Abstract
Objective
To examine the relationship between cardiac self-efficacy and health status, including symptom burden, physical limitation, quality of life, and overall health among outpatients with stable coronary heart disease (CHD). We hypothesized that lower self-efficacy would predict worse health status, independent of CHD severity and depression.
Methods
We performed a cross-sectional study of 1024 outpatients with CHD, who were recruited between 2000 and 2002 for the Heart and Soul Study. We administered a validated measure of cardiac self-efficacy, assessed cardiac function using exercise treadmill testing with stress echocardiography, and measured depressive symptoms using the Patient Health Questionnaire. Health status outcomes (symptom burden, physical limitation, and quality of life) were assessed using the Seattle Angina Questionnaire, and overall health was measured as fair or poor (versus good, very good, or excellent).
Results
After adjustment for CHD severity and depressive symptoms, each standard deviation (4.5-point) decrease in self-efficacy score was independently associated with greater symptom burden (adjusted odds ratio (OR) = 2.1, p = .001), greater physical limitation (OR = 1.8, p < .0001), worse quality of life (OR = 1.6, p < .0001), and worse overall health (OR = 1.9, p < .0001). Depressive symptoms and poor treadmill exercise capacity were also associated with poor health status, but left ventricular ejection fraction and ischemia were not.
Conclusions
Among patients with CHD, low cardiac self-efficacy is associated with poor health status, independent of CHD severity and depressive symptoms. Further study should examine if self-efficacy constitutes a useful target for cardiovascular disease management interventions.
Keywords: self-efficacy, health status, heart disease, epidemiology
INTRODUCTION
Improving patient-reported health status, including functional status and quality of life, is an important goal for the therapy of patients with chronic disease. In patients with cardiovascular disease, traditional studies have focused on improving physiological measures, such as left ventricular function or coronary graft patency. However, increasing attention has been directed toward health status outcomes as being equally, if not more, important for the well-being of patients with cardiac disease (1-4). Little is known about the determinants of health status in patients with cardiovascular disease (5-11). In particular, the extent to which health status is determined by psychological versus physiological measures of cardiac function is unclear (12-14).
Self-efficacy is a psychological construct based on social cognitive theory, which describes the interaction between behavioral, personal, and environmental factors in health and chronic disease (15-17). The theory of self-efficacy proposes that patients’ confidence in their ability to perform certain health behaviors influences their health outcomes. The construct of self-efficacy has extended far beyond the psychological arena and has been demonstrated to affect health behaviors and chronic disease management in many chronic disease settings (18-24). Importantly, self-efficacy is a modifiable characteristic; many health behavior interventions have been shown to improve patients’ self-efficacy (17,25-27).
In patients with cardiovascular disease, studies of self-efficacy have largely focused on its role in the successful rehabilitation of patients with cardiac disease (28-33). However, one previous study found that self-efficacy predicted health status in 198 patients undergoing cardiac angiography (34). We hypothesized that cardiac self-efficacy would be associated with health status, defined as an individual’s degree of wellness or illness with regard to cardiac symptom burden, physical limitation, quality of life, and overall health, within a broader population of 1024 outpatients with established coronary heart disease (CHD), and that this association would be independent of CHD severity and depressive symptoms.
METHODS
Participants
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients with coronary disease. Data collection methods have been described elsewhere (13,35). Administrative databases were used to identify patients with one of the following eligibility criteria: a) a history of myocardial infarction, b) angiographic evidence of at least 50% stenosis of ≥1 coronary vessels, c) evidence of ischemia by treadmill or nuclear stress testing, or d) a history of coronary revascularization. The exclusion criteria were a) an intention to move out of the area within 3 years, b) a history of myocardial infarction within the last 6 months, or c) exercise tolerance <1 block—all of which precluded completion of the study.
Between September 2000 and December 2002, we recruited 1024 participants with CHD from two Department of Veterans’ Affairs Medical Centers, one university hospital, and nine public health clinics in the San Francisco Bay Area. These participants constituted the subjects of the current cross-sectional analysis. The baseline appointment included a medical history interview, a physical examination, an exercise treadmill test with a stress echocardiogram, and a comprehensive health status questionnaire. The protocol was approved by the Institutional Review Boards at all participating facilities.
Health Status
Our outcome of interest was health status among patients with CHD. We used the Seattle Angina Questionnaire, based on Wilson and Cleary’s model (36), adapted for patients with CHD (37,38), to assess three components of health status: symptom burden, functional status, and disease-specific quality of life (13,39,40). As an additional measure of global health status, we also asked patients: “Compared with other people your age, how would you rate your overall health?” (41,42).
To facilitate comparison with other studies of cardiac health status (13,38,43,44), we divided the symptom burden scores into four categories reflecting daily (0–30), weekly (31–60), monthly (61–90), or absent (91–100) angina; the physical limitation scores into severe (0–24), moderate (25–49), mild (50–74), or minimal (75–100) physical limitation; and the quality-of-life scores into severely diminished (0–24), moderately diminished (25–49), mildly diminished (50–74), or good to excellent (75–100) quality of life. Higher scores for these measures reflected better health status. Responses to the overall health measure were categorized as fair or poor, good, very good, or excellent.
Cardiac Self-Efficacy
The main predictor of interest was cardiac self-efficacy, defined as participants’ confidence in their ability to take care of their health (15,16). We measured cardiac self-efficacy using Sullivan’s validated five-item summative “Maintain function” scale (24,29,34,45). Each item begins with the stem, “How confident are you that you know or can,” and assesses an aspect of daily life function, such as work and social activities (Table 1). The responses are a 5-level Likert scale from 0 = “not at all confident” to 4 = “completely confident.” The self-efficacy scores ranged between 0 and 20, with a higher score indicating better self-efficacy to maintain function.
TABLE 1. Cardiac Self-Efficacy Scale.
How confident are you that you can:
|
Responses (score): not at all confident (0); somewhat confident (1); moderately confident (2); very confident (3); and completely confident (4).
Cardiac Function
We hypothesized that participants’ cardiac function could influence self-reported health status. Therefore, we performed three physiologic measures of cardiac function: echocardiographic assessment of resting left ventricular ejection fraction (LVEF), exercise treadmill test for exercise capacity, and a stress echocardiogram for assessment of fixed and inducible ischemia (wall motion abnormalities). All of these variables are well-established measures of cardiac function (46).
A complete resting two-dimensional echocardiogram was performed on each participant. To determine the LVEF, standard two-dimensional parasternal short-axis and apical two-chamber and four-chamber views were used. Before and after exercise, we obtained apical two-chamber, four-chamber, and precordial long- and short-axis views to detect changes in wall motion or ventricular dilatation with exercise. To account for fixed and exertional wall motion defects (our measure of ischemia), we calculated the wall motion score at peak exercise. Each of 16 wall segments was evaluated for contractility at peak exercise, as follows: 1 = normal; 2 = hypokinetic; 3 = akinetic; 4 = dyskinetic; 5 = aneurysm. The scores for each segment are averaged to create an index from 1 to 16, with a higher score indicating worse contractility.
Other Participant Characteristics
Participants reported demographic characteristics, including age, ethnicity, education, and marital status. Patients reported their annual household income. Because we were interested in low income as a risk factor, we dichotomized responses into <$20,000 versus ≥$20,000 annual household income. The questionnaire assessed self-reported history of myocardial infarction, stroke, diabetes mellitus, or hypertension as well as alcohol and tobacco use. To account for other clinical characteristics that could affect health status, we recorded use of medications such as β blockers, statins, renin-angiotensin system inhibitors, and antidepressants. Body mass index (weight in kilograms divided by height in m2) was calculated for each participant.
Because health status can be affected by mood, stress, and social support (13,47-55), we also measured several psychosocial variables. We measured depressive symptoms using the Patient Health Questionnaire-9 (56), a validated measure in which a higher score indicates more depressive symptoms. We considered a score of ≥10 as consistent with depressive symptoms (57). To assess perceived stress, we used the 16-point, 4-item Perceived Stress Scale (58), in which experiencing at least one stressful symptom “fairly often” or a score of ≥9 indicates stress. To assess social support, we asked participants: “Do you have as much contact as you like with someone you feel close to, someone in whom you can trust and confide (yes/no)?” (59).
Statistical Analysis
We aimed to assess the contribution of cardiac self-efficacy to self-reported health status in the context of cardiac function. We examined bivariate associations between self-efficacy score as a continuous measure (per standard deviation (SD) change) and the four health status outcomes: symptom burden, functional status, disease-specific quality of life, and overall health status.
To further evaluate the independent association of self-efficacy with each health status outcome, we performed stepwise multivariate ordinal logistic regression. Potential predictors were grouped a priori into conceptually based blocks; each block of variables was entered sequentially, beginning with demographic variables and then adding medical history, medication use, psychosocial, and cardiac function variables. For consistency, we retained all potential predictors across all four models. To put these associations in context, we also reported the adjusted associations for other potential predictors of health status, including depressive symptoms and cardiac physiologic parameters. In all models, we tested for interactions between self-efficacy score and other psychosocial characteristics (depressive symptoms, social support, and perceived stress) and between self-efficacy and gender and nonwhite ethnicity and age. Results are reported as odds ratio (OR) with 95% confidence interval. Analyses were performed using SAS version 9 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
Patient Characteristics
Patients were older and predominantly male, with relatively low socioeconomic status (Table 2). The cohort has significant disease burden, with a majority of patients reporting history of myocardial infarction (54%) and coronary revascularization (59%).
TABLE 2. Characteristics of 1024 Participants With Coronary Heart Disease.
| n (%) or Mean ± SD | |
|---|---|
| Demographics | |
| Age | 67 ± 11 |
| Male | 839 (82) |
| White | 616 (60) |
| High school graduate | 891 (87) |
| Income < $20,000/year | 499 (49) |
| Married | 434 (43) |
| Medical history | |
| Hypertension | 723 (71) |
| Myocardial infarction | 548 (54) |
| Coronary revascularization | 604 (59) |
| Stroke | 147 (14) |
| Diabetes mellitus | 266 (26) |
| Body mass index | 28.4 ± 5.3 |
| Medication use | |
| β blocker | 594 (58) |
| Statin | 656 (64) |
| Renin-angiotensin system inhibitor | 524 (51) |
| Aspirin | 793 (77) |
| Antidepressant | 188 (18) |
| Psychosocial factors | |
| ≥ 10 depressive symptoms | 199 (19) |
| Current smoking | 202 (20) |
| Poor social support | 330 (32) |
| Regular alcohol use | 294 (29) |
| Perceived stress | 5.3 ± 3.2 |
| Cardiac function | |
| Left ventricular ejection fraction | 0.62 ± 0.10 |
| Wall motion score index | 1.2 ± 0.35 |
| Exercise capacity (METS) | 7.3 ± 3.3 |
SD = standard deviation.
Self-Efficacy and Health Status Outcomes
The mean self-efficacy score was 9.7 (SD = 4.5; range = 0–20; skewness = 0.18), corresponding to responses between “not at all confident” and “somewhat confident” for all of the scale items. The Cronbach α for the scale was 0.80.
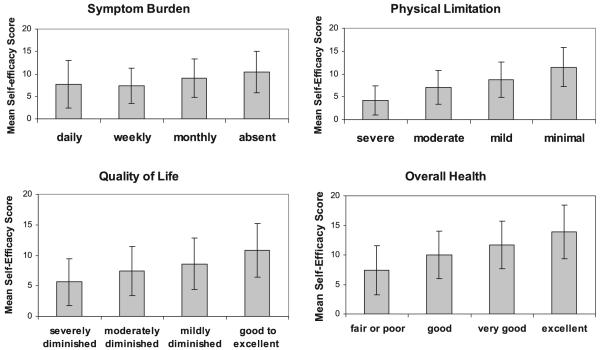
We observed a linear relationship between self-efficacy score and each of the four health status measures (Figure 1). Mean self-efficacy scores ranged from 7.7 to 10.4 in patients with daily to absent symptom burden, 4.2 to 11.5 in patients with severe to minimal physical limitation, 5.6 to 10.8 in patients with severely diminished to excellent quality of life, and 7.4 to 13.9 in patients with poor to excellent overall health (all p values <.0001).
Figure 1.
Mean self-efficacy scores by category of health status outcome (all p values for trend <.01). Error bars represent standard deviation.
With stepwise adjustment for demographics, medical history, medication use, psychosocial factors, and cardiac function, self-efficacy remained independently associated with all four health status measures. After adjustment for other psychosocial factors, the magnitude of self-efficacy and health status relationship decreased across all health status outcomes, but the direction and statistical significance of the relationships persisted (Table 3). In the fully adjusted models, with each SD (4.5-point) decrease in self-efficacy (Table 4), we found greater symptom burden (OR = 1.3, p = .001), physical limitation (OR = 1.9, p < .001), diminished quality of life (OR = 1.6, p < .001), and fair or poor overall health (OR = 2.1, p < .001).
TABLE 3. Association Between Self-Efficacy, Entered per SD (4.5-Point) Decrease, and Health Status in 1024 Participants With Coronary Disease, Adjusted for All Variables in Table 2.
| Odds Ratio (95% CI)* |
||||
|---|---|---|---|---|
| Greater Symptom Burden |
Greater Physical Limitation |
Worse Quality of Life |
Worse Overall Health |
|
| Unadjusted | 1.6 (1.4-1.8) | 2.6 (2.3-3) | 2.0 (1.8-2.3) | 2.5 (2.2-2.8) |
| Adjusted for demographic characteristics | 1.5 (1.3-1.8) | 2.6 (2.2-3) | 2.1 (1.8-2.4) | 2.7 (2.4-3.1) |
| Adjusted for above plus medical history | 1.5 (1.3-1.7) | 2.5 (2.1-2.9) | 2.0 (1.7-2.3) | 2.7 (2.3-3.1) |
| Adjusted for above plus medication use | 1.5 (1.3-1.7) | 2.4 (2.1-2.8) | 2.0 (1.7-2.3) | 2.6 (2.3-3) |
| Adjusted for above plus psychosocial factors | 1.3 (1.1-1.5) | 2.1 (1.8-2.5) | 1.6 (1.4-1.9) | 2.3 (2-2.7) |
| Adjusted for above plus cardiac function | 1.3 (1.1-1.5) | 1.9 (1.6-2.3) | 1.6 (1.4-1.9) | 2.1 (1.8-2.5) |
All p values <.001.
TABLE 4. Multivariate Associations of Self-Efficacy, Depressive Symptoms, and Cardiac Functions With Health Status in Participants With Coronary Heart Disease.
| Symptom Burden Odds Ratio (95% CI) |
Physical Limitation Odds Ratio (95% CI) |
Quality of Life Odds Ratio (95% CI) |
Overall Health Odds Ratio (95% CI) |
|
|---|---|---|---|---|
| Self-efficacy (per SD decrease) | 1.3 (1.1-1.5) | 1.9 (1.6-2.3) | 1.6 (1.4-1.9) | 2.1 (1.8-2.5) |
| Not a high school graduate | 0.71 (0.45-1.1) | 0.67 (0.43-1.1) | 1.1 (0.70-1.6) | 1 (0.66-1.5) |
| Poor social support | 1.4 (1.03-1.9) | 0.79 (0.57-1.1) | 1.3 (0.93-1.8) | 1.1 (0.85-1.5) |
| Depressive symptoms (PHQ ≤ 10) | 1.7 (1.2-2.5) | 2.7 (1.8-4.1) | 2.8 (1.9-4.1) | 1.6 (1.1-2.4) |
| Exercise capacity (per 3.3-MET decrease) | 1.2 (0.95-1.4) | 2.1 (1.7-2.6) | 1.2 (0.97-1.4) | 1.3 (1.1-1.6) |
| LVEF (per 10% decrease) | 1.01 (0.85-1.2) | 1 (0.83-1.2) | 0.97 (0.81-1.2) | 0.97 (0.82-1.1) |
| Wall motion score index (per 0.35 point increase) | 0.99 (0.83-1.2) | 1.1 (0.90-1.3) | 1.04 (0.87-1.2) | 1.1 (0.97-1.3) |
CI = confidence interval; SD = standard deviation; PHQ = Patient Health Questionnaire; LVEF = left ventricular ejection fraction.
Self-Efficacy, Depressive Symptoms, Cardiac Function, and Health Status
After adjustment for all other potential predictors of health status, with each SD decrease in self-efficacy, we observed higher odds of poor health status. Similarly, depressive symptoms were associated with poor health status (Table 4). Although decreased exercise capacity was an independent predictor of poor health status, low self-efficacy remained independently associated with poor health status after adjustment for exercise capacity. Decreased LVEF and impaired wall motion were not associated with any health status outcome (Table 4).
We evaluated the extent to which psychosocial factors (depressive symptoms, social support, and perceived stress) mediated the relationship between self-efficacy and health status. We found that self-efficacy was associated with depressive symptoms (p < .001), and depressive symptoms were predictive of worse health status (Table 4). Adjusting for psychosocial factors partly attenuated the association between self-efficacy and health status. However, even after adjustment for these psychosocial factors, lower self-efficacy remained independently associated with all four measures of health status (Table 3).
There were no interactions between self-efficacy and the other psychosocial measures (depressive symptoms, social support, and perceived stress). We also did not find interactions of self-efficacy score with gender, race/ethnicity, age, or cardiac function (all p values for interaction >.10).
DISCUSSION
We found a clear association between low self-efficacy, a modifiable risk factor, and poor health status in 1024 ambulatory patients with CHD. Specifically, low self-efficacy independently predicted four domains of disease-specific and general health status: greater symptom burden, greater physical limitation, worse quality of life, and worse overall health. Exercise capacity was also predictive of health status, but the association between self-efficacy and health status was independent of CHD severity. Two measures of cardiac function—LVEF and ischemia—were not even associated with health status. Although the causal pathways between self-efficacy and health status cannot be determined by this cross-sectional study and are almost certainly bidirectional, our study results suggest that self-efficacy is an important factor in the perceived health status of patients with CHD.
These findings are consistent with a growing body of evidence supporting an association between self-efficacy and physical health (17,23,25). Self-efficacy has been correlated with self-management behaviors for chronic conditions (19-22). In small studies of selected groups, including patients undergoing cardiac rehabilitation (28-33) and cardiac catheterization (34), patients with chronic obstructive pulmonary disease (24), and older women with congestive heart failure (23), investigators have found an association between self-efficacy and health status. Our study further elucidates the self-efficacy and health status relationship in several ways. First, we examined four distinct aspects of health status, using cardiac-specific and general health status measures, with very consistent results. Another strength of our study was our ability to adjust for other psychosocial characteristics known to be associated with health status, such as depression, perceived stress, and social support. We expected to find strong associations between these factors and self-efficacy. In our sample, these factors only partly accounted for the association between self-efficacy and health status. Depressive symptoms, perceived stress, and social support were partial mediators for the effect of self-efficacy on health status, but self-efficacy exerted an independent influence as well.
Finally, because all participants underwent a detailed cardiac evaluation, we were able to include several objective measures of cardiac function in our analysis.
To place these associations in context, we examined other potential predictors of the health status outcomes. Our finding that self-efficacy was lower among those with lower educational attainment, among women, among those with medical comorbidities, and older participants, are consistent with prior studies (20,31,60). As expected from prior studies of both cardiac and noncardiac conditions (12-14,61-65), depressive symptoms were associated with poor health status. From our results, self-efficacy and depressive symptoms seemed to have distinct relationships with health status; self-efficacy does not act as a significant mediator of the effect of depressive symptoms on health status. Moreover, in this patient sample, having low self-efficacy seemed to confer an even greater risk of poor health status than the presence of depressive symptoms. Finally, our patient population exhibited a strikingly low confidence in their ability to maintain function.
These findings suggest that interventions aimed to improve patient-centered aspects of health should study psychological as well as physiological factors. Self-efficacy is at least as important as cardiac function in the health of patients with CHD and may also be more easily modified. Multiple disease management programs have been shown to improve participant self-efficacy through successful performance of desired behaviors (17). Although individual interventions differ, the widely used Chronic Disease Self-Management Program has been shown to improve self-efficacy and improve clinical outcomes in varied settings (66-68). For example, in one study of 24 patients with heart failure, a home-based exercise protocol was found to improve patient self-efficacy compared with usual care (27). Similarly, a study of older women with heart failure demonstrated improved self-efficacy and improved medication adherence after a patient education intervention (23). Key components of this and other successful disease management programs include peer leadership, cognitive symptom management techniques, health communication training, and health-related problem solving (69,70). Taken together, these findings suggest further study to determine if improving self-efficacy mediates behavior change among patients with chronic disease.
Despite its strengths, our study also has several limitations. First, the relationship between self-efficacy, other psychosocial factors, and health status is likely to be reciprocal, and our cross-sectional data do not allow us to determine the causal directions of association. Second, most participants were older, lower-income males, and therefore the results may not be generalizable to other patient populations. Third, although a debate exists as to the usefulness of disease-specific versus global self-efficacy instruments (23,25), we elected to use a disease-specific measure. Finally, because of the variety of self-efficacy measures in the literature (ranging from single item to in-depth cognitive interviews), we cannot accurately compare the effect sizes for the self-efficacy and health status associations we found with those in other studies.
In summary, improving patient-centered outcomes is an important goal for the chronic management of patients with cardiovascular disease. We found that patient self-efficacy is strongly predictive of health status, including symptom burden, physical functioning, quality of life, and overall health. These results suggest that self-efficacy warrants further study as a potential target for cardiovascular disease management interventions.
Acknowledgments
This study is supported by National Research Service Awards Grant 1 T32 HP19025 (U.S). The Heart and Soul Study was also supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, and the Nancy Kirwan Heart Research Fund. None of these funding sources had any role in the collection of data, interpretation of results, or preparation of this manuscript.
Glossary
- CHD
coronary heart disease
- OR
odds ratio
- LVEF
left ventricular ejection fraction.
REFERENCES
- 1.Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery. Quality of life in patients randomly assigned to treatment groups. Circulation. 1983;68:951–60. doi: 10.1161/01.cir.68.5.951. [DOI] [PubMed] [Google Scholar]
- 2.Croog SH, Levine S, Testa MA, Brown B, Bulpitt CJ, Jenkins CD, Klerman GL, Williams GH. The effects of antihypertensive therapy on the quality of life. N Engl J Med. 1986;314:1657–64. doi: 10.1056/NEJM198606263142602. [DOI] [PubMed] [Google Scholar]
- 3.Hlatky MA, Rogers WJ, Johnstone I, Boothroyd D, Brooks MM, Pitt B, Reeder G, Ryan T, Smith H, Whitlow P, Wiens R, Mark DB, Bypass angioplasty revascularization investigation (BARI) investigators Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. N Engl J Med. 1997;336:92–9. doi: 10.1056/NEJM199701093360203. [DOI] [PubMed] [Google Scholar]
- 4.Pilote L, Lauzon C, Huynh T, Dion D, Roux R, Racine N, Carignan S, Diodati JG, Levesque C, Charbonneau F, Pouliot J, Joseph L, Eisenberg MJ. Quality of life after acute myocardial infarction among patients treated at sites with and without on-site availability of angiography. Arch Intern Med. 2002;162:553–9. doi: 10.1001/archinte.162.5.553. [DOI] [PubMed] [Google Scholar]
- 5.Gehi AK, Rumsfeld JS, Liu H, Schiller NB, Whooley MA. Relation of self-reported angina pectoris to inducible myocardial ischemia in patients with known coronary artery disease: the heart and soul study. Am J Cardiol. 2003;92:705–7. doi: 10.1016/s0002-9149(03)00831-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorkin L, Follick MJ, Geltman E, Hamm P, Sollano J, Sylvia S, Jacobson K, Jacobson MJ, Cochrane BS, Sussex B, Klein M, Gaudette G, Goldman D, Carrol D, Holcombe R, Abern DK, SAVE Investigators Quality of life among patients post-myocardial infarction at baseline in the survival and ventricular enlargement (SAVE) trial. Qual Life Res. 1994;3:111–9. doi: 10.1007/BF00435254. [DOI] [PubMed] [Google Scholar]
- 7.Mattera JA, De Leon CM, Wackers FJ, Williams CS, Wang Y, Krumholz HM. Association of patients’ perception of health status and exercise electrocardiogram, myocardial perfusion imaging, and ventricular function measures. Am Heart J. 2000;140:409–18. doi: 10.1067/mhj.2000.108518. [DOI] [PubMed] [Google Scholar]
- 8.Nelson CL, Herndon JE, Mark DB, Pryor DB, Califf RM, Hlatky MA. Relation of clinical and angiographic factors to functional capacity as measured by the Duke activity status index. Am J Cardiol. 1991;68:973–5. doi: 10.1016/0002-9149(91)90423-i. [DOI] [PubMed] [Google Scholar]
- 9.Sjoland H, Wiklund I, Caidahl K, Albertsson P, Herlitz J. Relationship between quality of life and exercise test findings after coronary artery bypass surgery. Int J Cardiol. 1995;51:221–32. doi: 10.1016/0167-5273(95)02424-u. [DOI] [PubMed] [Google Scholar]
- 10.Wiklund I, Comerford MB, Dimenas E. The relationship between exercise tolerance and quality of life in angina pectoris. Clin Cardiol. 1991;14:204–8. doi: 10.1002/clc.4960140306. [DOI] [PubMed] [Google Scholar]
- 11.Wenger NK. Improved quality of life after PTCA: generalizability and concerns. Cathet Cardiovasc Diagn. 1992;27:95–6. doi: 10.1002/ccd.1810270203. [DOI] [PubMed] [Google Scholar]
- 12.Lesman-Leegte I, Jaarsma T, Sanderman R, Linssen G, van Veldhuisen DJ. Depressive symptoms are prominent among elderly hospitalised heart failure patients. Eur J Heart Fail. 2006;8:634–40. doi: 10.1016/j.ejheart.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the heart and soul study. JAMA. 2003;290:215–21. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan AL, Masoudi FA, Havranek EP, Jones PG, Peterson PN, Krumholz HM, Spertus JA, Rumsfeld JS. Difficulty taking medications, depression, and health status in heart failure patients. J Card Fail. 2006;12:54–60. doi: 10.1016/j.cardfail.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 16.Bandura A. Self-efficacy: the exercise of control. W.H. Freedman and Co.; New York: 1997. [Google Scholar]
- 17.Lorig K, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 18.Holden G. The relationship of self-efficacy appraisals to subsequent health related outcomes: a meta-analysis. Soc Work Health Care. 1991;16:53–93. doi: 10.1300/j010v16n01_05. [DOI] [PubMed] [Google Scholar]
- 19.Aljasem L, Peyrot M, Wissow L, Rubin R. The impact of barriers and self-efficacy on self-care behaviors in type 2 diabetes. Diabetes Educator. 2001;27:393–404. doi: 10.1177/014572170102700309. [DOI] [PubMed] [Google Scholar]
- 20.Bernal H, Woolley S, Schenaul J, Dickinson J. Correlates of self-efficacy in diabetes self-care among Hispanic adults with diabetes. Diabetes Educator. 2000;26:673–80. doi: 10.1177/014572170002600415. [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh D, Gooley S, Wilson P. Prediction of adherence and control in diabetes. J Behav Med. 1993;16:509–23. doi: 10.1007/BF00844820. [DOI] [PubMed] [Google Scholar]
- 22.McCaul K, Glasgow R, Schafer L. Diabetes regimen behaviors: predicting adherence. Med Care. 1987;25:868–81. [PubMed] [Google Scholar]
- 23.Clark NM, Dodge JA. Exploring self-efficacy as a predictor of disease management. Health Educ Behav. 1999;26:72–89. doi: 10.1177/109019819902600107. [DOI] [PubMed] [Google Scholar]
- 24.Arnold R, Ranchor AV, DeJongste MJ, Koeter GH, Ten Hacken NH, Aalbers R, Sanderman R. The relationship between self-efficacy and self-reported physical functioning in chronic obstructive pulmonary disease and chronic heart failure. Behav Med. 2005;31:107–15. doi: 10.3200/BMED.31.3.107-115. [DOI] [PubMed] [Google Scholar]
- 25.Lorig K, Ritter P, Stewart A, Sobel D, Brown B, Jr, Bandura A, Gonzales V, Laurent D, Holman H. Chronic disease self-management program: 2-year health status and health utilization outcomes. Med Care. 2001;39:1217–23. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Maddigan S, Majumdar S, Guirguis L, Lewanczuk R, Lee T, Toth E, Johnson J. Improvements in patient-reported outcomes associated with an intervention to enhance quality of care for rural patients with type 2 diabetes. Diabetes Care. 2004;27:1306–12. doi: 10.2337/diacare.27.6.1306. [DOI] [PubMed] [Google Scholar]
- 27.Oka RK, DeMarco T, Haskell WL. Effect of treadmill testing and exercise training on self-efficacy in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4:215–9. doi: 10.1016/j.ejcnurse.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Ewart CK, Taylor CB, Reese LB, DeBusk RF. Effects of early postmyocardial infarction exercise testing on self-perception and subsequent physical activity. Am J Cardiol. 1983;51:1076–80. doi: 10.1016/0002-9149(83)90348-x. [DOI] [PubMed] [Google Scholar]
- 29.Berkhuysen MA, Nieuwland W, Buunk BP, Sanderman R, Rispens P. Change in self-efficacy during cardiac rehabilitation and the role of perceived overprotectiveness. Patient Educ Couns. 1999;38:21–32. doi: 10.1016/s0738-3991(98)00115-3. [DOI] [PubMed] [Google Scholar]
- 30.Foster C, Oldridge NB, Dion W, Forsyth G, Grevenow P, Hansen M, Laughlin J, Plichta C, Rabas S, Sharkey RE, Schmidt DH. Time course of recovery during cardiac rehabilitation. J Cardiopulm Rehabil. 1995;15:209–15. doi: 10.1097/00008483-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Gardner JK, McConnell TR, Klinger TA, Herman CP, Hauck CA, Laubach CA., Jr. Quality of life and self-efficacy: gender and diagnoses considerations for management during cardiac rehabilitation. J Cardiopulm Rehabil. 2003;23:299–306. doi: 10.1097/00008483-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Izawa KP, Watanabe S, Omiya K, Hirano Y, Oka K, Osada N, Iijima S. Effect of the self-monitoring approach on exercise maintenance during cardiac rehabilitation: a randomized, controlled trial. Am J Phys Med Rehabil. 2005;84:313–21. doi: 10.1097/01.phm.0000156901.95289.09. [DOI] [PubMed] [Google Scholar]
- 33.Carlson JJ, Norman GJ, Feltz DL, Franklin BA, Johnson JA, Locke SK. Self-efficacy, psychosocial factors, and exercise behavior in traditional versus modified cardiac rehabilitation. J Cardiopulm Rehabil. 2001;21:363–73. doi: 10.1097/00008483-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan MD, LaCroix AZ, Russo J, Katon WJ. Self-efficacy and self-reported functional status in coronary heart disease: a six-month prospective study. Psychosom Med. 1998;60:473–8. doi: 10.1097/00006842-199807000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Ruo B, Rumsfeld JS, Pipkin S, Whooley MA. Relation between depressive symptoms and treadmill exercise capacity in the heart and soul study. Am J Cardiol. 2004;94:96–9. doi: 10.1016/j.amjcard.2004.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 37.Rumsfeld JS. Health status and clinical practice: when will they meet? Circulation. 2002;106:5–7. doi: 10.1161/01.cir.0000020805.31531.48. [DOI] [PubMed] [Google Scholar]
- 38.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–9. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 39.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–4. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 40.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 41.Cunny KA, Perri M., 3rd Single-item vs multiple-item measures of health-related quality of life. Psychol Rep. 1991;69:127–30. doi: 10.2466/pr0.1991.69.1.127. [DOI] [PubMed] [Google Scholar]
- 42.Guyatt G, Feeny D, Patrick D. Issues in quality-of-life measurement in clinical trials. Control Clin Trials. 1991;12:81S–90S. doi: 10.1016/s0197-2456(05)80014-5. [DOI] [PubMed] [Google Scholar]
- 43.Parashar S, Rumsfeld JS, Spertus JA, Reid KJ, Wenger NK, Krumholz HM, Amin A, Weintraub WS, Lichtman J, Dawood N, Vaccarino V. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166:2035–43. doi: 10.1001/archinte.166.18.2035. [DOI] [PubMed] [Google Scholar]
- 44.Rumsfeld JS, Magid DJ, Plomondon ME, Sales AE, Grunwald GK, Every NR, Spertus JA. History of depression, angina, and quality of life after acute coronary syndromes. Am Heart J. 2003;145:493–9. doi: 10.1067/mhj.2003.177. [DOI] [PubMed] [Google Scholar]
- 45.Salamah M, Wahl S, Abriam-Yago K. Life situations of elderly people with heart disease: the impact of self-efficacy on self-care. Internet J Adv Nurs Practice. 2003:5. [Google Scholar]
- 46.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, 3rd, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC., Jr. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction—summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina) J Am Coll Cardiol. 2002;40:1366–74. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 47.Cossette S, Frasure-Smith N, Lesperance F. Clinical implications of a reduction in psychological distress on cardiac prognosis in patients participating in a psychosocial intervention program. Psychosom Med. 2001;63:257–66. doi: 10.1097/00006842-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Miller MD, Schulz R, Paradis C, Houck PR, Mazumdar S, Frank E, Dew MA, Reynolds CF., 3rd Changes in perceived health status of depressed elderly patients treated until remission. Am J Psychiatry. 1996;153:1350–2. doi: 10.1176/ajp.153.10.1350. [DOI] [PubMed] [Google Scholar]
- 49.Fortin M, Bravo G, Hudon C, Lapointe L, Almirall J, Dubois MF, Vanasse A. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15:83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- 50.Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: relationships with global, cancer-related, and life event stress. Psychooncology. 2004;13:211–20. doi: 10.1002/pon.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kornblith AB, Herndon JE, 2nd, Zuckerman E, Viscoli CM, Horwitz RI, Cooper MR, Harris L, Tkaczuk KH, Perry MC, Budman D, Norton LC, Holland J. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer. 2001;91:443–54. doi: 10.1002/1097-0142(20010115)91:2<443::aid-cncr1020>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 52.Shyu YI, Tang WR, Tsai WC, Liang J, Chen MC. Emotional support levels can predict physical functioning and health related quality of life among elderly Taiwanese with hip fractures. Osteoporos Int. 2005:1–6. doi: 10.1007/s00198-005-0020-y. [DOI] [PubMed] [Google Scholar]
- 53.Spertus JA, McDonell M, Woodman CL, Fihn SD. Association between depression and worse disease-specific functional status in outpatients with coronary artery disease. Am Heart J. 2000;140:105–10. doi: 10.1067/mhj.2000.106600. [DOI] [PubMed] [Google Scholar]
- 54.Spitzer RL, Kroenke K, Linzer M, Hahn SR, Williams JB, DeGruy Fr, Brody D, Davies M. Health-related quality of life in primary care patients with mental disorders. Results from the PRIME-MD 1000 study. JAMA. 1995;274:1511–7. [PubMed] [Google Scholar]
- 55.Sullivan MD, LaCroix AZ, Spertus JA, Hecht J. Five-year prospective study of the effects of anxiety and depression in patients with coronary artery disease. Am J Cardiol. 86:1135–8. doi: 10.1016/s0002-9149(00)01174-7. A2000;6:A9. [DOI] [PubMed] [Google Scholar]
- 56.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. patient health questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 57.McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the heart and soul study) Am J Cardiol. 2005;96:1076–81. doi: 10.1016/j.amjcard.2005.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 59.Williams RB, Barefoot JC, Califf RM, Haney TL, Saunders WB, Pryor DB, Hlatky MA, Siegler IC, Mark DB. Prognostic importance of social and economic resources among medically treated patients with angiographically documented coronary artery disease. JAMA. 1992;267:520–4. published erratum appears in JAMA 1992;268:2652. [PubMed] [Google Scholar]
- 60.Connell C, Davis W, Gallant M, Sharpe P. Impact of social support, social cognitive variables, and perceived threat on depression among adults with diabetes. Health Psychol. 1994;13:263–73. doi: 10.1037//0278-6133.13.3.263. [DOI] [PubMed] [Google Scholar]
- 61.Alonso J, Permanyer-Miralda G, Cascant P, Brotons C, Prieto L, Soler-Soler J. Measuring functional status of chronic coronary patients. Reliability, validity and responsiveness to clinical change of the reduced version of the Duke activity status index (DASI) Eur Heart J. 1997;18:414–9. doi: 10.1093/oxfordjournals.eurheartj.a015260. [DOI] [PubMed] [Google Scholar]
- 62.Dwosh IL, Giles AR, Ford PM, Pater JL, Anastassiades TP. Plasmapheresis therapy in rheumatoid arthritis. A controlled, double-blind, crossover trial. N Engl J Med. 1983;308:1124–9. doi: 10.1056/NEJM198305123081903. [DOI] [PubMed] [Google Scholar]
- 63.Frimodt-Moller PC, Jensen KM, Iversen P, Madsen PO, Bruskewitz RC. Analysis of presenting symptoms in prostatism. J Urol. 1984;132:272–6. doi: 10.1016/s0022-5347(17)49587-5. [DOI] [PubMed] [Google Scholar]
- 64.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–8. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 65.Nerenz DR, Repasky DP, Whitehouse FW, Kahkonen DM. Ongoing assessment of health status in patients with diabetes mellitus. Med Care. 1992;30:MS112–24. doi: 10.1097/00005650-199205001-00010. [DOI] [PubMed] [Google Scholar]
- 66.Fu D, Fu H, McGowan P, Shen YE, Zhu L, Yang H, Mao J, Zhu S, Ding Y, Wei Z. Implementation and quantitative evaluation of chronic disease self-management programme in Shanghai, China: randomized controlled trial. Bull World Health Organ. 2003;81:174–82. [PMC free article] [PubMed] [Google Scholar]
- 67.Lorig KR, Ritter PL, Jacquez A. Outcomes of border health Spanish/English chronic disease self-management programs. Diabetes Educ. 2005;31:401–9. doi: 10.1177/0145721705276574. [DOI] [PubMed] [Google Scholar]
- 68.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4:256–62. [PubMed] [Google Scholar]
- 69.Lorig K, Holman H, Sobel DS, Laurent D, Minor M, Gonzales V. Living a healthy life with chronic conditions. Bull Publishing Company; Palo Alto: 1993. [Google Scholar]
- 70.McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med. 2001;110:378–84. doi: 10.1016/s0002-9343(00)00743-9. [DOI] [PubMed] [Google Scholar]