Abstract
Objective
Most HF patients are older adults, yet the associations of low serum potassium and outcomes in these patients are unknown. We studied the effect of low serum potassium in a propensity-matched population of elderly HF patients.
Methods
Of the 7788 patients in the Digitalis Investigation Group trial, 4036 were ≥65 years. Of these, 3598 had data on baseline serum potassium and 324 with potassium ≥5mEq/L were excluded. Remaining patients we categorized into low (<4 mEq/L; n=590) and normal (4–4.9 mEq/L; n=2684) potassium groups. Propensity scores for low-potassium, calculated for each patient, were used to match 561 low-potassium and 1670 normal-potassium patients. Association of low potassium and outcomes were assessed using matched Cox regression analyses.
Results
Patients had a mean (±SD) age of 72 (±6) years, 29% were women and 12% were non-whites. Of the 561 low-potassium patients, 500 had low-normal (3.5–3.9 mEq/L) potassium. All-cause mortality occurred in 37% (rate, 1338/10,000 person-years) normal-potassium and 43% (rate, 1594/10,000 person-years) low-potassium patients (hazard ratio {HR} for low-potassium, 1.22; 95% confidence interval {CI}, 1.04–1.44; p=0.014). Low-normal (3.5–3.9 mEq/L) potassium levels had a similar association with mortality (HR, 1.19, 95% CI, 1.00–1.41, p=0.049). Low (HR, 1.10; 95% CI, 0.96–1.25; p=0.175) or low-normal (HR =1.09, 95% CI =0.95–1.25, p=0.229) serum potassium levels were not associated with all-cause hospitalization.
Conclusions
In a propensity-matched population of elderly ambulatory chronic HF patients, well-balanced in all measured baseline covariates, low and low-normal serum potassium were associated with increased mortality but had no association with hospitalization.
Keywords: Heart failure, elderly, potassium, mortality, hospitalization, propensity score
Hypokalemia is common in heart failure (HF) and is associated with increased mortality [1–3]. A recent study of propensity-matched population of ambulatory chronic HF suggested that serum potassium <4 mEq/L may be associated with increased mortality without any effect on hospitalization. Most HF patients are older adults and yet the effect of low serum potassium in older adults with HF has not been well-studied. A subgroup analysis of the above study found no difference in the effect of low serum potassium on mortality between patients <65 years and those ≥65 years [2]. However, patients in that subgroup analysis were not propensity-matched, and that subgroup analysis did not provide data on other outcomes.
Older adults are often excluded from clinical trials and studies and evidence for these patients is often extrapolated from subgroup analyses. However, because propensity-matched studies can be conduced in a cost-efficient manner, these studies can be used to derive evidence for elderly patients [2, 4, 5]. Thus, the objective of this study was to determine the long-term effects of low serum potassium on mortality and hospitalization in a cohort of propensity score matched chronic systolic and diastolic HF patients 65 years of age or older.
Methods
Study design
We conducted a non-randomized propensity-matched study of the Digoxin Investigation Group (DIG) trial, which was a randomized clinical trial of digoxin in HF conducted in 302 centers (186 in the United States and 116 in Canada) over 32 months during 1991–1993.[6] Detailed descriptions of the rationale, design, implementation, and results of the DIG trial have been reported elsewhere [6].
Study patients
All of the 7788 DIG participants were ambulatory chronic systolic and diastolic HF patients in normal sinus rhythm. Of these, 6800 had left ventricular ejection fraction ≤45%. Most DIG participants were receiving angiotensin-converting enzyme (ACE) inhibitors and diuretics. Beta-blockers were not approved for HF during the DIG trial and data on beta-blocker use were not collected. Of the 7788 patients, 4036 (52%) patients were aged ≥65 years of age, and of them 3598 (89%) had valid data on baseline serum potassium levels. We excluded patients with a serum potassium level of ≥5mEq/L from our analysis based on a preliminary analysis that suggested an increased risk of death associated with serum potassium at these levels. Thus we restricted our analysis to a subset of 590 low-potassium patients (<4 mEq/L) and 2684 normal-potassium (4–4.9 mEq/L) patients, of whom a matched cohort of 2231 patients were used for main analyses.
Low serum potassium
Serum potassium values below 4 mEq/L have been suggested as low in HF [7], and these patients may be at increased risk of death [2]. Therefore, we defined low serum potassium as <4 mEq/L. Of the 3274 patients in our analysis, 590 (18%) had serum potassium <4 mEq/L, of whom 561 (95%) were included in the matched analysis. Of the 561 patients with serum potassium <4 mEq/L, 61 patients had serum potassium <3.5 mEq/L, and of these only 12 patients had serum potassium <3 mEq/L.
Study outcomes
The primary outcomes were all-cause mortality and all-cause hospitalization. We also studied mortality and hospitalizations due to cardiovascular causes and HF. All study outcomes were ascertained by blinded study investigators. DIG participants were followed for a median of 38 months and vital status data were complete for 99% of the patients.
Statistical analysis
There were significant imbalances in baseline patient characteristics between patients with low (<4 mEq/L) and normal (4–4.9 mEq/L) serum potassium levels (Table 1, pre-match data). We used propensity scores to balance baseline covariates between patients with low (<4 mEq/L) and normal (4–4.9 mEq/L) serum potassium. The propensity score for hypokalemia of a patient may be defined as the conditional probability of that patient’s developing hypokalemia given his/her baseline covariates. We estimated propensity scores for low serum potassium for each of the 3274 patients using a non-parsimonious multivariable logistic regression model [3, 8, 9]. In the model, low serum potassium was used as the dependent variable, and 32 measured baseline patient characteristics (Figure 1, except for chronic kidney disease, which was a derived variable) were included as covariates. Clinically plausible interaction terms were tested but were not included in the final model due to lack of significant interactions [10]. We then used the propensity scores to match 561 (95% of 590) patients with low serum potassium with 1670 (62% of 2684) patients with normal serum potassium levels.
Table 1.
Baseline patient characteristics, by serum potassium, before and after propensity score matching
| n (%) or mean (±SD) | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Serum potassium 4–4.9 mEq/L (N=2684) | Serum potassium <4 mEq/L (N=590) | P Value | Serum potassium 4–4.9 mEq/L (N=1670) | Serum potassium <4 mEq/L (N=561) | P Value | |
| Age | 72 ± 5 | 72 ± 6 | 0.158 | 72 ± 6 | 72 ± 6 | 0.943 |
| Female | 705 (26%) | 213 (36%) | <0.001 | 559 (34%) | 189 (34%) | 0.925 |
| Non-white | 263 (10%) | 79 (13%) | 0.010 | 198 (12%) | 74 (13%) | 0.403 |
| Body mass index, kg/m2 | 26 ± 5 | 26 ± 5 | 0.406 | 26 ± 5 | 26 ± 6 | 0.927 |
| Duration of Heart failure(months) | 29 ± 37 | 29 ± 37 | 0.997 | 29 ± 38 | 30 ± 37 | 0.789 |
| Primary cause of Heart failure | ||||||
| Ischemic | 1987 (74%) | 394 (67%) | <0.001 | 1,165 (70%) | 385 (69%) | 0.962 |
| Hypertensive | 277 (10%) | 97 (16%) | 229 (14%) | 80 (14%) | ||
| Idiopathic | 289 (11%) | 70 (12%) | 201 (12%) | 69 (12%) | ||
| Others | 131 (5%) | 29 (5%) | 75 (5%) | 27 (5%) | ||
| Prior myocardial infarction | 1,778 (66%) | 355 (60%) | 0.005 | 1,039 (62%) | 344 (61%) | 0.705 |
| Current angina pectoris | 752 (28%) | 169 (29%) | 0.759 | 497 (30%) | 160 (29%) | 0.577 |
| Hypertension | 1271 (47%) | 336 (57%) | <0.001 | 915 (55%) | 310 (55%) | 0.847 |
| Diabetes mellitus | 767 (29%) | 153 (26%) | 0.196 | 449 (27%) | 147 (26%) | 0.752 |
| Chronic kidney disease | 1,588 (59%) | 357 (61%) | 0.548 | 1,019 (61%) | 336 (60%) | 0.637 |
| Medications | ||||||
| Pre-trial digoxin use | 1113 (42%) | 251 (43%) | 0.632 | 705 (42%) | 239 (43%) | 0.872 |
| Trial use of digoxin | 1335 (50%) | 291 (49%) | 0.854 | 823 (49%) | 277 (49%) | 0.969 |
| ACE inhibitors | 2,485 (93%) | 535 (91%) | 0.117 | 1,521 (91%) | 513 (91%) | 0.792 |
| Hydralazine & nitrates | 42 (1.6%) | 10 (1.7%) | 0.819 | 30 (2%) | 9 (2%) | 0.764 |
| Diuretics | 2,141 (80%) | 507(86%) | 0.001 | 1436 (86%) | 478 (85%) | 0.646 |
| Potassium sparing diuretics | 180 (7%) | 51 (9%) | 0.096 | 135 (8%) | 49 (9%) | 0.628 |
| Potassium supplement | 824 (31%) | 241 (41%) | <0.001 | 635 (38%) | 217 (39%) | 0.782 |
| Symptoms and signs of Heart failure | ||||||
| Dyspnea at rest | 571 (21%) | 136 (23%) | 0.342 | 370 (22%) | 127 (23%) | 0.812 |
| Dyspnea on exertion | 2,057 (77%) | 448 (76%) | 0.714 | 1,257 (75%) | 423 (75%) | 0.950 |
| Limitation of activity | 2057 (77%) | 453 (77%) | 0.942 | 1,278 (77%) | 429 (77%) | 0.978 |
| Jugular venous distension | 336 (13%) | 94 (16%) | 0.026 | 235 (14%) | 81 (14%) | 0.829 |
| Third heart sound | 597 (22%) | 143 (24%) | 0.294 | 410 (25%) | 133 (24%) | 0.687 |
| Pulmonary râles | 488 (18%) | 122 (21%) | 0.159 | 334 (20%) | 112 (20%) | 0.985 |
| Lower extremity edema | 565 (21%) | 161 (27%) | 0.001 | 416 (25%) | 140 (25%) | 0.983 |
| NYHA functional class | ||||||
| Class I | 353 (13%) | 71 (12%) | 0.476 | 204 (12%) | 71 (13%) | 0.967 |
| Class II | 1451 (54%) | 306 (52%) | 878 (53%) | 291 (52%) | ||
| Class III | 823 (31%) | 199 (34%) | 554 (33%) | 186 (33%) | ||
| Class IV | 57 (2%) | 14 (2%) | 34 (2%) | 13 (2%) | ||
| Heart rate (/minute), | 77 ± 12 | 79 ± 13 | 0.020 | 78 ± 12 | 78 ± 12 | 0.887 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 130 ± 21 | 131 ± 22 | 0.251 | 130 ± 21 | 130 ± 21 | 0.931 |
| Diastolic | 74 ± 11 | 75 ± 11 | 0.066 | 74 ± 11 | 74 ± 11 | 0.754 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 385 (14%) | 95 (16%) | 0.275 | 254 (15%) | 88 (16%) | 0.786 |
| Cardiothoracic ratio >0.5 | 1,659 (62%) | 412 (70%) | <0.001 | 1,150 (69%) | 386 (69%) | 0.980 |
| Serum creatinine (mg/dL) | 1.34 ± 0.37 | 1.34 ± 0.42 | 0.834 | 1.34 ± 0.38 | 1.34 ± 0.41 | 0.889 |
| Estimated glomerular filtration rate, ml/min per1.73 m2 | 57 ± 17 | 57 ± 18 | 0.602 | 57 ± 18 | 57 ± 18 | 0.522 |
| Ejection fraction (%) | 33 ± 13 | 34 ± 14 | 0.156 | 34 ± 14 | 34 ± 14 | 0.951 |
| Ejection fraction ≤45% | 2,277 (85%) | 486 (82%) | 0.136 | 1,378 (83%) | 465 (83%) | 0.840 |
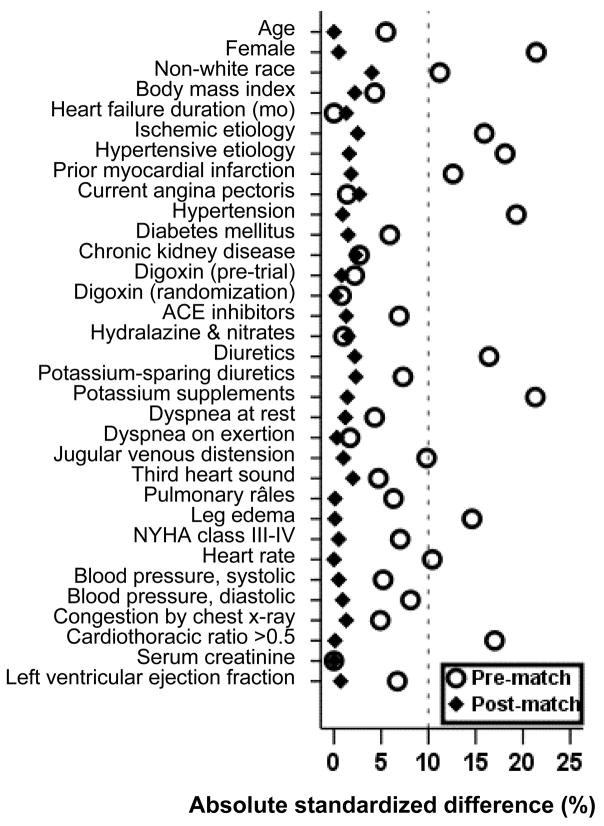
Figure 1.
Absolute standardized differences before and after propensity score matching comparing covariate values for patients with potassium levels 4–4.9 and <4
Pre-match imbalances in baseline covariates between groups and post-match balance achieved were assessed by estimating pre- and post-match absolute standardized differences of covariates between the two groups [2, 11–13]. Standardized differences directly quantify biases in the means (or proportions) of covariates across the groups, and are expressed as percentages of the pooled standard deviations. An absolute standardized difference of 0% on a covariate indicates no residual bias for that covariate, and an absolute standardized difference below 10% suggests inconsequential residual bias [12]. We then compared baseline characteristics of matched patients using McNemar and paired sample t tests.
We used Kaplan-Meier plots and matched Cox regression analysis to estimate associations of low potassium with various outcomes. We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves. We then repeated our analyses using serum potassium as a continuous variable. To determine whether the loss of sample size in the matching process affected our results, we estimated the effect of low potassium on outcomes in the full pre-match cohort of 3274 patients using three different approaches: (1) unadjusted, (2) adjusted for raw propensity scores, and (3) adjusted for all covariates used in the propensity score model. We also repeated these analyses using serum potassium as a continuous variable.
We conducted subgroup analyses to determine the homogeneity of the associations of low potassium with all-cause mortality. We first calculated absolute risk differences, and then estimated the effect of low potassium on mortality in each subgroup using Cox regression model, in each case adjusting for propensity score for low potassium. Finally, we formally tested for first-order interactions using Cox proportional hazards models, entering interaction terms and adjusting for propensity scores, separately for each subgroup. All statistical tests were evaluated using two-tailed 95% confidence levels, and data analyses were performed using SPSS for Windows version 14 [14].
Results
Patient characteristics
The mean (±SD) age of the 2231 matched patients was 72 (±6) years, 748 (29%) were women and 272 (12%) were non-whites. Before matching, low-potassium patients were more likely to be women, non-white, and have hypertension, elevated jugular venous pressure and leg edema, cardiomegaly, and be receiving diuretics and potassium supplements. They were less likely to have diabetes or a prior myocardial infarction (Table 1). After matching, normal- and low-potassium patients were more similar in regards to all measured baseline covariates (Table 1 and Figure 1). Our propensity score matching reduced standardized differences for all observed covariates below 5% in absolute value, demonstrating substantial improvement in covariate balance across the treatment groups (Figure 1).
Low potassium and mortality
All-cause mortality occurred in 37% of normal-potassium (rate, 1338/10000 person-years) and 43% of low potassium (rate, 1594/10,000 person-years) patients (hazard ratio {HR} when low-potassium group was compared with normal-potassium group, 1.22, 95% confidence interval {CI}, 1.04–1.44, P=0.014; Table 2 and Figure 2a). When we repeated our analysis after excluding patients with very low (<3.5 mEq/L) serum potassium, compared with normal (≥4 mEq/L) potassium, low-normal (3.5–3.9 mEq/L) serum potassium was associated with increased mortality (HR, 1.19, 95% CI, 1.00–1.41, P=0.049).
Table 2.
Cause-specific mortality in heart failure patients age ≥65 after matching by propensity scores for serum potassium <4 mEq/L.
| Serum potassium 4–4.9 mEq/L (N=1670) | Serum potassium <4 mEq/L (N=561) | Absolute rate difference* (per 10000 person-years) | Hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Rate, per 10000 person-years(Events/total follow up years) | |||||
| All-cause | 1338 (625/4672) | 1594 (242/1518) | + 256 | 1.22 (1.04–1.44) | 0.014 |
| Cardiovascular | 1021 (477/4672) | 1219 (185/1518) | + 198 | 1.19 (0.99–1.43) | 0.058 |
| Progressive heart failure | 467 (218/4672) | 626 (95/1518) | + 159 | 1.31 (1.01–1.69) | 0.045 |
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the serum potassium 4–4.9 mEq/L group from the event rates in the serum potassium <4 mEq/L group (before values were rounded).
Figure 2.
Kaplan-Meier plots for (a) all-cause mortality, and (b) all-cause hospitalization
In the full pre-match cohort (n =3274 patients), 43% of patients with low potassium and 37% of patients with normal-potassium died (unadjusted HR, 1.21, 95% CI, 1.06–1.39; P=0.007). The association remained essentially unchanged when adjusted for raw propensity scores (HR =1.19, 95% CI =1.03–1.37, P=0.016) or all baseline covariates (HR, 1.22, 95% CI, 1.06–1.40, P=0.006).
When we repeated our analysis using serum potassium as a continuous variable, the unadjusted, propensity-adjusted, and multivariable adjusted HR for all-cause mortality for every mEq/L increase in serum potassium were respectively 0.88 (95% CI, 0.77–1.00, P=0.050); 0.89 (95% CI =0.78–1.02, P=0.096) and 0.84 (95% CI, 0.74–0.96, P= 0.012).
Death due to cardiovascular causes occurred in 29% (rate, 1021/10,000 person-years) normal-potassium and 33% (rate, 1219/10,000 total person-years) low-potassium patients (HR, 1.19, 95% CI, 0.99–1.43, P=0.058; Table 2). Death from progressive HF occurred in 13% (rate, 467/10,000 person-years) and 17% (rate, 626/10,000 person-years) of respectively normal- and low-potassium group (HR, 1.31, 95% CI, 1.01–1.69, P=0.045 (Table 2).
Low potassium and hospitalization
All-cause hospitalization occurred in 70% (rate, 4482/10,000 person-years) normal-potassium and 70% (rate, 4752/10,000 person-years) low-potassium patients (HR, 1.10, 95% CI, 0.96–1.25, P=0.175; (Table 3, Figure 2b). A similar lack of association was observed when were compared low-normal (3.5–3.9 mEq/L) potassium with normal-potassium group (HR, 1.09, 95% CI, 0.95–1.25, P=0.229). There was no difference in the rates of hospitalization for cardiovascular causes or worsening HF between the normal and low-potassium cohorts (Table 3).
Table 3.
Hospitalizations† by causes in heart failure patients age ≥65 years after matching by propensity scores for serum potassium < 4.1 mEq/L.
| Serum potassium 4–4.9 mEq/L (N=1670) | Serum potassium <4 mEq/L (N=561) | Absolute rate difference* (per 10000 person-years) | Hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Rate, per 10000 person-years (Events/total follow up years) | |||||
| All-cause | 4482 (1173/2617) | 4752 (393/827) | +270 | 1.10 (0.96–1.25) | 0.175 |
| Cardiovascular | 2761 (884/3202) | 3134 (314/1002) | + 373 | 1.08 (0.94–1.25) | 0.272 |
| Worsening heart failure | 1384 (538/3887) | 1521 (190/1249) | + 137 | 1.01 (0.85–1.21) | 0.878 |
Data shown include the first hospitalization of each patient for each cause.
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the serum potassium 4–4.9 mEq/L group from the event rates in the serum potassium <4 mEq/L group (before values were rounded).
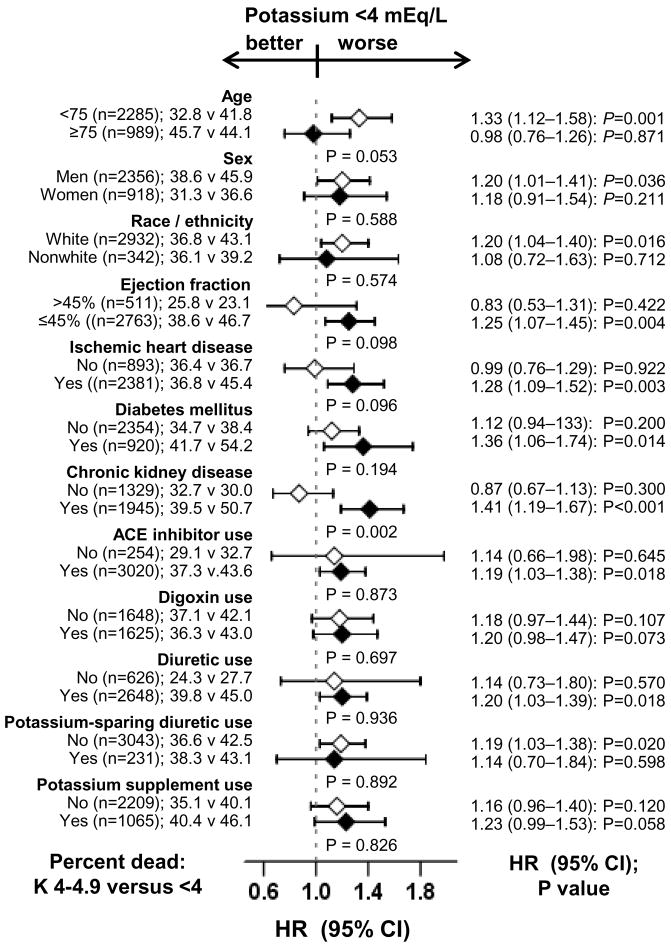
Subgroup analyses
The associations of low potassium and all-cause mortality were observed in a wide spectrum of HF patients (Figure 3). There were no significant interactions between low potassium and any of the subgroups, except for in chronic kidney disease (p for interaction 0.002). Of interest, low potassium had similar effect on mortality regardless of the use of digoxin (43% and 42% deaths respectively among patients receiving and not receiving digoxin; Figure 3). The effects of low potassium on cardiovascular and HF mortality were also similar in patients receiving and not receiving digoxin (data not shown).
Figure 3.
Association of serum potassium <4 mEq/L and all-cause mortality in subgroups of propensity score matched heart failure patients (p values or interactions are in the middle of the figure)
(ACE=angiotensin-converting enzyme; CI= confidence interval; HR=hazard ratio)
Discussion
The findings from the current analysis demonstrate that in a propensity-matched population of ambulatory older adults with chronic HF, serum potassium level <4 mEq/L was associated with increased mortality, but had no effect on hospitalization. Low serum potassium was also associated with increased mortality from progressive HF but had no effect on hospitalization due to worsening HF. These apparent dissociations between mortality and hospitalization suggest that most deaths related to low serum potassium may have been caused by potentially preventable fatal ventricular arrhythmias that may have precluded hospital admissions. These findings are important as most patients in the low-potassium groups had serum potassium between 3.5 and 4 mEq/L, suggesting that serum potassium levels considered normal to low-normal by most laboratory standards may be potentially harmful in chronic HF.
Possible mechanistic explanations
Increased mortality associated with low serum potassium may be due to increased sudden cardiac death in that group. Even though we had no data on sudden cardiac death, there is evidence that low serum potassium may enhance membrane excitability leading to increased cardiac automaticity and ventricular repolarization delays leading to potential fatal arrhythmias [15, 16]. This notion is supported by our observation that low potassium was associated with increased mortality, but not with increased hospitalization, indicating fatal arrhythmias and sudden cardiac death as possible mechanistic explanations.
Hypokalemia may also be a marker of disease progression. Data from animal and human models also suggest low potassium may cause diastolic dysfunction, endothelial dysfunction, and increased rates of thrombosis and platelet aggregation [17–21]. Angiotensin II, aldosterone, and noradernaline activation is associated with hypokalemia [22–25]. Alternately, inhibitors of these neurohormones may raise serum potassium levels [26–30]. Thus, hypokalemia may be a marker of increased disease progression and/or lack of neurohormonal inhibition. Over 90% of our matched patients were receiving ACE inhibitors. However, we had no data on the use of other neurohormonal antagonists including beta-blockers.
Hypokalemia can also be a surrogate marker for symptomatic HF. More symptomatic HF patients are likely to receive diuretic in higher dosages and thus at greater risk of developing hypokalemia. Although we had no data on diuretic dosages, our matched patients were well-balanced in all measured baseline covariates including symptom burden and use of diuretics. We do not know why hypokalemia would be more harmful in HF patients with chronic kidney disease (Figure 3). HF patients with chronic kidney disease often require high-dose diuretics and yet clinicians may be less aggressive with hypokalemia in these patients for fear of causing hypokalemia.
Clinical implications
Low serum potassium, well tolerated in healthy adults, is believed to increase risks of mortality in patients with cardiovascular disease, especially the elderly [7, 31]. However, the effect of low potassium on outcomes in elderly HF patients is not well-studied. Our findings suggest that maintaining serum potassium of 4–4.9 mEq/L may improve survival in chronic HF. Spironolactone (or eplerenone for those who are unable to tolerate spironolactone) may be preferable to potassium supplement as in addition to correcting hypokalemia, it may also correct other electrolyte imbalances such as hypomagnesemia, associated with aldosterone activation in HF [26, 27]. For those patients unable to tolerate an aldosterone receptor antagonist, potassium supplementation should be used to avoid hypokalemia and maintain normokalemia [32]. Serum potassium levels should be monitored in elderly HF patients receiving aldosterone antagonists or potassium supplement to maintain normokalemia, especially in those with renal insufficiency and/or receiving ACE inhibitors, angiotensin-receptor blockers, or beta-blockers.
Limitations
Patients in our study were predominantly white male patients in normal sinus rhythm with few receiving beta-blockers and aldosterone antagonists. We also had no data on dose of diuretics, magnesium levels, and sudden cardiac deaths. Finally, most patients in the low-potassium group had low-normal (3.5–3.9 mEq/L) serum potassium. However, the association observed in our study may be stronger in those with very low (<3.5 mEq/L) serum potassium.
Conclusions
Low-normal to low serum potassium levels were associated with increased mortality without any association with hospitalization in a wide spectrum of well-balanced propensity-matched ambulatory older adults with chronic HF. Serum potassium levels in elderly HF patients should be maintained between 4 and 4.9 mEq/L. An aldosterone antagonist may be preferable over potassium supplements to correct hypokalemia and maintain normokalemia.
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–9. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed A, Zannad F, Love TE, et al. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–43. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed A, Young JB, Love TE, Levesque R, Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed A. Digoxin and reduction in mortality and hospitalization in geriatric heart failure: importance of low doses and low serum concentrations. J Gerontol A Biol Sci Med Sci. 2007;62:323–9. doi: 10.1093/gerona/62.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A, Aronow WS. A propensity-matched study of the association of physical function and outcomes in geriatric heart failure. Arch Gerontol Geriatr. 2008;46(2):161–72. doi: 10.1016/j.archger.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43:155–61. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A, Pitt B, Rahimtoola SH, et al. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: a propensity-matched study of the DIG trial. Int J Cardiol. 2008;123:138–46. doi: 10.1016/j.ijcard.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sui X, Gheorghiade M, Zannad F, Young JB, Ahmed A. A propensity matched study of the association of education and outcomes in chronic heart failure. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.05.029. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13:841–53. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: a propensity score analysis. Am Heart J. 2006;152:956–66. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 13.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–7. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 14.SPSS. SPSS 15 for Windows. rel 14. Chicago IL: 2007. [Google Scholar]
- 15.Coca SG, Perazella MA, Buller GK. The cardiovascular implications of hypokalemia. Am J Kidney Dis. 2005;45:233–47. doi: 10.1053/j.ajkd.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Schulman M, Narins RG. Hypokalemia and cardiovascular disease. Am J Cardiol. 1990;65:4E–9E. doi: 10.1016/0002-9149(90)90244-u. discussion 22E–23E. [DOI] [PubMed] [Google Scholar]
- 17.Ascherio A, Rimm EB, Hernan MA, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98:1198–204. doi: 10.1161/01.cir.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 18.Lin H, Young DB. Interaction between plasma potassium and epinephrine in coronary thrombosis in dogs. Circulation. 1994;89:331–8. doi: 10.1161/01.cir.89.1.331. [DOI] [PubMed] [Google Scholar]
- 19.McCabe RD, Bakarich MA, Srivastava K, Young DB. Potassium inhibits free radical formation. Hypertension. 1994;24:77–82. doi: 10.1161/01.hyp.24.1.77. [DOI] [PubMed] [Google Scholar]
- 20.McCabe RD, Young DB. Potassium inhibits cultured vascular smooth muscle cell proliferation. Am J Hypertens. 1994;7:346–50. doi: 10.1093/ajh/7.4.346. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava TN, Young DB. Impairment of cardiac function by moderate potassium depletion. J Card Fail. 1995;1:195–200. doi: 10.1016/1071-9164(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 22.Brilla CG, Rupp H, Funck R, Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur Heart J. 1995;16 Suppl O:107–9. doi: 10.1093/eurheartj/16.suppl_o.107. [DOI] [PubMed] [Google Scholar]
- 23.Ramires FJ, Mansur A, Coelho O, et al. Effect of spironolactone on ventricular arrhythmias in congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am J Cardiol. 2000;85:1207–11. doi: 10.1016/s0002-9149(00)00729-3. [DOI] [PubMed] [Google Scholar]
- 24.Zannad F, Dousset B, Alla F. Treatment of congestive heart failure: interfering the aldosterone-cardiac extracellular matrix relationship. Hypertension. 2001;38:1227–32. doi: 10.1161/hy1101.099484. [DOI] [PubMed] [Google Scholar]
- 25.Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta2-receptor stimulation by circulating epinephrine. N Engl J Med. 1983;309:1414–9. doi: 10.1056/NEJM198312083092303. [DOI] [PubMed] [Google Scholar]
- 26.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 27.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 28.Petch MC, McKay R, Bethune DW. The effect of beta, adrenergic blockade on serum potassium and glucose levels during open heart surgery. Eur Heart J. 1981;2:123–6. doi: 10.1093/oxfordjournals.eurheartj.a061171. [DOI] [PubMed] [Google Scholar]
- 29.Rosa RM, Silva P, Young JB, et al. Adrenergic modulation of extrarenal potassium disposal. N Engl J Med. 1980;302:431–4. doi: 10.1056/NEJM198002213020803. [DOI] [PubMed] [Google Scholar]
- 30.Frost L, Bottcher M, Botker HE, Kristensen SD, Norgaard A. Enalapril and exercise-induced hyperkalemia. A study of patients randomized to double-blind treatment with enalapril or placebo after acute myocardial infarction. Int J Cardiol. 1992;37:401–5. doi: 10.1016/0167-5273(92)90273-6. [DOI] [PubMed] [Google Scholar]
- 31.Gennari FJ. Hypokalemia. N Engl J Med. 1998;339:451–8. doi: 10.1056/NEJM199808133390707. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed A, Adamopoulos C, Sui X, Love TE. Potassium supplement use may increase hospitalization without affecting mortality in chronic heart failure: Implications for use of aldosterone antagonists to maintain potassium balance in chronic heart failure. Circulation. 2007;116:II-766. [Google Scholar]