Abstract
The goal of this study was to quantify the microbial load (enterococci) contributed by the different animals that frequent a beach site. The highest enterococci concentrations were observed in dog feces with average levels of 7.4 × 106 CFU/g; the next highest enterococci levels were observed in birds averaging 3.3 × 105 CFU/g. The lowest measured levels of enterococci were observed in material collected from shrimp fecal mounds (2.0 CFU/g). A comparison of the microbial loads showed that 1 dog fecal event was equivalent to 6,940 bird fecal events or 3.2 × 108 shrimp fecal mounds. Comparing animal contributions to previously published numbers for human bather shedding indicates that one adult human swimmer contributes approximately the same microbial load as one bird fecal event. Given the abundance of animals observed on the beach, this study suggests that dogs are the largest contributing animal source of enterococci to the beach site.
Keywords: enterococci, animal feces, enumeration, recreational water, bacteria indicators, non-point pollution sources
Introduction
Marine beach advisories are based upon measurements of enterococci in the water column (U.S. EPA 1986) and, in many cases, the cause of elevated levels of this fecal indicator bacteria is unknown, especially for beaches characterized by non-point sources of pollution. The most obvious ultimate source of fecal indicator bacteria to a beach site are humans (Hanes and Fossa, 1970; Smith and Dufour, 1993; Gerba 2000) and animals including wildlife (Dunlap and Thies 2002; Harwood et al. 1999), shorebirds (Graczyk et al. 1998; Jones et al. 1978; Lévesque et al. 1993, 2000; Fujioka et al. 1988; Davies et al. 1995; Hatch 1996; Alderisio and DeLuca 1999; Jones and Obiri-Danso 1999; Obiri-Danso and Jones 2000), and domestic animals (Calci et al. 1998; Cox et al. 2005; Kühn et al. 2003; Meals and Braun 2006). Recent studies have indicated that bird feces may be a primary cause for elevated fecal indicator levels, specifically within some recreational waters (Boehm et al. 2005; Haack et al. 2003; Wither et al. 2005; Edge and Hill 2007) as well as beach sands which influence recreational waters (Bonilla et al. 2007). In addition to shorebirds, dog feces have been identified in very few studies as a potential significant contributor to fecal indicator contamination. For example, Martin and Gruber (2005) found that decomposing marine vegetation containing remnants of dog feces along the wrack or seaweed line were shown to have elevated enterococci concentrations (5 × 105 MPN/g) in comparison to marine vegetation without fecal waste (5 × 104 MPN/g). In addition, shrimp within the sea bed and their fecal pellets (Ziebis et al. 1996; Manning and Kumpf 1959) represent an as yet-unexamined source of fecal indicator microbes to the water column.
Although several studies have measured the concentration of fecal indicator bacteria in the feces of animals, few provide enough information for establishing a mass balance approach for evaluating the relative contributions of different non-point sources to a recreational water body. A mass balance approach requires establishing a microbial load per human/animal source in conjunction with enumerating each of these contributing sources. The product of the two provides the total load from each source. Studies which measure animal loads using such an approach are very limited in that the number of animals is difficult to quantify and typically requires the use of cameras to document variations in numbers in time and space. Furthermore, when establishing the load for a particular animal, most studies focus on measuring the microbe concentration of feces and in many cases the mass of the feces, which is necessary for a mass balance analysis, is not included. Although one study is available that utilized a completed mass balance approach to establish that birds are a significant source of fecal indicator bacteria (Grant et al. 2001), few, if any, have evaluated dogs and none have evaluated the significance of aquatic organisms such as shrimp. The few studies which provide a fecal load per dog (U.S. EPA 2001) typically provide the load for another fecal indicator such as fecal coliform instead of enterococci which is the primary indicator microbe for monitoring recreational marine waters in the U.S.
The objective of the current study was to evaluate the direct enterococci inputs from animals at a recreational marine beach using a mass balance approach. For comparative purposes, the inputs from humans that frequent the beach were also estimated. The animal loads provided in this paper (which includes the enumeration of enterococci concentrations plus mass of feces) are intended to add to the extensive literature focusing on bird contributions and the limited literature which describes contributions from dogs and shrimp. These animal loading rates can be coupled with counts at other beach sites to establish similar mass balance comparisons between different sources at other non-point source beaches. Furthermore human and animal count information, in particular the relative distribution between humans and animals and the spatial distribution of animals between land and water may be relevant to other studies, as such information is lacking within the literature.
Materials and Methods
Site Description
The study recreational beach is located within a subtropical climate characterized by an average ambient temperature of 24.8°C (27.6°C during the summer months and 22.0°C during the winter) and annual average rainfall of 149 cm (total of 109 cm during the wet season (May through September) months and 39 cm during the dry season (October through April). The site is located on Virginia Key, a small island on the eastern edge of Biscayne Bay that is immediately east from the coast of Miami, Florida, U.S.A. (Fig. 1). This site was chosen because 30 swimming advisory/warnings have been issued for the recreational bathing beach since the inception of the Florida Healthy Beaches Program in August 2000 (Fl. Dept. of Health, 2007), and previous research had shown elevated levels of enterococci (Shibata et al. 2004, Durbin et al. 2005) which have been attributed to non-point sources. The study site is also the only beach within Miami-Dade County where people are allowed to bring their pets including dogs. Dog owners are not required to clean up their dog waste, even though the beach is a designated recreational swimming area. In addition to dogs and humans, birds had been observed to gather near the shoreline in particular during the early morning hours, and shrimp fecal mounds were readily observable in submerged beach sediments throughout the study site.
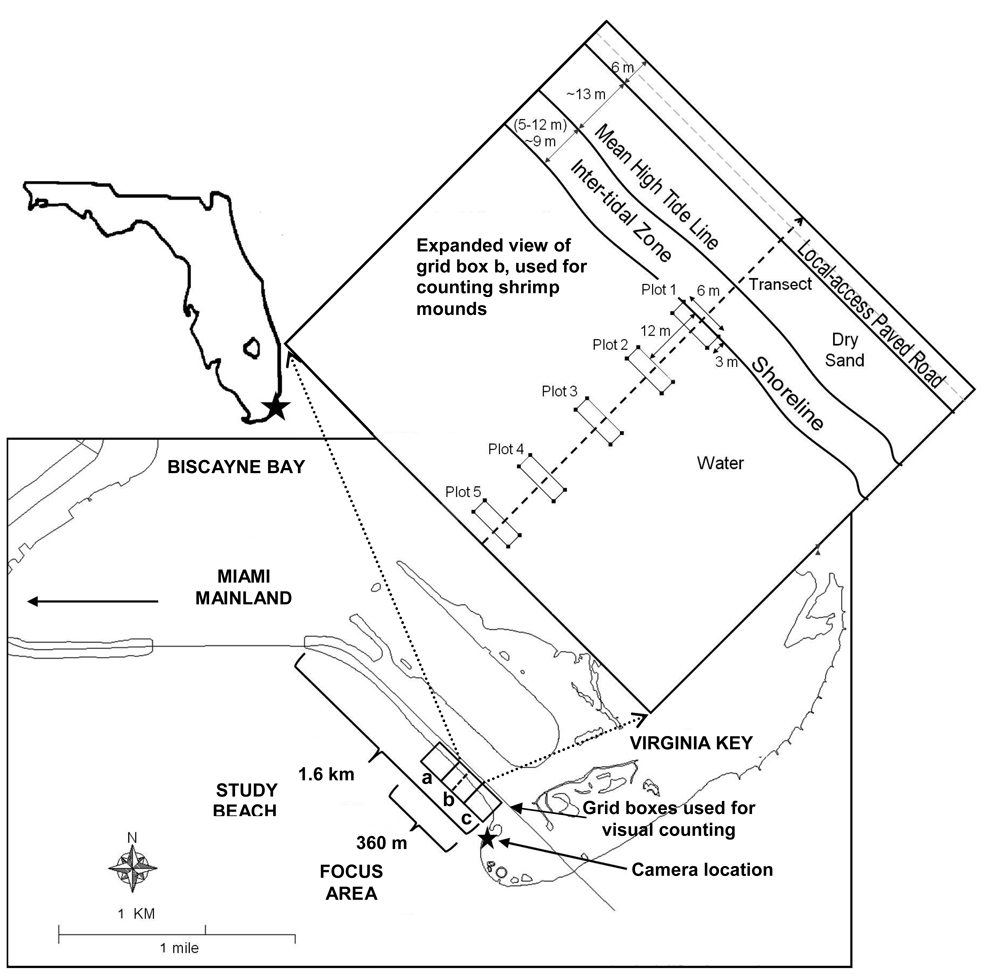
Figure 1.
The study beach was located within Biscayne Bay on Virginia Key, east of Miami, Fl. The focus area is identified as the eastern most 360 m. Bottom inset shows location of camera. Boxes within the bottom inset show grid boxes used for in-field visual counting surveys for dogs and humans. Top inset shows transect and plots used for the in-field visual counting surveys for shrimp.
The study beach is approximately 1.6 km long; the focus area of this study was the eastern 360 m of the beach that faces towards the southwest, on the bay side of the island. The width of the beach was defined by a local-access paved road that runs parallel to the beach at a distance of approximately 13 meters from mean high tide. One bathroom facility was located at the northwestern end of the beach. There were no storm drains at the site and runoff from the paved road flowed directly to the beach. The beach was characterized by relatively weak water circulation because the bottom slope is small and headland features are found at both ends. Movement of water near the shoreline was dominated by tidal action. The average fluctuation in tidal height at the study site was 58 cm. This tidal height resulted in a 5 to 12 meter horizontal translation of the instantaneous shoreline between high and low tide given the relatively shallow slope (approximately 0.06m/m) within this area of the beach. The sand area that received periodic wetting and drying during the tidal cycles is herein referred to as the “inter-tidal zone.”
The opposite side of Virginia Key from the study beach is also home to a wastewater treatment plant. The outfall from this plant is located approximately 3.25 km further out in the Atlantic Ocean. Observations show, however, that remote sources, such as this outfall do not impact enterococci levels at the study site; prior studies have consistently shown a decreasing gradient offshore from the site with mostly below detection levels of enterococci in chest deep waters, whereas the shallow water near the shoreline was characterized by higher levels (Shibata et al. 2004). Thus, the major source of enterococci to this site comes from the inter-tidal zone (or swash zone). Of note, sand at this site has been shown to have high levels of enterococci within the inter-tidal zone (39 ± 20 CFU/g dry sand) and within the “dry zone” (380 ± 200 CFU/g dry sand) located 1 m above the inter-tidal zone (Durbin et al. 2005). The cause of the elevated indicator levels in the sand is not yet fully understood. Suspected sources to the sand as previously mentioned include direct and indirect animal and human inputs, and potential survival and regrowth of enterococci along the shoreline in the sand.
Sample Collection
Feces collection occurred over a period of 8 months between June 2005 and January 2006. Specifically, fecal samples from dogs, birds, and shrimp were analyzed for enterococci concentration and were weighed for total fecal mass; the product of the two provided the total enterococci load per animal fecal event. During collection, all samples were aseptically transferred into pre-weighed Whirl-Pak® sampling bags or pre-sterilized tin containers using scoops or spoons. Sample collection occurred carefully to minimize the inclusion of non-fecal matter (such as sand, twigs and rocks). All samples were transported from their respective collection sites to the laboratory in an iced cooler and analyzed within 4 hours.
Prior to the enterococci enumeration, the total mass was determined for each sample, where the weight of the container was subtracted from the total mass of the container plus feces. After weighing, an aliquot of the fecal sample was used for enterococci enumeration, and a separate aliquot was used for water content analysis to allow reporting of enterococci numbers in units of colony forming units (CFU) per gram of dry feces.
Dog feces were collected by asking a dog owner:1) if a fecal event occurred; 2) the time it occurred; and 3) the location of the event or by following the dog until a fecal event occurred. In some cases, the researchers also observed the defecation. Once the fecal event was identified, the sample was then collected. Dogs were identified as either large (> 9 kg) or small (< 9 kg). Dog owners were asked if the dog had any medical condition or was currently taking any medications. (No participant dogs were known to have medical conditions, and none were taking medications.) Information about the dog’s age, weight and breed were also documented. A total of 9 samples were collected from the study site. In addition to the above mentioned samples, the total mass of a dog fecal event was measured for two dogs, one large (23 kg) and one small (3.2 kg), each of which were monitored for a week. During this week, each dog fecal event was collected, and then weighed to obtain the total mass of feces produced per day by each dog.
Bird feces were collected from the beach, a zoo, and a native bird rehabilitation center within Miami-Dade County. The sample collected at the beach was obtained by watching birds early in the morning at sunrise, and waiting for a fecal event to occur. At the beach, only one sample from an Ibis (Eudocimus albus, E. ruber, Plegadis falcinellus, or P. chihi) was collected after several attempts of sample collection over a span of two weeks. Due to this difficulty, sampling efforts were expanded to include native birds from a local zoo and a local native bird rehabilitation center, both of which were fed a wild diet. The native bird exhibit at the local zoo consisted of an artificial lake that included a small island located in the center of the lake and a concrete border that surrounded the lake. Samples were collected from a plastic tarp that was placed under a tree on the island. In the afternoon once the zoo closed to the public, the plastic tarp was sanitized, and the concrete border was repeatedly scrubbed and washed. The following morning six feces samples from birds, Ibis and Heron (Ardea herodias, Butorides striatus, Egretta caerulea, Egretta tricolor, Nycticorax nycticorax and N. violaceus), known to congregate overnight in the tree on the island were collected from the tarp. Seven fecal samples were collected from the concrete border corresponding to Ibis, a Heron, Duck (unidentified species), and Coot (Fulica americana). For the bird rehabilitation center, fecal samples were collected from: a) cages located indoors which housed Pelicans (Pelicanus occidentalis and carolinensis) (4 samples, n=4); b) a concrete pad located outdoors where Pelicans congregate (n=2); and c) dock facility where Gulls (Larus atricilla and delawarensis) and Pigeons (Columba leucocephala) frequent (n=6). Indoor cages were sanitized and lined with newspaper and a plastic mat prior to housing the birds. The concrete pad was washed with water prior to sample collection; the dock had no prior cleaning. A total of 26 bird fecal samples were collected and analyzed from the 3 study sites.
Ghost shrimp fecal pellets (sand mounds which contained shrimp (Callianassa californiensis; Murphy and Kremer 1992) were collected by scooping the sand directly into a Whirl-Pak® bag underwater to minimize the loss of fine sediments. Preliminary studies showed no statistical difference when only a portion of the mound (with fecal pellets) or entire mound was collected, resulting in the entire mound being used for analysis. Nine mounds were collected from the study site out to a distance of 50 m within the inter-tidal zone where colonies of shrimp live on the ocean floor. Mounds were easily identified above water due to the shallow and clear water (3.6 Nephelometric Turbidity Units, NTU).
Laboratory Methods
Enterococci were extracted from the fecal samples using a modified version of the procedure outlined by Van Elsas and Smalla (1997). The method required two basic steps. The first step was to measure the water content of the feces. Water content was determined by measuring the weight difference of feces before and after drying (110°C for 24 h). The second step required the extraction of enterococci from the feces to a predefined volume of sterile water. In order to accomplish this, approximately 2.0, 2.6 and 4.6 g for dogs, birds, and shrimp, respectively, of fresh un-dried feces (wet fecal matter) were aseptically removed from the sampling bags and placed into sterile pre-weighed jars. Approximately 30 to 50 ml of sterile phosphate buffer dilution water (PBS) was then added to each jar. The jars were manually shaken for 30 seconds, and then placed in a sterile graduated cylinder and raised up to a volume of 100 ml with PBS. This solution was then analyzed for enterococci concentration using a standardized membrane filtration (MF) method (U.S. EPA 2002, Method 1600). In brief, the MF method was based upon a selective medium (mEI agar, Becton Dickinson, Sparks, MD) and incubation of filters at 41°C for 24 hours. All colonies that were blue or characterized by a blue halo were recorded as “enterococci colonies.”
Animal Enumeration
Two methods were used to enumerate the instantaneous number of animals (humans, dogs, birds, and shrimp mounds) at any given time during daylight hours: camera image analysis, and in-field visual counting surveys. The camera image analysis was based upon the use of a digital camera (C-8080WZ, Olympus) with pan, tilt and zoom capabilities. This camera was placed in an environmental housing and mounted near the top of a pole, approximately 3 m from the ground. The pole was located across the bay on private property, approximately 440 m from the study site (Fig. 1). The camera took 3 images of the beach area every 15 minutes. The 3 images were subsequently assembled into one panorama for disk storage. Each panorama corresponded to the eastern 360 m of the 1.6 km total beach length and the numbers from the panorama were thus multiplied by 4.4 to obtain total numbers for the entire beach. The numbers obtained from these images were representative of the entire beach as the distribution of people and animals is relatively uniform because of the long narrow geometry. Fifty images were analyzed over a period of 16 months (beginning April 2005 and ending July 2006), with the addition of 16 images analyzed during Labor Day weekend due to increased human volume at the study site (September 2005). The study camera database contained the following animal counts: people within the backshore zone of the beach, people in the water, small and large dogs in the backshore zone and in the water, and birds (Crows, Ibis, Gulls, Anhingas, Pelicans, Herons and Vultures).
Two general approaches were used for in-field visual counting surveys. The first method was used to count humans and dogs, and the second method was used to count shrimp mounds. Humans and dogs were enumerated at one instant during the day using a grid consisting of 3 boxes within the study site (Fig. 1). Humans and dogs within each box were counted to find the total number of people both in the water and on the beach. The numbers from each of the three boxes were added together giving the total within the 360 m long focus area of the study. These numbers were then scaled to obtained the number for the entire 1.6 km length of beach. Humans and dogs were counted from images collected during 13 days from June 2004 and May 2005. All days were non-holiday weekday counts, except for Memorial Day (Monday) 2005. The second in-field visual counting survey method, used for counting shrimp fecal mounds, was based upon establishing a set of five 6 m by 3 m plots which extended out from the shoreline within grid box “b” (Fig. 1). Shrimp mounds were then counted during high tide and during low tide within each plot during two consecutive days (two high tide counts and two low tide counts during the 2-day period). The entire procedure was conducted twice, once during the summer (August 2 and 3, 2006) and once during the winter (December 21 and 22, 2006).
In order to compare contributions between non-point sources, the assumption was made that the presence of an animal represented a fecal event, meaning one dog had one fecal event while at the beach and one bird had one fecal event, one shrimp mound equals one fecal event. Humans were assumed to contribute enterococci three times per day from skin shedding directly into the water while bathing according to the values estimated by Elmir et al. (2007) who conducted a human shedding study at this same study site.
Results
Enteroccoci
Highest enterococci concentrations on average were found in dog feces (3.9 × 107 CFU/g dry feces), then bird feces (3.3 × 105 CFU/g), with the lowest counts in shrimp fecal mounds (2.0 CFU/g). All averages included only those samples that did not exceed the detection limit. The microbial load from dog feces, bird feces, and shrimp fecal mounds were measured at 3.2 × 109 CFU per dog event, 4.7 × 105 CFU per bird fecal event, and 10 CFU per shrimp fecal mound. These results indicated that one dog fecal event had the same enterococci loading as 6,940 bird fecal events and 3.2 × 108 shrimp fecal mounds.
Of the nine dog fecal samples collected, two were above the detection limit. The range for the samples that were within detection limits was from 5.7 × 104 to 2.4 × 108 CFU/g dry feces. The median concentration (which included the samples that were above detection limits) was 1.0 × 107 CFU/g feces (Table 2). The two samples that measured above the detection limit were observed to contain greater than 4.9 × 107 CFU/g feces. The average enterococci concentration for large dogs was 6.4 × 107 CFU/g feces (n=4), while the average for small dogs was 5.9 × 106 CFU/g feces (n=3). The mass of dog feces in terms of dry weight per event was 79 g for large dogs and 28 g for small dogs. The microbial load was computed at 5.6 × 109 CFU/event for large dogs and 1.5 × 108 CFU/event for small dogs, on average. For large and small dogs evaluated in this study, the computed microbial load was 3.3 × 109 CFU/event overall. The results from the collection and weighing of dog feces over a period of a week showed fecal masses within the same order of magnitude. The total mass (grams of dry feces) produced per day from a large dog was 52 g, while the small dog produced 7.6 g daily.
Table 2.
Dry masses, enterococci concentrations, and enterococci loads from dog feces evaluated in this study
| Description of Dog | Weight of Dog (kg) |
Weight of Feces (g) |
Enterococci Concentration (CFU/g dry feces) |
Enterococci Load per fecal event (CFU) |
|---|---|---|---|---|
| Boxer | 16 | 32.3 | >2.8 × 108 | >8.8 × 109 |
| Boxer | 27 | 69.1 | >4.9 × 107 | >3.4 × 109 |
| Labrador Retriever | 38 | 88.5 | 2.5 × 108 | 2.2 × 1010 |
| Arabian Kanubau Mix | 29 | 53.6 | 1.0 × 107 | 5.5 × 108 |
| Chesapeake Bay | >9 | 130 | 2.0 × 105 | 2.7 × 107 |
| Labrador Retreiver | 27 | 45.1 | 5.7 × 104 | 2.6 × 106 |
| Small Dog | <9 | 49.7 | 7.5 × 106 | 3.7 ×108 |
| Small Dog | <9 | 6.6 | 1.0 × 107 | 6.5 × 107 |
| Small Dog | <9 | 26.8 | 1.1 × 105 | 3.0 × 106 |
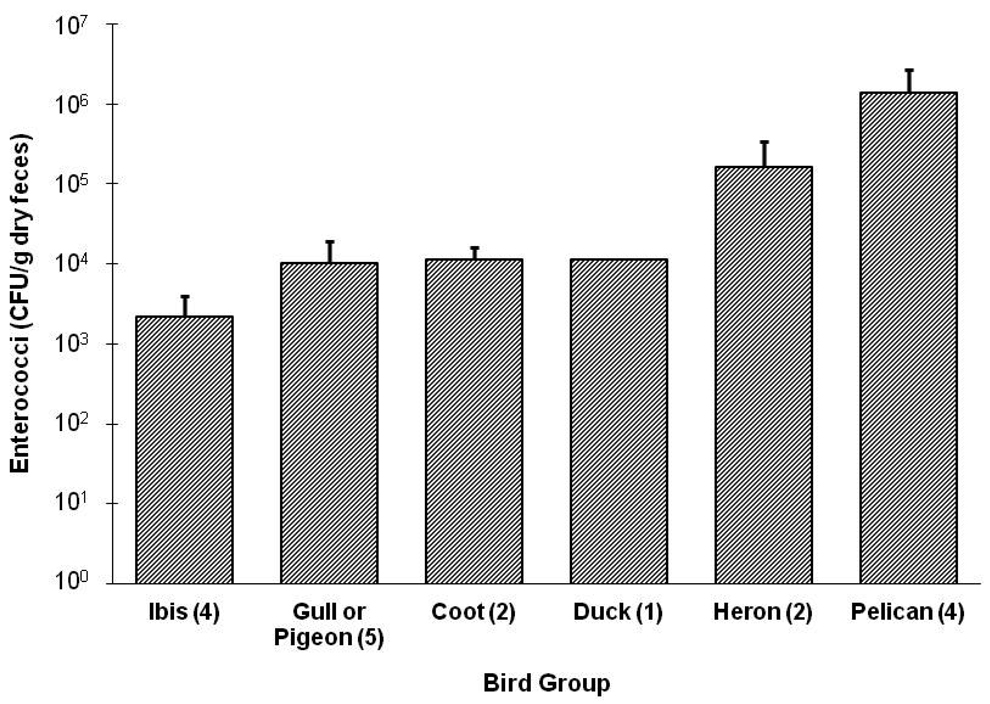
Bird feces showed variable concentrations of enterococci ranging from 347 to 3.1 × 106 CFU/g dry feces, with a median value of 2.0 × 104 CFU/g feces for the entire 26 sample data set. Of the 26 samples, eight were above the detection limit, which resulted in a total sample size of 18 within detection limits. Enterococci concentrations by bird group ranked from lowest to highest included: Ibis, Gull or Pigeon, Coot, Duck, Heron, and Pelicans, respectively (Fig. 2). The combined average concentration for Ibis, Coot, Duck, Gull and Pigeons was 8.0 × 103 CFU/g feces; the combined average for Heron and Pelicans was 9.7 × 105 CFU/g feces. The average dry mass of a bird fecal event was 0.82 g, with the smallest mass of 0.027 g for a Gull or Pigeon and the largest for a pelican (1.7 g). The smallest contribution in terms of microbial load was from a Gull or Pigeon (53 CFU/event), and the largest contribution was calculated from Pelicans (5.1 × 106 CFU/event). The average microbial load from all types of birds was 4.7 × 105 CFU/event.
Figure 2.
Enterococci concentrations (per gram of dry feces) from feces from different types of birds and the respective sample size in parenthesis. Error bars represent the standard deviation for the respective type of bird within that sample size.
Shrimp fecal mound concentrations ranged from 0.65 to 7.7 CFU/g, with a median value of 0.95 CFU/g. The shrimp mounds contained fecal pellets mixed with sand. Durbin et al. (2005) measured the enterococci levels of sand within the inter-tidal zone sand at the study site as 39 ± 20 CFU/g dry sand, thus the material used by the shrimp in developing their mounds appeared to contain less enterococci than the surface sediment within the inter-tidal zone. The average mass of shrimp mound samples was 6.9 g, resulting in an average microbial load of 10 CFU per mound.
Animal Enumeration
Human enumeration results from both camera image analysis and in-field counting surveys (instantaneous values at the time of counting) demonstrated that the highest human count during weekdays was 276 (avg. 55 ± 19, 95% confidence limit) and during a non-holiday weekend day was 351. The largest number of people was observed during holiday weekends (2,640 people maximum at one time, with an average of 1,330). The maximum population of 2,640 people corresponded to a density of 1 person per 8 square meter of beach. The overall average number of people during weekends on the beach (considering both non-holiday and holiday weekends) was 1090. During the weekends, more people were observed along the shore out of the water (695 on average) compared to in the water (392 on average). For data collected during weekdays, the distribution between people who were in the water versus on dry land was available only from the camera image analysis. Camera images indicated that the average number of people at the beach during weekdays was 46 people, with 33 people on the shore and 13 people in the water.
Of the 65 days of data (from both camera image analysis and field sampling days), 36 days corresponded to no dogs. For days when dogs were observed, the overall average number of dogs during weekdays was found to be 12 dogs, with the highest number at 89 dogs. During the one non-holiday weekend, 13 dogs were observed at the beach at any given time; during holiday weekends, the maximum number observed was 196, with an average of 83 dogs. For weekends as a whole (including non-holiday and holidays), 66 dogs were observed on average.
Dog counts were separated by size for camera image analysis only. From the camera images, large dogs were observed to represent the majority of the population, with their proportion varying from 60% to 90%. The location of dogs was documented from the visual counting survey conducted during a holiday weekend. This survey showed roughly 57% of the dogs on shore, with the remaining dogs in the water.
Thirty-three of the 53 days of camera images evaluated contained countable birds. No counts were taken visually in-field. The average number of birds (combined species) documented per camera image over the 53 days was 150, with a maximum number of 587 birds. The number of birds (when present) was typically in the 1 to 50 range. An additional peak of bird frequency was observed within the range of 301 to 350, suggesting that birds frequented the site predominantly as individuals and in flocks of over 300. Gulls were observed most frequently (31 days), and were characterized by the highest counts (maximum of 587); the average count was 151 per day during these 31 days. Pelicans and Anhinga (Anhinga anhinga) were seen four days, and averaged 22 and 4 per day, while Vultures were observed three days and averaged 12 per day when present. Herons were observed two days, and had an average count of 13. The bird species observed the least were Crows and Ibis which were documented only one time each, with counts of 89 and 9, respectively.
Shrimp fecal mounds, as estimated from in-field counting surveys, showed an overall average of 984 mounds for the entire 1.6 km of beach, with a median value of 919. The overall maximum count occurred during high tide (1,837 mounds). The average number of mounds observed during high tide was 1,180, while during low tide the average was 787 mounds. The maximum number of mounds observed during low tide was 1,310.
Discussion
The highest numbers observed during the enumeration process for all animals (including humans) was combined with the mean microbial load to provide the potential contribution of enterococci to the beach from non-point sources evaluated as part of this study (Table 1). This evaluation estimates that during the weekend the enterococci contribution to the beach could be as high as 6.4 × 1011 CFU. Calculating an average (average enumeration numbers multiplied by the mean microbial load) for the weekend resulted in a mean weekend contribution per image of 2.1 × 1011 CFU. The highest weekday enterococci contribution (2.9 × 1011 CFU per image) and average weekday contribution (3.6 × 1010 CFU per image) were between a factor of 3 to 10 times lower than the corresponding weekend values.
Table 1.
Results for the enterococci load per event, animal enumeration, and total contribution for people, dogs, birds, and shrimp mounds given the instantaneous enumeration results. Maximum contributions per image correspond to the product of the average value of the enterococci contribution per event and the maximum numbers of animals or humans observed.
| Source | Enterococci | Enumeration | Enterococci Load (CFU per image) | |||
|---|---|---|---|---|---|---|
| Type | Daya | (CFU/event) | Maximum | Average | Maximum | Average |
| W | 2,644 | 1,088 | 4.6 × 109 | 1.9 × 109 | ||
| People | D | 1.7 × 106, b | 276 | 55 | 4.8 × 108 | 9.6 × 107 |
| A | 2,644 | 129 | 2.5 × 109 | 9.9 × 108 | ||
| W | 196 | 66 | 6.3 × 1011 | 2.1 × 1011 | ||
| Dogs | D | 3.2 × 109 | 89 | 11 | 2.9 × 1011 | 3.6 × 1010 |
| A | 196 | 19 | 4.6 × 1011 | 1.2 × 1011 | ||
| Birds | A | 4.7 × 105 | 587 | 150 | 2.7 × 108 | 7.0 × 107 |
| Shrimp | D | 1.0 × 101 | 1,837 | 984 | 1.9 × 104 | 1.0 × 104 |
W = Overall weekend (non-holiday weekend and holiday weekends), D = Weekday daylight hours, A= All 7 days of the week
Refers to one person swimming 3 times during a beach visit
When comparing the amount of enterococci shed from humans via swimming to enterococci concentrations in animal feces, the results (CFU per event) indicated that one dog feces equated to 1,872 people, 6,940 bird feces, and 3.2 × 108 shrimp fecal mounds. The enterococci contribution from dog feces far exceeded the contribution from human shedding or from bird feces. This observation was especially significant as people, birds, and shrimp outnumbered the quantity of dogs. The enterococci contribution from dog feces was most significant because of the high concentration of enterococci in the feces coupled with the large mass, resulting in an exceedingly large contribution per dog event.
The microbial load for dog feces could be highly variable. The literature is very limited with respect to documenting the enterococci concentration associated with dog feces. Although data were available with respect to total bacterial and fecal coliform levels (Calci et al. 1998) and data existed concerning the characterization of enterococci isolates (Rodriques et al. 2002; De Leener et al. 2005; De Graef et al. 2005; Delgado et al. 2007), only one such study had measured total enterococci counts for one dog fecal sample (1.13 × 104 CFU/g feces in wet weight; Anderson et al. 1997). Using the moisture contents measured in the current study, the 1.13 × 104 CFU/g in wet weight is estimated at 2.3 × 104 CFU/g feces in units of dry weight. The current study found that enterococci concentrations from dogs can be variable (5.7 × 104 to 2.4 × 108 CFU/g dry feces) and high in comparison to the value reported by Anderson et al. (1997). Of note, Anderson et al. (1997) observed up to 5 orders of magnitude difference in the enterococci concentrations of other animal species and the range in enterococci concentration observed in the current study among dogs is consistent with the variability observed by Anderson et al. (1997) in other animals.
In the current study, the mass of dog feces used to estimate the total daily load corresponded to fecal masses that were collected and analyzed at the study site. This was an accurate assessment in terms of the contribution of enterococci from those dogs present during that sampling event. However, the mass of dog feces was subjective and directly corresponded to the size of the dog and the dog’s food consumption. The literature suggests that a large-sized dog (weights ranging from 19 to 32 kg) produces approximately 40 g per day of dry feces (range of 32 to 49 g) (Spears et al. 2004, Murray et al. 1997, NRC 1985). The dog feces weights measured over the course of a week in this study (52 g for a large dog and 7.6 g for a small dog) was consistent with the published literature but the variation in masses can add another order of magnitude in the variability of the enterococci contribution from dogs.
Enterococci concentrations in bird fecal samples from the current study (1.2 × 104 CFU/g dry feces for ducks and 1.0 × 104 CFU/g dry feces for gulls or pigeon) were low when compared to the literature. For ducks, Roll and Fujioka (1997) reported 1.4 × 106 CFU/g dry feces and Anderson et al. (1997) reported 3.5 × 104 to 1.7 × 107 CFU/g equivalent dry weight; thus indicating that the concentration observed in duck feces within the current study was in the low range of that observed in other studies. For pigeons, Oshiro and Fujioka (1995) found 4.0 × 105 CFU/g (unspecified whether dry or wet weight) feces and Anderson et al. 1997 found 4.5 × 106 CFU/g equivalent dry fecal weight, while Haack et al. (2003) found enterococci in gull and duck feces, on average, at 5 × 107 CFU/g wet feces. These values converted to enterococci per gram dry feces using the water contents measured in this study resulted in much higher concentrations by four orders of magnitude (1.2 × 108 CFU/g dry feces). Most studies found wide ranges in enterococci levels in bird fecal samples. Fogarty et al. (2003) found that gull feces from the Great Lakes region contain between 104 to 108 CFU/g dry feces and Haack et al. (2003), for all birds, found a range of 1.2 × 102 to 3.2 × 1010 CFU/g equivalent dry feces, the maximum values being much higher than concentrations reported in previous research and in the current study. The enterococci concentrations on average (3.3 × 105 CFU/g dry feces) observed in bird feces from this study appear to be in the lower range and contribute somewhat to the decreased significance of bird fecal contributions to this particular study site.
The overall mass of bird feces observed in this study ranged from 0.027 to 1.6 g, dry weight. Kushlan (1977, 1979) suggested that an overall daily average mass of fecal matter on dry basis for an Ibis was approximately 10 g. The current study measured 1.7 g for an Ibis for one event suggesting that an Ibis has on the order of 5 to 6 fecal events per day. Bedard et al. (1980) found that a housed ring-billed gull produced 8.3 g of fecal matter and Gould and Fletcher (1978) found that the average amount of feces excreted by a gull per day ranged from 11.2 to 24.9 g. The current study documented 0.11 g per fecal event for gulls, which suggested many fecal events per day for gulls. The assumption made (i.e. one observed bird equates to one fecal event) is potentially incorrect. It is possible that birds may deposit more than one fecal event per visit. However, given the difficulty in finding bird fecal samples on the beach, the assumption of one event per bird was assumed to be adequate for the purposes of this study.
Conclusion
Shrimp fecal mounds were found to be negligible, but dog contributions indicated an overall concern. The results (combined weekdays and weekends) showed that the average contribution from dogs was 1.2 × 1011 CFU per image. Enterococci load from humans and birds were smaller at 9.9 × 108 and 7.0 × 107 CFU for the images evaluated. The load of enterococci from shrimp fecal mounds was even lower at 1.9 × 104 CFU (avg. 1.0 × 104). Given these values, dogs are believed to contribute the bulk of the total enterococci load, whereas humans, birds, and shrimp contributed a much smaller amount.
Results from this study provide evidence that dog feces represent the largest animal source to the study site. Improved management of dog feces at the beach could potentially reduce enterococci inputs to the beach, thereby decreasing the number of advisories for beach sites which are frequented by significant numbers of dogs In order to better estimate the relative contributions of enterococci from animals and humans to the site, a greater number of camera images should be analyzed so that daily, weekly and seasonal variations in populations can be established. Once these variations are established, the results from the current study (CFU/animal) should be integrated over time to estimate the enterococci contribution per day or per year from each animal/human source. With such estimates, the relative contribution of dogs relative to other sources would be more accurate as it takes into consideration the amount of time each animal spends at the beach. Furthermore, the development of a beach management plan would also benefit from the evaluation of additional indirect sources of enterococci, such as enumerating the contribution from rainwater runoff and potential regrowth of enterococci within beach sands. The relative contribution from these in-situ sources should be compared with direct animal contributions as such information would be useful for establishing policies for improving water quality.
Acknowledgments
This work was funded by NSF-NIEHS Oceans and Human Health Program (NSF OCE0432368 and NIEHS P50 ES12736-01) and the NSF-REU Program (OCE 0432368). We would like to thank all the students, scientists and professionals, including the individuals from the bird rehabilitation center, Miami Metro Zoo, and Miami Seaquarium who participated in the logistics and sample collection for this project. Environmental Specialists, Gary Miller and Sudhir Oberoi, from the Miami-Dade County Health Department assisted considerably. We thank John D. Wang for insightful comments on the manuscript and we would like to thank Amir Abdelzaher, Cristina Ortega, Colleen Block, Kelly Jackson, Eduardo Lam, Karina Vega, Jonathan Fernandes, Adrianne Vargas, and Freddy Nava for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderisio KA, DeLuca N. Seasonal enumeration of fecal coliform bacteria from the feces of ring-billed gulls (Larus delawarensis) and Canada geese (Branta canadensis) Applied and Environmental Microbiology. 1999;65(12):5628–5630. doi: 10.1128/aem.65.12.5628-5630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Turner SJ, Lewis GD. Enterococci in the New Zealand environment: implications for water quality monitoring. Water Science and Technology. 1997;35(11–12):325–331. [Google Scholar]
- Bedard J, Therriault JC, Berube J. Assessment of the importance of nutrient recycling by searbirds in the St. Lawrence estuary. Canadian Journal of Fisheries and Aquatic Sciences. 1980;37:583–588. [Google Scholar]
- Boehm AB, Keymer DP, Shellenbarger GG. An analytical model of enterococci inactivation, grazing, and transport in the surf zone of a marine beach. Water Research. 2005;39(2005):3565–3578. doi: 10.1016/j.watres.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Bonilla TD, Nowosielski K, Cuvelier M, Hartz A, Green M, Esiobu N, McCorquodale DS, Fleisher JM, Rogerson A. Prevalence and distribution of fecal indicator organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Marine Pollution Bulletin. 2007;54:1472–1482. doi: 10.1016/j.marpolbul.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Calci KR, Burkhardt W, III, Watkins WD, Rippey SR. Occurrence of male-specific bacteriophage in feral and domestic animal wastes, human feces, and human-associated wastewaters. Applied and Environmental Microbiology. 1998;64(12):5027–5029. doi: 10.1128/aem.64.12.5027-5029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P, Griffith M, Angles M, Deere D, Ferguson C. Concentrations of pathogens and indicators in animal feces in the Sydney watershed. Applied and Environmental Microbiology. 2005;71(10):5929–5934. doi: 10.1128/AEM.71.10.5929-5934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CM, Long JA, Donald M, Ashbolt NJ. Survival of fecal microorganisms in marine and freshwater sediments. Applied and Environmental Microbiology. 1995;61(5):1888–1896. doi: 10.1128/aem.61.5.1888-1896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graef EM, Devrisde LA, Baele M, Vancanneyt M, Seings J, Haesebrouck F. Identification of enterococcal, streptococcal and Weissella species in the faecal flora of individually owned dogs. Journal of Applied Microbiology. 2005;99:348–353. doi: 10.1111/j.1365-2672.2005.02588.x. [DOI] [PubMed] [Google Scholar]
- De Leener E, DeCostere A, De Graef EM, Moyaert H, Haesebrouck F. Presence and mechanism of antimicrobial resistance among enterococci from cats and dogs. Microbial Drug Resistance. 2005;11:395–403. doi: 10.1089/mdr.2005.11.395. [DOI] [PubMed] [Google Scholar]
- Delgado M, Neto I, Correia JH, Pomba C. Antimicrobial resistance and evaluation of susceptibility testing amoung pathogenic enterococci isolated from dogs and cats. International Journal of Antimicrobial Agents. 2007;30(1):98–100. doi: 10.1016/j.ijantimicag.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Dunlap BG, Thies ML. Giardia in beaver (Castor canadensis) and nutria (Myocastor coypus) from east Texas. The Journal of Parasitology. 2002;88(6):1254–1258. doi: 10.1645/0022-3395(2002)088[1254:GIBCCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Durbin ME, Zaher AM, Heybeck NF, Solo-Gabriele HM, Elmir S, Goodwin KD, Sinigalliano C. The source of enterococci to a subtropical recreational beach is the inter-tidal zone. Proceedings of the 105th General Meeting of the American Society for Microbiology. 2005 (Abstract) [Google Scholar]
- Edge TA, Hill S. Multiple lines of evidence to identify the sources of fecal pollution at a freshwater beach in Hamilton Harbour, Lake Ontario. Water Research. 2007;41:3585–3594. doi: 10.1016/j.watres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Elmir SM, Wright ME, Abdelzaher A, Solo-Gabriele HM, Fleming LE, Miller G, Rybolowik M, Shih P, Pillai SP, Cooper JA, Quaye EA. Quantitative evaluation of bacteria released by bathers in a marine water. Water Research. 2006;41:3–10. doi: 10.1016/j.watres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florida Department of Health. [20 Feb. 2007];Florida Healthy Beaches Program, MyFlorida.com. 2007 http://esetappsdoh.doh.state.fl.us/irm00beachwater/default.aspx.
- Fogarty LR, Haack SK, Wolcott MJ, Whitman RL. Abundance and characteristics of the recreational water quality indicator bacteria Escherichia coli and enterococci in gull faeces. Journal of Applied Microbiology. 2003;94(5):865–878. doi: 10.1046/j.1365-2672.2003.01910.x. [DOI] [PubMed] [Google Scholar]
- Fujioka RS, Tenno K, Kansako S. Naturally occurring fecal coliforms and fecal streptococci in Hawaii’s freshwater streams. Toxicity Assessment. 1988;3(5):613–630. [Google Scholar]
- Gerba CP. Assessment of enteric pathogen shedding by bathers during recreational activity and its impact on water quality. Quantitative Microbiology. 2000;2(1):55–68. [Google Scholar]
- Gould DJ, Fletcher MR. Gull droppings and their effects on water quality. Water Research. 1978;12:665–672. [Google Scholar]
- Graczyk TK, Fayer R, Trout JM, Lewis EJ, Farley CA, Sulaiman I, Lal AA. Giardia sp. cysts and infectious Cryptosporidium parvum oocysts in the feces of migratory Canada geese (Branta canadensis) Applied and Environmental Microbiology. 1998;64(7):2736–2738. doi: 10.1128/aem.64.7.2736-2738.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SB, Sanders BF, Boehm AB, Redman JA, Kim JH, Mrše RD, Chu K, Gouldin M, McGee CM, Gardiner NA, Jones BH, Svejkovsky J, Leipzig GV, Brown A. Generation of enterococci bacteria in a coastal saltwater marsh and its impact on surf zone water quality. Environmental Science and Technology. 2001;35(12):2407–2416. doi: 10.1021/es0018163. [DOI] [PubMed] [Google Scholar]
- Haack SK, Fogarty LR, Wright C. Escherichia coli and enterococci at beaches in the Grand Traverse Bay, Lake Michigan: sources, characteristics, and environmental pathways. Environmental Science and Technology. 2003;37(15):3275–3282. doi: 10.1021/es021062n. [DOI] [PubMed] [Google Scholar]
- Hanes NB, Fossa AJ. A qualitative analysis of the effects of bathers in recreational water quality. Advances in Water Pollution Research. 1970;5:9/1–9/9. [Google Scholar]
- Harwood VJ, Butler J, Parrish D, Wagner V. Isolation of fecal coliform bacteria from the diamondback terrapin (Malaclemys terrapin centrata) Applied Environmental Microbiology. 1999;65(2):865–867. doi: 10.1128/aem.65.2.865-867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch JJ. Threat to public health from gulls. International Journal of Environmental Health Research. 1996;6:5–16. [Google Scholar]
- Jones F, Smith P, Watson DC. Pollution of water supply catchment by breeding gulls and the potential environmental health implications. Journal of the Institute of Water Engineers and Scientists. 1978;32:469–482. [Google Scholar]
- Jones K, Obiri-Danso K. Non-compliance of beaches with the EU directives of bathing water quality: evidence of non-point sources of pollution in Morecambe Bay. Journal of Applied Microbiology Symposium Supplement. 1999;85:101S–107S. doi: 10.1111/j.1365-2672.1998.tb05288.x. [DOI] [PubMed] [Google Scholar]
- Kühn I, Iversen A, Burman LG, Olsson-Liljequist B, Franklin A, Finn M, Aarestrup F, Seyfarth AM, Blanch AR, Vilanova X, Taylor H, Cerrero IA, Möllby R. Comparison of enterococcal populations in animals, humans, and the environment – a european study. International Journal of Food Microbiology. 2003;88(2003):133–145. doi: 10.1016/s0168-1605(03)00176-4. [DOI] [PubMed] [Google Scholar]
- Kushlan JA. Population energetics of the White Ibis. American Ornithologists' Union. 1977;94:114–122. [Google Scholar]
- Kushlan JA. Feeding ecology and prey selection in the White Ibis. Condor. 1979;81(4):376–389. [Google Scholar]
- Lévesque B, Brousseau P, Simard P, Dewailly E, Meisels M, Joly J. Impact of a ring-billed gull (Larus delawarensis) on the microbiology quality of recreational water. Applied and Environmental Microbiology. 1993;59(4):1228–1230. doi: 10.1128/aem.59.4.1228-1230.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque B, Brousseau P, Bernier F, Dewailly E, Joly J. Study of the bacterial content of ring-billed gull droppings in relation to recreational water quality. Water Research. 2000;34(4):1089–1096. [Google Scholar]
- Manning RB, Kumpf HE. Preliminary investigations of the fecal pellets of certain invertebrates of the South Florida area. Bulletin of Marine Science of the Gulf and Caribbean. 1959;9(3):291–309. [Google Scholar]
- Martin A, Gruber S. Weston Solutions Technical Paper 0507. Rancho Cordova, CA: 2005. Amplification of Indicator Bacteria in Organic Debris on Southern California Beaches. [Google Scholar]
- Meals DW, Braun DC. Demonstration of methods to reduce E. coli runoff from dairy manure application sites. Journal of Environmental Quality. 2006;35(4):1088–1100. doi: 10.2134/jeq2005.0380. [DOI] [PubMed] [Google Scholar]
- Murray SM, Patil AR, Fahey GC, Jr, Merchen NR, Hughes DM. Raw and rendered animal by-products as ingredients in dogs diets. Journal of Animal Science. 1997;75(9):2497–2505. [PubMed] [Google Scholar]
- Murphy RC, Kremer JN. Benthic community metabolism and the role of deposit-feeding callianassid shrimp. Journal of Marine Research. 1992;50:321–340. [Google Scholar]
- National Research Council. Nutrient Requirements of Dogs. Washington, D.C: National Academy of Sciences; 1985. pp. 2–41. [Google Scholar]
- Obiri-Danso K, Jones J. Intertidal sediments as reservoirs for hippurate negative camplybacters, salmonellae and faecel indicators in three EU recongnized bathing waters in North West England. Water Research. 2000;34(2):519–527. [Google Scholar]
- Oshiro R, Fujioka R. Sand, soil, and pigeon droppings: sources of indicator bacteria in the waters of Hanauma Bay, Oahu, Hawaii. Water Science Technology. 1995;31(5–6):251–254. [Google Scholar]
- Rodriques J, Poeta P, Martins A, Costa D. The importance of pets as reservoirs of resistant Enterococcus strains with special reference to vancomycin. Journal of Veterinary Medicine Series B-Infectious Diseases and Veterinary Public Health. 2002;49(6):278–280. doi: 10.1046/j.1439-0450.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- Roll BM, Fujioka RS. Sources of faecal indicator bacterica in a brackish, tropical stream and their impact on recreational water quality. Water Science and Technology. 1997;35(11–12):179–186. [Google Scholar]
- Shibata T, Solo-Gabriele HM, Fleming LE, Elmir S. Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Research. 2004;38(2004):3119–3131. doi: 10.1016/j.watres.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BG, Dufour AP. Effects of the microbiological quality of recreational waters: a simulation study; American Society for Microbiology 93rd General Meeting; May 16–20, 1993; Atlanta, Georgia. 1993. [Google Scholar]
- Spears JK, Grieshop CM, Fahey GC., Jr Evaluation of stabilized rice bran as an ingredient in dry extruded dog diets. Journal of Animal Science. 2004;82(4):1122–1135. doi: 10.2527/2004.8241122x. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Ambient water quality criteria for bacteria. Washington, D.C: U.S. Environmental Protection Agency; 1986. EPA A440/5-84-002. [Google Scholar]
- U.S. Environmental Protection Agency. Protocol for Developing Pathogen TMDLs. (First Edition) 2001 EPA 841-R-00-002 http://www.epa.gov/owow/tmdl/pathogen_all.pdf.
- U.S. Environmental Protection Agency. Method 1600: membrane filter test method for enterococci in water. Washington, D.C: U.S. Environmental Protection Agency; 2002. EPA-821-R-02-022. [Google Scholar]
- Van Elsas JD, Smalla K. Methods for sampling soil microbes. In: Hurst CJ, Knudsen GR, McInernery MJ, Stetzenbach LD, Walter MV, editors. Manual of Environmental Microbiology. Washington, D.C: ASM Press; 1997. pp. 383–390. [Google Scholar]
- Wither A, Rehfisch M, Austin G. The impact of bird populations on the microbiological quality of bathing waters. Water Science and Technology. 2005;51(34):199–208. [PubMed] [Google Scholar]
- Ziebis W, Forster S, Huettel M, Jorgensen BB. Complex burrows of the mud shrimp Callianassa truncata and their geochemical impact in the sea bed. Nature. 1996;382(6592):619–622. [Google Scholar]