed with moderate decrements in GFR.2,16 Detection of mild kidney disease in routine clinical practice is problematic, however. Serum creatinine concentrations4–6 and the Modification of Diet and Renal Disease study formula27,32 are both insensitive to mild decrements in kidney function. Whereas measured creatinine clearance by timed urine collection may be more sensitive, this test is fraught with inaccuracy and is no longer recommended to routinely assess kidney function in the ambulatory setting.22 Because cystatin C is sensitive to mild decrements of GFR and may not be affected by age, sex, and muscle mass, it may have clinical utility in accurately detecting mild kidney disease among ambulatory persons.8,9,32 We hypothesized that it might also allow more accurate cardiovascular risk prediction among persons with CHD.
In the present study, we demonstrate that among persons with cystatin C concentrations in the highest quartile of our cohort (>1.3 mg/L), one fourth had estimated GFR >60 mL/min per 1.73 m2, and two thirds had no microalbuminuria. These participants were demonstrated to have increased risks for adverse clinical outcomes, and the relative hazards were similarly increased among persons with or without low estimated GFR or microalbuminuria. These results demonstrate that the risk of adverse events attributable to kidney disease is not completely captured by estimates of kidney function routinely used in clinical practice. More accurate characterization of kidney function in persons with CHD could allow targeted therapies that might improve prognosis and limit use of tests or procedures that have high risk for acute renal failure and associated consequences.
Although previous studies demonstrate that cystatin C predicts all-cause mortality,15–17 cardiovascular events,16 and incident CHF18 among elderly ambulatory cohorts predominantly without CHD, the predictive value of cystatin C among outpatients with prevalent CHD has not been studied extensively. Jernberg and colleagues33 determined that elevated cystatin C was associated with mortality among persons hospitalized with acute coronary syndrome, and Koenig and colleagues34 demonstrated that elevated cystatin C predicted second cardiovascular events among a cohort participating in an in-hospital rehabilitation program shortly after acute myocardial infarction or coronary revascularization. Our present study adds to the existing literature by demonstrating that cystatin C concentrations predict not only cardiovascular events but also mortality and incident heart failure among ambulatory persons with CHD.
Among the strengths of the present study are measurements of multiple potential confounding variables and availability of albuminuria measurement among an ambulatory cohort with CHD. Several limitations should be considered when our results are interpreted, however. Although 2 blinded adjudicators reviewed all medical records, the diagnosis of cardiovascular events and incident CHF may be subject to misclassification. Any misclassification should have biased our results toward the null and should not have been influenced by serum cystatin C concentrations. We cannot exclude the possibility of residual confounding. However, any unmeasured confounder would need to be highly prevalent in our cohort and strongly associated with cystatin C and each outcome measure to explain the relatively large hazard ratios we observed in the present study. The number of outcome events within each quartile was relatively small; therefore, these findings should be confirmed in future studies. For the cardiovascular events and incident heart failure outcomes, the number of covariates included in multivariable models was >1 for each 10 outcome events. Therefore, it remains possible that the models were overfitted. Finally, the present study participants were elderly and predominantly male. Therefore, our results may not be generalizable to younger or female patients.
In conclusion, we found that higher serum cystatin C concentrations were associated with all-cause mortality, cardiovascular events, and incident CHF among ambulatory persons with CHD. Moreover, higher cystatin C concentrations predicted increased risk of these adverse clinical outcomes even among persons without microalbuminuria or low estimated GFR. If confirmed in future studies, serum cystatin C measurement may prove to be useful for the prediction of cardiovascular events among persons with coronary heart disease.
CLINICAL PERSPECTIVE.
Previous epidemiological studies have demonstrated associations of moderate kidney disease with mortality, cardiovascular events, and heart failure among diverse populations. However, studies evaluating whether milder kidney disease is associated with these adverse outcomes have been limited by the use of serum creatinine and associated estimating equations because they are insensitive to mild decrements in glomerular filtration rate. Cystatin C is a novel endogenous biomarker of kidney function that may be more sensitive to mild decrements in glomerular filtration rate. Whether cystatin C concentrations are associated with adverse clinical events among persons with coronary heart disease has not been studied extensively. In the present study, we demonstrate that higher cystatin C concentrations are associated with mortality, cardiovascular events, and incident heart failure, independent of traditional cardiovascular risk factors, among 990 persons with coronary heart disease. Higher cystatin C concentrations predicted these outcomes whether or not participants had low estimated glomerular filtration rate or microalbuminuria. If confirmed in future studies, cystatin C may have clinical utility in identifying persons at high risk for adverse outcomes among populations with coronary heart disease.
Acknowledgments
The authors thank Eric Vittinghoff, PhD, and David Glidden, PhD, for their assistance in statistical consultation.
Sources of Funding Dr Ix was funded by grants from the University of California at San Francisco Academic Senate and the American Heart Association Fellow-to-Faculty Transition Award. Dr Shlipak was funded by the American Federation for Aging Research and National Institute on Aging (Paul Beeson Scholars Program), the Robert Wood Johnson Foundation (Generalist Faculty Scholars Program), and National Institutes of Health grant R01 DK066488. Dr Whooley was funded by the Department of Veterans Affairs, the American Federation for Aging Research, the Robert Wood Johnson Foundation, the Ischemia Research and Education Foundation, the Nancy Kimran Heart Research Fund, and Dade Behring, Inc. Dade Behring supported the cystatin C measurements in the Heart and Soul cohort.
Footnotes
Disclosures Dr Shlipak has received a modest honorarium from Amgen, Inc. Dr Chertow has received significant research support from Amgen, Inc. Dr Whooley has received significant research support from Roche Pharmaceuticals, Amgen, Inc, and Dade Behring, Inc. The other authors report no conflicts.
References
- 1.Clase CM, Garg AX, Kiberd BA. Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2002;13:1338–1349. doi: 10.1097/01.asn.0000013291.78621.26. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD. Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol. 2001;38:705–711. doi: 10.1016/s0735-1097(01)01450-4. [DOI] [PubMed] [Google Scholar]
- 4.Kassirer JP. Clinical evaluation of kidney function– glomerular function. N Engl J Med. 1971;285:385–389. doi: 10.1056/NEJM197108122850706. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 6.Seliger SL, Davis C, Stehman-Breen C. Gender and the progression of renal disease. Curr Opin Nephrol Hypertens. 2001;10:219–225. doi: 10.1097/00041552-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 8.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C: a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 9.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 10.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 11.Coll E, Botey A, Alvarez L, Poch E, Quinto L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 12.Keevil BG, Kilpatrick ES, Nichols SP, Maylor PW. Biological variation of cystatin C: implications for the assessment of glomerular filtration rate. Clin Chem. 1998;44:1535–1539. [PubMed] [Google Scholar]
- 13.Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function: a review. Clin Chem Lab Med. 1999;37:389–395. doi: 10.1515/CCLM.1999.064. [DOI] [PubMed] [Google Scholar]
- 14.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 15.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Fyr CL Wassel, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 18.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 19.Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the Heart and Soul Study. J Am Soc Nephrol. 2003;14:3233–3238. doi: 10.1097/01.asn.0000095642.25603.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II system. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 23.Toto RD. Microalbuminuria: definition, detection, and clinical significance. J Clin Hypertens (Greenwich) 2004;6(suppl 3):2–7. doi: 10.1111/j.1524-6175.2004.4064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beattie MS, Shlipak MG, Liu H, Browner WS, Schiller NB, Whooley MA. C-reactive protein and ischemia in users and nonusers of beta-blockers and statins: data from the Heart and Soul Study. Circulation. 2003;107:245–250. doi: 10.1161/01.cir.0000044387.23578.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 28.Angeja BG, Shlipak MG, Go AS, Johnston SC, Frederick PD, Canto JG, Barron HV, Grady D. Hormone therapy and the risk of stroke after acute myocardial infarction in postmenopausal women. J Am Coll Cardiol. 2001;38:1297–1301. doi: 10.1016/s0735-1097(01)01551-0. [DOI] [PubMed] [Google Scholar]
- 29.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 30.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 31.Ix JH, Mercado N, Shlipak MG, Lemos PA, Boersma E, Lindeboom W, O’Neill WW, Wijns W, Serruys PW. Association of chronic kidney disease with clinical outcomes after coronary revascularization: the Arterial Revascularization Therapies Study (ARTS) Am Heart J. 2005;149:512–519. doi: 10.1016/j.ahj.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation. 2004;110:2342–2348. doi: 10.1161/01.CIR.0000145166.44942.E0. [DOI] [PubMed] [Google Scholar]
- 34.Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 2005;51:321–327. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]