Abstract
Although much has been learned in the last few decades concerning the molecular mechanisms and pathways associated with the development of familial as well as sporadic Parkinson disease (PD), the precise mechanisms and specific proteins responsible for mediating these effects remain to be elucidated. Thus, the identification and biological evaluation of novel proteins involved in these pathways is critical to providing a more comprehensive understanding of PD pathogenesis. Previously, in a cellular model of PD, we identified nucleolin as a protein interacting with α-synuclein and DJ-1, two critical proteins involved in PD pathogenesis. In our current study, we found the expression levels of nucleolin were dramatically reduced in the substantia nigra pars compacta of human PD subjects, compared with controls. Furthermore, manipulation of nucleolin in an in vitro model of PD resulted in significant alterations in generation of oxidative stress as well as proteasomal inhibition following rotenone exposure. Interestingly, nucleolin expression did not influence mitochondrial complex I activity, suggesting a selective specificity for oxidative stress and proteasomal pathways.
Keywords: mitochondria, nucleolin, oxidative stress, Parkinson disease, proteasome, substantia nigra pars compacta
INTRODUCTION
The precise molecular mechanisms underlying development of Parkinson disease (PD) are largely unknown, complicating the initiation of a proper therapeutic intervention aimed at attenuating or abrogating the progression of the disease. To identify novel mechanisms involved in PD, we, as well as others, have focused on the proteins interacting with α-synuclein (SNCA), LRRK2, DJ-1, Pink-1, and Parkin whose mutations result in familiar PD [9]. To this end, we have applied a robust proteomics technique, SILAC (stable isotope labeling of amino acids in cell culture) to characterize novel proteins that interact with SNCA and DJ-1[16]. Of the proteins identified and confirmed in the initial analysis is nucleolin, a protein previously not known to be associated with PD pathogenesis.
The reasons for further analysis of nucleolin in the current study are several; including 1) although unreported in PD, levels of nucleolin appear to be altered in Alzheimer disease (AD), Down Syndrome (DS) and progressive supranuclear palsy (PSP) [6, 7, 13, 23]; 2) studies have demonstrated an involvement of nucleolin in the posttranscriptional regulation of amyloid precursor protein (APP) mRNA, a key protein associated with AD pathogenesis [27-29]; and 3) nucleolin, a protein observed within the nucleus, cytoplasm, as well as on the plasma membrane [6, 12, 16], appears to be involved in several aspects of cellular homeostasis, including cell proliferation, ribosomal biogenesis, signal transduction, chaperone/shuttling of cellular components, and apoptosis [4, 5, 8, 11, 12, 22].
Here, we have, for the first time, demonstrated a reduction in nucleolin protein expression in the substantia nigra pars compact (SNpc) of PD brains. Furthermore, manipulation of nucleolin levels in a cellular model of PD demonstrated an altered sensitivity to the neurotoxic effects of rotenone. These results suggest a potential role for nucleolin in PD pathogenesis.
MATERIALS AND METHODS
Human brain samples and fractionation of SNpc
Substantia nigra pars compacta tissue was obtained from ten pathologically verified PD and ten healthy control subjects from Duke University, University of California at Los Angeles, University of Michigan, and University of Washington. All subjects were age, gender, and post mortem interval (PMI<12hr) matched and their use was approved by The Human Subjects Division at each of the universities. The neuropathological diagnosis of PD and normal controls was based on established criteria that are routinely used in our laboratory [21, 31]. The SNpc was dissected from control and PD patients; an equal amount of tissue from each subject was pooled into two samples (i.e. control and PD SNpc) followed by isolation of cytosolic-, nuclear-and mitochondrial-enriched fraction using a method previously described [15]. Individual samples saved before pooling were analyzed again after demonstration of significant changes between PD and controls. Briefly, nigral tissues (about 100 mg) were suspended in 1 mL sucrose buffer containing 20 mM HEPES (pH 7.5), 320 mM sucrose, 1 mM PMSF, protease inhibitor cocktail (Sigma, St. Louis, MO) and phosphatase inhibitors (0.2 mM Na3VO3 and 1 mM NaF). The suspension was homogenized with 10 strokes using a glass Potter homogenizer, and then centrifuged at 800×g for 10 min at 4°C to sediment crude nuclei and supernatant. The supernatant was further centrifuged at 10,000×g for 15 min to obtain the supernatant as the cytosolic-enriched fraction. Protein concentrations were determined by BCA assay prior to performing further analysis.
Western blotting of nucleolin
A comparison of the relative abundance of nucleolin in the SNpc of control and PD patients was achieved by Western immunoblotting using rabbit polyclonal anti-nucleolin antibody (Novus Biologicals). Twenty micrograms of protein from cytosolic-enriched, mitochondria-enriched, and nuclear-enriched fractions of pooled human SNpc, or from the cytosolic-enriched fraction in individual samples were subjected to 8-16% SDS-PAGE and transferred to PVDF, blocked, and probed overnight at 4°C with rabbit anti-nucleolin antibody (1:4,000); peroxidase-conjugated secondary antibody was added at 1:40,000, and developed with enhanced chemiluminescence.
Cellular model of PD and nucleolin expression in cytosolic fraction
A DAergic neuron cell line, MES (a gift from Dr. Le at the Baylor College of Medicine), has been widely used in PD-related experiments [15, 30, 32]. Detailed methods for culturing MES cells have been previously described [30]. For a PD cellular model, MES cells were seeded overnight and then treated with 20 nM rotenone (a potent inhibitor of mitochondrial complex I) or vehicle (0.1% DMSO) for 1, 2 and 3 days. Cells were harvested, sonicated in RIPA buffer and centrifuged at 10,000xg for 10 min to obtain the cytosolic fraction. Protein concentrations were determined by the BCA assay. To measure the alteration of nucleolin expression level in the cytosolic fraction, 20 μg of protein was subjected to 8-16% SDS-PAGE and transferred to PVDF, blocked and probed for nucleolin.
Transfection of Nucleolin in MES cells
Flag-tagged nucleolin was expressed from pCMV-tag2B-nucleolin. Cells were transfected with pCMV-tag2B-nucleolin or pCMV-tag2B (empty vector) using Fugene transfection reagent, according to manufacturer’s specifications (Fugene 6, Roche Applied Science). Nucleolin expression levels were assessed by Western blot.
Nucleolin SiRNA transfection in MES Cells
MES cells were transfected with 5 nM Rat nucleolin-specific SiRNA (Rn_Ncl1_HP siRNA (Qiagen, Valencia, CA)) constructs or nonspecific control SiRNA constructs (Qiagen) using HiPerFect transfection reagent (Qiagen) according to the manufacturer’s instructions. Forty-eight hours after SiRNA transfection, cells were treated with vehicle (DMSO) or rotenone 10 nM for 24 hours.
Measurement of neurite growth
MES cells were seeded (1×104) overnight on chamber slides (Lab-Tek II, Nagle, Nunc) previously coated with ploy-D-lysine and fibronectin. Cells were transfected with pCMV-tag2B-nucleolin or pCMV-tag2B (empty vector), SiRNA, or nonspecific SiRNA followed by treatment with 10 nM rotenone or vehicle for 24 hours. Cells were washed in PBS and fixed overnight in 4% paraformaldehyde followed by overnight incubation with rabbit anti-microtubule-associated protein 2 (MAP2) primary antibody (Chemicon/Millipore, Temecula, CA) and then incubated with secondary antibody (1:400 Flex-Fluor ® 488) goat anti-rabbit IgG (Molecular Probes, Eugene, OR). Images of five randomly selected fields were then captured using a laser scanning confocal microscope (Bio-Rad LS2000, Hercules, CA), and total neurite length was quantified using the Neurolucida software (version 8.0, MicroBrightField, Williston, VT).
Measurement of Mitochondrial Complex I Activity
MES cells were transfected and treated as above. Mitochondria were isolated and assayed for complex I activity using methods described previously [10]. Complex I (NADH-ubiquinone oxidoreductase) activity was measured by monitoring the loss of absorbance at 340nm (ε= 6.81mM-1 · cm-1) resulting from the oxidation of n-decylubiquinone (130 μM) at 30°C.
Measurement of Proteasomal Activity
20S chymotrypsin peptidase proteasomal activities were determined by measuring the fluorescence of 7-amido-4-methylcouramin liberated from peptide-7-amido-4-methylcouramin-linked substrates [19]. The results, expressed as fluorescence units/min/mg of protein, were normalized against inhibition of total proteasomal function by 10 μM lactacystin.
Protein Carbonyl Assay for Oxidative Stress
Protein oxidation was measured by quantifying total protein carbonyl contents using 2,4-dinitrophenylhydrazine (DNPH). The spectrum difference between DNPH-treated and control samples was determined, and results were expressed as nanomoles of DNPH incorporated/mg of protein based on the absorption at 375 nm (ε= 21.0 mM-1 · cm-1). Protein concentration was determined by standard BCA assay.
Statistical Analysis
All cell culture experiments represent a minimum of three independent experiments performed in triplicate. Statistical analysis was performed on raw data by one-way ANOVA. Post hoc analysis was performed using Student-Newman Keuls test. Significance is reported at p < 0.05.
RESULTS
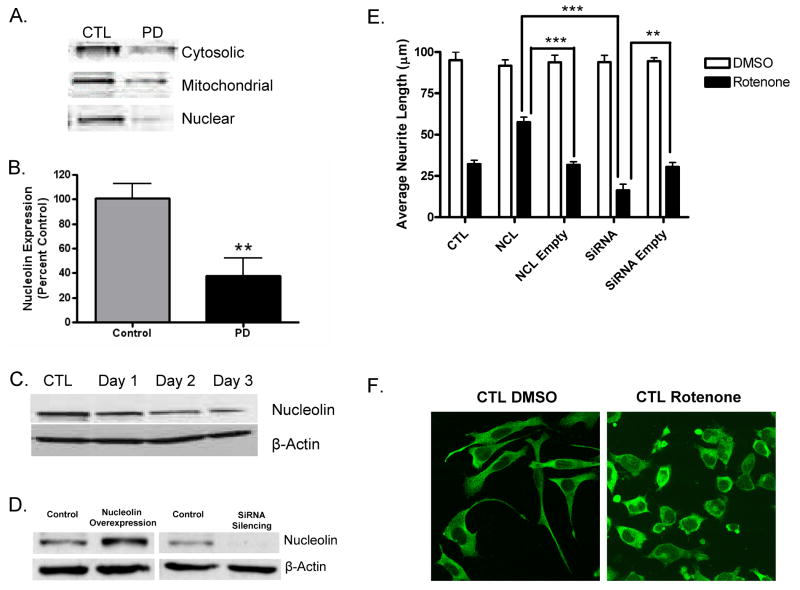
As the first approach towards verifying the involvement of nucleolin in human PD, we examined the levels of nucleolin in the SNpc of human tissue. Western blot analysis was carried out on cytosolic, where the interaction was initially defined between nucleolin and SNCA/DJ-1 in a cellular model of PD, as well as mitochondrial and nuclear fractions [16], isolated from the SNpc of PD patients (n=10) and healthy controls (n=10). The results clearly demonstrate a substantial reduction in nucleolin levels in PD patients as compared to controls, whether examined with pooled samples from cytosolic, mitochondrial, or nuclear fractions (Fig. 1A) or cytosolic fractions from individual cases (Fig. 1B).
Figure 1.
Nucleolin expression in human SNpc and MES cells. A). Representative nucleolin expression in pooled cytosolic, mitochondrial, and nuclear fractions from the SNpc of control and PD subjects. B). Nucleolin levels are significantly reduced in the cytosolic fraction from individual PD SNpc (n=10) compared with individual control (n=10) subjects (p=0.005). C). In vitro model of nucleolin reduction in MES cells treated with 20 nM rotenone for 1, 2, or 3 days. Manipulation of nucleolin levels influenced cell viability. D). Expression of nucleolin in MES cells following transfection with nucleolin expression plasmid or nucleolin-SiRNA. E). Neurodegeneration induced by rotenone was assessed by evaluating neurite growth following exposure to 10 nM rotenone or DMSO. MES cells were transfected with empty versus expression plasmid or nonspecific-SiRNA versus nucleolin-SiRNA for 48 hours prior to treatment with rotenone for 24 hours. F). Representative confocal microscopy images of untransfected MES cells treated with DMSO or 10 nM rotenone for 24 hours. **, p≤0.01; ***, p≤0.001. No difference was observed between groups receiving DMSO. Abbreviations: CTL=Control, NCL=Nucleolin, Empty=Empty Vector, DMSO=Dimethyl Sulfoxide.
Given the decrease in nucleolin levels found in the SNpc of PD patients, we decided to extend these findings to a cellular model of PD. We, as well as others, have previously demonstrated the utility of rotenone in generating a cellular model of PD, via inhibition of mitochondrial complex I, and production of oxidative stress [15, 16, 26]. Consistent with the results obtained in our human SNpc samples, exposure of MES cells to 20 nM rotenone produced a time dependent reduction in nucleolin expression (Fig 1C). It should be noted that in our original paper, nucleolin expression was also decreased in the protein complex (containing nucleolin, SNCA, DJ-1 along with other proteins) after MES cells were treated with rotenone [16]. Taken together, these results suggest a role for nucleolin in the maintenance of healthy neurons.
To gain a more in depth understanding of the role of nucleolin in PD, we manipulated the expression of nucleolin in MES cells and evaluated these effects on rotenone-mediated neurotoxicity. Overexpression of the nucleolin gene results in a 100% increase in expression compared with controls transfected with the empty vector, while silencing of nucleolin expression resulted in a 90% reduction two days after transfection (Fig. 1D). The effects of these manipulations on cell viability were evaluated in MES cells exposed to 10 nM rotenone for 24 hours. Using neurite growth as a cell viability endpoint we found that nucleolin overexpression or silencing had no effect on neurite growth in vehicle-treated cells (Fig. 1E). However, overexpression and silencing significantly influenced the neurotoxic response to rotenone exposure. Specifically, reduction of nucleolin resulted in decreased cell viability, while overexpression appeared to attenuate the neurotoxic effects of rotenone, when compared with their respective rotenone treated empty vector controls (Fig. 1E). No difference was observed between cells receiving only DMSO and vehicle treated cells transfected with empty or their respective coding vectors. Similarly, no difference was seen between control cells treated with rotenone compared with cells transfected with the empty vectors, followed by exposure to rotenone (Fig. 1E).
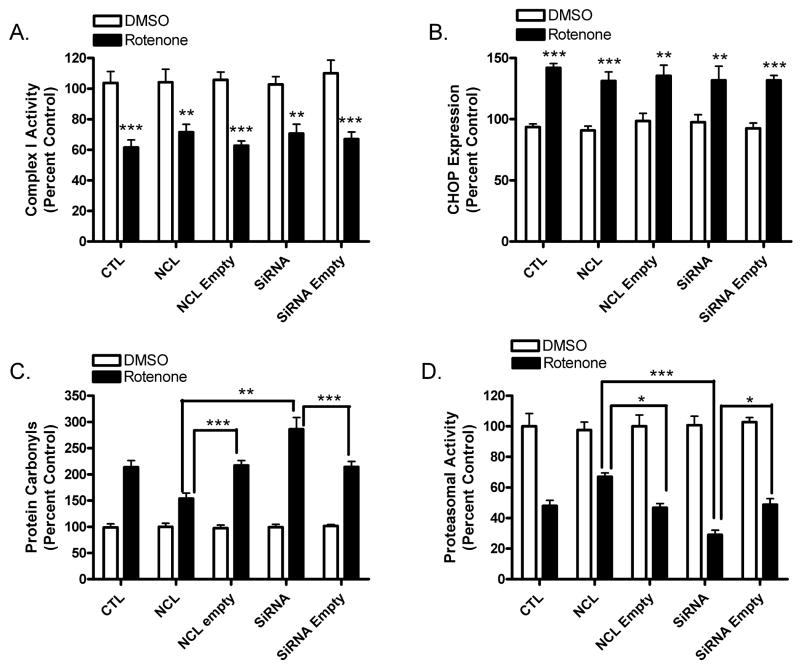
While the results shown above suggest a role for nucleolin in mediating rotenone neurotoxicity the specific mechanisms and pathways remain to be determined. Given the well-characterized mitochondrial deficits and activation of ER stress in PD, we went on to determine the effects of nucleolin manipulation on complex I activity and ER stress [24], respectively, following exposure of MES cells to 10 nM rotenone. Figure 2A demonstrates that, as expected, a significant reduction in complex I activity was observed in all cells receiving rotenone, compared with their respective vehicle treated controls. Surprisingly, overexpression or silencing of nucleolin had no effect on complex I activity, following rotenone treatment. Similarly, as predicted, two markers of ER stress (CHOP, a pro-apoptotic transcription factor and Grp78, an ER chaperone (data not shown)) were significantly increased after rotenone exposure. However, overexpression or silencing of nucleolin did not significantly affect the levels of these proteins (Fig. 2B).
Figure 2.
Effects of nucleolin expression on markers of rotenone neurotoxicity. A). Exposure of MES cells to rotenone caused a significant reduction in complex I activity in all treatment groups, compared with their respective DMSO treated group. Nucleolin expression did not affect mitochondrial complex I activity of MES cells transfected with expression plasmid or nucleolin-siRNA for 48 hours, followed by exposure to 10 nM rotenone for 24 hours. Rotenone induced toxicity was assessed by mitochondrial complex I activity. B). In addition, rotenone exposure resulted in a significant increase in the expression of CHOP, a marker of ER stress and apoptosis, compared with respective DMSO groups. However, nucleolin expression did not affect the expression of CHOP, compared to their respective empty vector controls treated with rotenone. In contrast, nucleolin expression significantly affected rotenone-mediated inhibition of proteasomal activity and generation of oxidative stress. C). The extent of oxidative stress was assessed by measuring the generation of protein carbonyls. Overexpression of nucleolin significantly attenuated the formation of protein carbonyls, while silencing of nucleolin exacerbated carbonyl formation, compared with their respective rotenone treated empty vector controls. D). MES cells were transfected with expression plasmid or nucleolin-siRNA and treated with 10 nM rotenone for 24 hours. Overexpression of nucleolin reduced rotenone-mediated inhibition of proteasomal activity, while silencing potentiated the inhibition, compared to their respective rotenone treated empty vector controls. *,p≤0.05; **,p≤0.01; ***, p≤0.001.
Another important aspect of human PD, as well as the rotenone-induced cellular model of PD, is increased oxidative stress and decreased proteasomal activity [1]. Thus, we sought to evaluate the role of nucleolin in oxidative stress and proteasomal activity following rotenone treatment. As previously reported, exposure of untransfected cells to rotenone caused a significant reduction in proteasomal activity, in addition to a substantial increase in oxidative stress, as measured by inhibition of the 20S chymotrypsin peptidase and the generation of protein carbonyls, respectively (Fig. 2C & D). Interestingly, silencing of nucleolin levels further potentiated both proteasomal inhibition and the production of protein carbonyls, while overexpression of nucleolin significantly attenuated both measures. As in Figure 1E, we did not observe a difference between cells receiving only DMSO and vehicle treated cells transfected with empty or their respective coding vectors. Similarly, no difference was seen between control cells treated with rotenone compared with cells transfected with the empty vectors, followed by exposure to rotenone.
DISCUSSION
In the current investigation, nucleolin levels were found to be significantly reduced in the SNpc of human PD samples, as well as in an in vitro model of PD. Manipulation of nucleolin expression in a cellular model of PD demonstrated a role for nucleolin in cell viability as well as oxidative stress and proteasomal activity. However, nucleolin did not affect mitochondrial complex I function or the ER stress response, in vitro.
As discussed in the Introduction, although this represents the first report of involvement of nucleolin in PD, previous studies have demonstrated an association of nucleolin with AD as well as other neurodegenerative diseases. Specifically, Dranovsky and colleagues showed a reduction in nucleolin expression in the temporal lobe of AD patients compared with control [6]. Furthermore, as nucleolin expression was reduced in AD samples, they demonstrated a coincident increase in levels of phosphorylated nucleolin, which appeared to associate with NFT in AD, as well as numerous other neurodegenerative diseases demonstrating aberrant accumulations of tau [13]. It should be noted that in Husseman’s paper, phosphorylated nucleolin was not detected in the SNpc neurons of PD patients [13].
The significance of a reduction in nucleolin expression in human PD was inquired using a cellular model of PD. Rotenone was chosen as a toxicant in our model based upon its ability to recapitulate several of the pathological features of human PD, including mitochondrial deficits, oxidative stress, and altered ER stress and proteolysis [2, 14, 20, 25]. Exposure of dopaminergic MES cells to rotenone for three days demonstrated a time dependent reduction in nucleolin levels, similar to those seen in human SNpc (Fig. 1C). Additionally, manipulation of nucleolin expression appeared to have significant influence on cellular viability, exhibiting an attenuated or potentiated neurotoxic response following overexpression and silencing of nucleolin levels, respectively (Fig. 1E). These results argue that nucleolin expression influences DAergic cell viability following a neurotoxic challenge with rotenone.
As to the mechanisms by which nucleolin regulates cell viability, we observed that nucleolin did not play a role in mitochondrial complex I function (Fig. 2A) or in the ER stress response (Fig. 2B), suggesting that the cellular effects of nucleolin function are either downstream or independent of complex I inhibition and ER stress. This observation is fairly intriguing, given that rotenone is traditionally believed to exert its neurotoxicity primarily through inhibition of mitochondrial complex I of the electron transport chain, resulting in a series of downstream events, e.g. increased ER and oxidative stress, and eventual cell death [2, 3, 26].
In contrast to these results, alteration of nucleolin levels had significant effects on oxidative stress and proteasomal activity, as overexpression of nucleolin resulted in an increase in activity of the 20S proteasomal subunit and a reduction in the generation of protein carbonyls (Fig. 2C & D). These results, along with a negative result with respect to mitochondrial inhibition, are significant at least on several grounds. First, increased oxidative stress and decreased proteasomal activity, both of which have been demonstrated in PD patients, are not only critical to rotenone-mediated neurotoxicity but also appear to be sensitive to the modulation of nucleolin expression. Second, the source of rotenone-mediated oxidative stress, a component regulated by nucleolin, appears to be independent of mitochondrial respiration, although impairment of complex I activity exacerbates ROS production [18, 26]. Finally, the possibility that nucleolin directly affects the proteasome, rather than as an indirect result of attenuated oxidative stress, remains to be determined.
While one might argue that the change in nucleolin expression demonstrated in the SNpc of PD patients could just be a general phenomenon of cell loss, given that there is usually a significant loss of neurons in the SNpc of PD patients at autopsy, it should be noted that our proteomics analysis clearly indicated that not all proteins are decreased in PD tissue ([17] and Caudle et al., unreported results). Furthermore, as nucleolin expression has been demonstrated in astroglia, microglia, in addition to neurons [13], identifying the specific cell population(s) that contributes to the involvement of nucleolin in neurodegeneration remains to be determined. More importantly, the fact that manipulation of nucleolin levels in DAergic cells influences multiple mechanisms and pathways known to be involved in PD pathogenesis indicates that it is likely that nucleolin plays a role in DAergic neurodegeneration. The hypothesis certainly needs to be further tested in a more biologically sophisticated experimental system, e.g. primary cultures or even in an animal model of PD, with manipulation of nucleolin levels in DAergic neurons specifically vs. other neurons or glia.
In summary, our study demonstrated a significant reduction in nucleolin in the SNpc of PD patients. Further evaluation of the role of nucleolin in PD pathogenesis found a substantial effect on the generation of oxidative stress and proteasomal function. However, mitochondrial complex I activity and the ER stress response were unaffected by nucleolin expression. These results suggest that nucleolin influences specific molecular mechanisms known to be associated with PD pathogenesis. However, further studies will be necessary to gain a more in depth understanding of the role of nucleolin in the development of PD.
Acknowledgments
This work was supported by National Institutes of Health grants AG025327 and ES012703 (JZ) and T32007032 (WMC) as well as grants from the Michael J. Fox Foundation, American Parkinson’s Disease Association, and the C.-M. Shaw Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson’s disease: Effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiology of disease. 2006 doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT. Mechanistic approaches to Parkinson’s disease pathogenesis. Brain Pathol. 2002;12:499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 4.Bogre L, Jonak C, Mink M, Meskiene I, Traas J, Ha DT, Swoboda I, Plank C, Wagner E, Heberle-Bors E, Hirt H. Developmental and cell cycle regulation of alfalfa nucMs1, a plant homolog of the yeast Nsr1 and mammalian nucleolin. Plant Cell. 1996;8:417–428. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CM, Chiang SY, Yeh NH. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–7758. [PubMed] [Google Scholar]
- 6.Dranovsky A, Vincent I, Gregori L, Schwarzman A, Colflesh D, Enghild J, Strittmatter W, Davies P, Goldgaber D. Cdc2 phosphorylation of nucleolin demarcates mitotic stages and Alzheimer’s disease pathology. Neurobiol Aging. 2001;22:517–528. doi: 10.1016/s0197-4580(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 7.Dubois T, Zemlickova E, Howell S, Aitken A. Centaurin-alpha 1 associates in vitro and in vivo with nucleolin. Biochemical and biophysical research communications. 2003;301:502–508. doi: 10.1016/s0006-291x(02)03010-3. [DOI] [PubMed] [Google Scholar]
- 8.Fang SH, Yeh NH. The self-cleaving activity of nucleolin determines its molecular dynamics in relation to cell proliferation. Exp Cell Res. 1993;208:48–53. doi: 10.1006/excr.1993.1221. [DOI] [PubMed] [Google Scholar]
- 9.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 10.Fiskum G, Starkov A, Polster BM, Chinopoulos C. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Annals of the New York Academy of Sciences. 2003;991:111–119. doi: 10.1111/j.1749-6632.2003.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 11.Ginisty H, Amalric F, Bouvet P. Nucleolin functions in the first step of ribosomal RNA processing. Embo J. 1998;17:1476–1486. doi: 10.1093/emboj/17.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112(Pt 6):761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 13.Husseman JW, Nochlin D, Vincent I. Mitotic activation: a convergent mechanism for a cohort of neurodegenerative diseases. Neurobiol Aging. 2000;21:815–828. doi: 10.1016/s0197-4580(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 14.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36-28. [DOI] [PubMed] [Google Scholar]
- 15.Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, Zhang J. Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease. Mol Cell Proteomics. 2006;5:1193–1204. doi: 10.1074/mcp.M500382-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Jin J, Li GJ, Davis J, Zhu D, Wang Y, Pan C, Zhang J. Identification of novel proteins associated with both alpha-synuclein and DJ-1. Mol Cell Proteomics. 2007;6:845–859. doi: 10.1074/mcp.M600182-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Kitsou E, Pan S, Zhang JP, Shi M, Zabeti A, Dickson DW, Albin R, Gearing M, Kashima DT, Beyer RP, Wang Y, Zhou Y, Pan C, Caudle WM, Zhang J. Identification of proteins in human substantia nigra. Proteomics-Clinical Applications. 2008;2:776–782. doi: 10.1002/prca.200800028. [DOI] [PubMed] [Google Scholar]
- 18.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. The Biochemical journal. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNaught KS, Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neuroscience letters. 2001;297:191–194. doi: 10.1016/s0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- 20.McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P. Failure of the ubiquitin-proteasome system in Parkinson’s disease. Nat Rev Neurosci. 2001;2:589–594. doi: 10.1038/35086067. [DOI] [PubMed] [Google Scholar]
- 21.Michotte A. Recent developments in the neuropathological diagnosis of Parkinson’s disease and parkinsonism. Acta neurologica Belgica. 2003;103:155–158. [PubMed] [Google Scholar]
- 22.Ohmori H, Murakami T, Furutani A, Higashi K, Hirano H, Gotoh S, Kuroiwa A, Masui A, Nakamura T, Amalric F. Simultaneous activation of heat shock protein (hsp 70) and nucleolin genes during in vivo and in vitro prereplicative stages of rat hepatocytes. Exp Cell Res. 1990;189:227–232. doi: 10.1016/0014-4827(90)90240-b. [DOI] [PubMed] [Google Scholar]
- 23.Reiser G, Bernstein HG. Neurons and plaques of Alzheimer’s disease patients highly express the neuronal membrane docking protein p42IP4/centaurin alpha. Neuroreport. 2002;13:2417–2419. doi: 10.1097/00001756-200212200-00008. [DOI] [PubMed] [Google Scholar]
- 24.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schapira AH. Mitochondrial dysfunction in neurodegenerative disorders. Biochimica et biophysica acta. 1998;1366:225–233. doi: 10.1016/s0005-2728(98)00115-7. [DOI] [PubMed] [Google Scholar]
- 26.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westmark CJ, Malter JS. Extracellular-regulated kinase controls beta-amyloid precursor protein mRNA decay. Brain Res Mol Brain Res. 2001;90:193–201. doi: 10.1016/s0169-328x(01)00112-7. [DOI] [PubMed] [Google Scholar]
- 28.Westmark CJ, Malter JS. Up-regulation of nucleolin mRNA and protein in peripheral blood mononuclear cells by extracellular-regulated kinase. J Biol Chem. 2001;276:1119–1126. doi: 10.1074/jbc.M009435200. [DOI] [PubMed] [Google Scholar]
- 29.Zaidi SH, Malter JS. Nucleolin and heterogeneous nuclear ribonucleoprotein C proteins specifically interact with the 3’-untranslated region of amyloid protein precursor mRNA. J Biol Chem. 1995;270:17292–17298. doi: 10.1074/jbc.270.29.17292. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Kravtsov V, Amarnath V, Picklo MJ, Graham DG, Montine TJ. Enhancement of dopaminergic neurotoxicity by the mercapturate of dopamine: relevance to Parkinson’s disease. Journal of neurochemistry. 2000;74:970–978. doi: 10.1046/j.1471-4159.2000.0740970.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Montine TJ, Smith MA, Siedlak SL, Gu G, Robertson D, Perry G. The mitochondrial common deletion in Parkinson’s disease and related movement disorders. Parkinsonism & related disorders. 2002;8:165–170. doi: 10.1016/s1353-8020(01)00041-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Price JO, Graham DG, Montine TJ. Secondary excitotoxicity contributes to dopamine-induced apoptosis of dopaminergic neuronal cultures. Biochemical and biophysical research communications. 1998;248:812–816. doi: 10.1006/bbrc.1998.9044. [DOI] [PubMed] [Google Scholar]