Abstract
The primary motor cortex (M1) plays a critical role in early aspects of motor skill learning. Given the notion of inter-hemispheric competition, unilateral disruption of M1 may increase excitability of the unaffected motor cortex and thus improve motor learning with the ipsilateral hand. We applied slow-frequency repetitive transcranial magnetic stimulation (rTMS) before the initiation of practice of a simple motor skill. Participants were randomly divided into three stimulation groups: i) ipsilateral M1; ii) contralateral M1; and Cz (control site). Mean execution time and error rate were recorded in 4 sessions distributed over 2 days. Disruption of M1 with rTMS slowed down skill acquisition with the contralateral hand, but paradoxically enhanced learning with the ipsilateral hand. This was evidenced by a significant decrease of execution time at the end of day 1 in the group that received rTMS over the ipsilateral M1 compared to both control groups (Cz and contralateral M1 stimulation). This supports the notion of inter-hemispheric competition and provides novel insights that may be applicable to neurorehabilitation.
Keywords: Transcranial magnetic stimulation, Plasticity, Motor practice, Paradoxical facilitation
Introduction
Motor skill learning is a dynamic process occurring over time and involving plastic changes in different brain regions. The primary motor cortex (M1) plays a critical role in early stages of the acquisition of various motor skills (Pascual-Leone et al., 1994; Karni et al., 1995; Brashers-Krug et al., 1996; Muellbacher et al., 2002; Walker et al., 2003; Antal et al., 2004; Baraduc et al., 2004; Lu & Ashe, 2005). During early stages after practice, the memory of a new motor skill is vulnerable to interference, for example by practice of another motor skill (Brashers-Krug et al., 1996; Walker et al., 2003) or disruption of M1 by transcranial magnetic stimulation (TMS; Muellbacher et al., 2002; Baraduc et al., 2004). On the other hand, under certain circumstances learning of specific motor tasks can be facilitated by modulation of M1 activity (Bury & Jones, 2002; Antal et al., 2004).
The two cerebral hemispheres are functionally coupled and balanced (Oliveri et al., 1999; Hilgetag et al., 2001). Unilateral dysfunction disrupts this balance and can lead to the release of the unaffected hemisphere, resulting in a paradoxical functional enhancement (Oliveri et al., 1999; Hilgetag et al., 2001; Kobayashi et al., 2004). Repetitive TMS (rTMS) of M1 can induce a temporary reduction of excitability in the targeted M1, and modify voluntary movement-related cortical activity beyond the duration of the rTMS train (Rossi et al., 2000). TMS-induced disruption of M1 activity can enhance motor performance with the ipsilateral hand, presumably by modulating activity in the unaffected M1 (Kobayashi et al., 2004). In the present study, we investigated whether transient suppression of the hand representation in M1 by low-frequency rTMS can modulate early aspects in motor skill learning with the ipsilateral hand.
Subjects and Methods
Subjects, motor task and study design
Twenty-four healthy right-handed volunteers (9 men, 15 women, mean age 25.1±5.1 years) participated in this study, which had been approved by the institutional review board. Subjects were randomly divided into three groups, matched for age and gender distributions. All were naive to the task, and were instructed to press four keys on a response box as quickly and precisely as possible using the left index finger, following a defined sequence (Fig.1). This sequence was repeated 12 times (48 key presses in total) in each block of the task. Mean execution time for each key press and error rate per block was calculated. Each block of trials was separated by a 20 seconds rest period, and 10 blocks composed a session. Each session lasted approximately 5 minutes. All subjects completed two sessions (S1 and S2) on day1, and another two (S3 and S4) on day2. Before S1, subjects received rTMS over the left M1 (ipsilateral to the used index finger), over the right M1 (contralateral), or over a control scalp position (Cz; as defined by the 10-20 International System for EEG electrodes). Mirror movements were ruled out by on-line monitoring of continuous EMG recordings. As an additional experiment, another 8 subjects with similar age and gender distributions (3 men, 5 women, mean age 26.3±5.2 years) were recruited and performed the same experiments without any TMS, in order to investigate the effect of rTMS itself.
Figure 1.
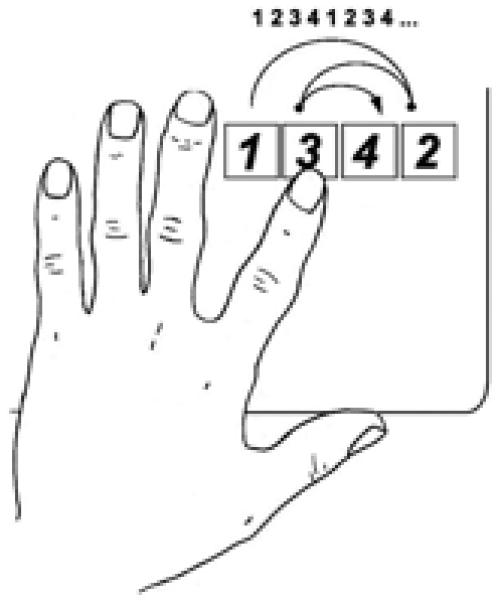
Finger movement sequence. Subjects were instructed to press the keys in the numbered order with their left index finger, as accurately and quickly as possible.
Transcranial magnetic stimulation
Subjects were seated in a reclining chair. A tight-fitting white lycra swimming cap was placed on their head to mark the optimal scalp site from which TMS elicited MEPs of the largest amplitude in the contralateral first dorsal interosseous (FDI). Optimal scalp site was defined following recommended guidelines (Rossini & Rossi, 1998) TMS was performed with a 70 mm figure-eight-coil and a Magstim Rapid stimulator (Magstim, Dyfed, UK). MEPs were recorded from the FDI using surface electrodes and a Dantec Counterpoint electromyography (Dantec, Skovlunde, Denmark) with a band pass of 20-2000Hz. Motor threshold (MT) was defined as the minimum TMS intensity required to induce MEPs of >50 microvolt peak-to-peak amplitude in at least 5 of 10 trials in the contralateral target muscle, determined with TMS delivered to the optimal scalp site for induction of MEPs from the contralateral FDI (Rossini & Rossi, 1998). The coil was held tangentially to the scalp, with the handle pointing 45° postero-laterally. Each rTMS session consisted of a 10 min train at 1 Hz frequency and an intensity of 90 % of the individual’s resting MT. For the control position over the vertex (Cz), the intensity was set in reference to the highest MT of right or left hemisphere. Such low-frequency rTMS can temporarily suppress local cortical activity beyond the duration of the rTMS train (Chen et al., 1997).
Data analysis
Mean (± standard deviation) execution time and error rate were calculated from the 48 key-presses in each block of trials. Across groups of subjects changes in execution time and error rate for the first and last block of trials in the four sessions were subjected to a mixed analysis of variance (ANOVA) with group and block as factors. The changes in execution time over the course of the first session were also analyzed. Paired t-tests (Fisher’s PLSD) were used for post-hoc analysis.
Results
None of the subjects experienced any adverse effects during or following rTMS. The mixed ANOVA revealed a significant effect of block on execution time (F7,147 = 56.37, p < 0.001) and a significant interaction between block and group (F14,147 = 1.94, p < 0.05; Figure 2a). Post-hoc tests demonstrated a significant decrease of execution time in the group that received rTMS to the ipsilateral M1 at the end of Day-1 (after S2), as compared with either of the other two groups (p<0.05, Fisher’s PLSD test). Thus, while all subjects showed learning over time, subjects who received ipsilateral M1 stimulation learned faster than the control group, as indexed by quicker shortening of execution time and more rapid achievement of a performance plateau. Conversely, the group with rTMS to the contralateral M1 improved their execution time slower than the control and ipsilateral TMS groups, but this effect was not significant. At the end of the last session (S4) on the second day, there was no significant difference in performance among the groups of subjects (Figure 2a).
Figure 2.
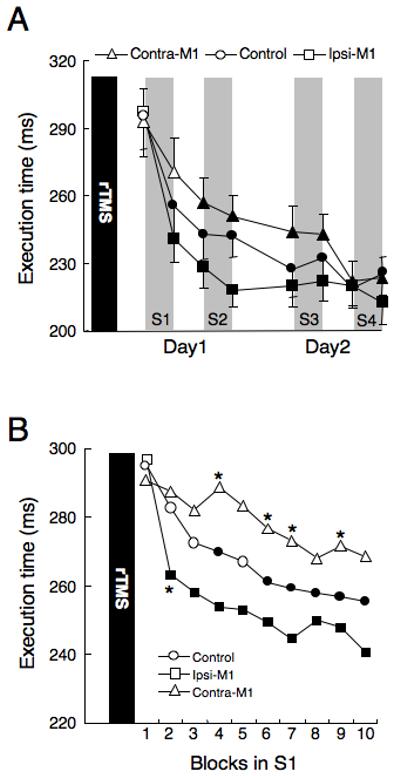
A) Changes in execution time. Each plot represents mean execution time in one practice block. Data from the initial block on Day-1 (baseline), the first block on Day-2, and the last one in each practice session are plotted. Execution time improved with practice sessions. In the group with rTMS to the ipsilateral M1, the execution time at the end of Day-1 (after S2) was significantly shorter than in the other two groups (*, p < 0.05). The group with rTMS of the contralateral M1 improved their execution times slower than the other two groups, but not significantly. Filled symbols indicate significantly shorter values than baseline (p < 0.05). Error bars represent standard error. B) Changes of execution time during the ten blocks of the first session (S1). Subjects with rTMS to the ipsilateral M1 improved their execution times faster than the other two groups. Conversely, subjects with rTMS to the contralateral M1 improved their execution times slower than the other two groups. Asterisks show the values that are significantly different from those of controls, indicating shorter or longer values. Filled symbols represent significantly shorter values than baseline, indicating significantly improved performance.
Figure 2b shows changes of execution time during the ten blocks of the first session (S1). Repeated measures ANOVA revealed a significant effect of block on execution time (F9,189 = 20.77, p < 0.001) and a significant interaction between block and group (F18, 189 = 2.02, p < 0.05). Post-hoc tests revealed that subjects with rTMS to the ipsilateral M1 significantly shortened their execution time after the second block of trials, the control group shortened it by the fourth to sixth block, while the group with rTMS to the contralateral TMS did not improved significantly at any block of S1 (p<0.05, Fisher’s PLSD test). By the second block, subjects with ipsilateral rTMS performed faster than the control group, while subjects with contralateral rTMS performed slower than the control group at the fourth, sixth, seventh and ninth blocks (p < 0.05, Fisher’s PLSD test).
In a follow-up experiment, task performance in subjects without any rTMS was compared with the control group receiving rTMS to Cz. Repeated measures ANOVA revealed a significant effect of block (F7, 91 = 49.87, p < 0.0001), but no significant difference between the two groups nor an interaction of block and group, indicating that rTMS to Cz did not have any significant effect on motor skill learning. Furthermore, the data across subjects with ipsilateral, contralateral and no rTMS were subjected to statistical analysis, which showed similar results to those obtained with rTMS to Cz as control. Mean error rates for the three groups in the first block of day1 and the last block of day2 were 1.13±1.96% and 1.47±1.76%, respectively. There was no significant difference in error rates according to subject group or practice session.
Discussion
In the present study, we showed that suppression of M1 activity with slow-frequency rTMS enhances ipsilateral learning of a simple motor task while at the same time disrupting learning in the contralateral hand. These data add to a previous study by our group where it was shown that rTMS of the motor cortex improved execution time during a learned task performed with the ispilateral hand (Kobayashi et al., 2004). Our data are also in agreement with those of a study in rats revealing that experimental unilateral M1 lesions enhanced learning of skills with the ipsilateral forepaw (Bury & Jones, 2002). A possible mechanism for this paradoxical facilitation is that disrupting activity in M1 suppresses motor control and learning with the contralateral hand, while transcallosally disinhibiting the un-stimulated M1 and thus increasing cortico-spinal drive for the ipsilateral extremity. In humans, a unilateral stroke involving M1 can disinhibit excitability of the unaffected M1 (Shimizu et al., 2002), and unilateral suppression of excitability in M1 with rTMS leads to a demonstrable change in cortical excitability and metabolic rates in the un-stimulated M1 (Chouinard et al., 2003). The effects of rTMS on motor learning could also involve effects of M1 stimulation on frontal eye-fields or parietal lobes, thus affecting visual tracking or spatial attention (Pascual-Leone et al., 1994; Hilgetag et al., 2001; Antal et al., 2004). However, while focality of rTMS is limited, studies combining rTMS with brain imaging in humans and animals reveal that when applied to M1 the effects of rTMS appear restricted to M1 and its impact along specific motor network connections, with negligible spread to prefrontal or parietal regions (Bestmann et al., 2003; Chouinard et al., 2003; Valero-Cabre et al., 2005).
In addition to the ipsilateral effects of rTMS, stimulation of the contralateral M1 disrupted skill acquisition although it did not reach statistical significance. Several TMS studies have failed to show a significant M1 contribution to skill acquisition. Rather, it was found that TMS prior or during learning interfered with retention of specific motor skills. For example, Richardson et al. (2006) reported that rTMS over M1 prior to adaptation to a velocity-dependent force field did not disrupt initial learning, whereas subsequent testing 24 hours after learning revealed increased error rates in stimulated subjects compared to control participants. Along the same lines, Hadipour-Niktarash et al. (2007) showed that single-pulse TMS over M1 led to no apparent changes in the rate of adaptation to a visuomotor rotation task. The effects of TMS were seen in the deadaptation period: participants that received TMS showed faster deadaptation rates than those that did not, suggesting again that TMS interfered with retention of the motor skill and not acquisition. It remains an open question as to why skill acquisition was enhanced ipsilaterally to rTMS while there was no significant change contralaterally (altough there was a clear trend). In light of reports suggesting that M1 involvement in motor learning is task-specific (Baraduc et al., 2004), it would be of great interest to determine if the effect reported here can generalize to other types of motor behavior.
Constraint-induced therapy for post-stroke rehabilitation, i.e. immobilization of the healthy arm, can enhance functional recovery of the paretic hand by increasing motor learning (Liepert et al., 2000). This benefit appears to involve decreased activity in the undamaged M1 (contralateral to the immobilization), reduced interhemispheric inhibition, and increased of the excitability in the affected M1 (ipsilateral to the immobilization; (Liepert et al., 2000). Direct suppression of ipsilateral M1 excitability by rTMS may thus represent “central constraint-induced therapy”. It should be mentioned, however, that the effects of rTMS reported here were short lived, such that performance at the end of the last session of the second day (S4) was not different between the three groups. Unlike healthy individuals, stroke patients may not be able to re-acquire lost motor skills in a few days, needing several weeks to reach best motor performance. As such, modulation of ipsilateral M1 activity in stroke rehabilitation may contribute to functional recovery when it is accompanied with appropriate physical or occupational therapy over an extended period of time. In this setting, rTMS could be used to “prime” M1 and allow for more efficient physical therapy. Clinical studies are needed to determine if this procedure can produce lasting recovery from motor deficits.
Acknowledgements
This work was conducted at the Harvard-Thorndike General Clinical Research Center, supported by the National Center for Research Resources (MO1RR01032). The study was partly supported by grants from the NIH (K24RR018875, RO1NS20068, R01EB 005047, RO1NS47754, RO1DC05672) and the Uehara Memorial Foundation (M.K.).
References
- Antal A, Nitsche MA, Kincses TZ, Kruse W, Hoffmann KP, Paulus W. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur. J. Neurosci. 2004;19:2888–2892. doi: 10.1111/j.1460-9568.2004.03367.x. [DOI] [PubMed] [Google Scholar]
- Baraduc P, Lang N, Rothwell JC, Wolpert DM. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Curr. Biol. 2004;14:252–256. doi: 10.1016/j.cub.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 2003;20:1685–1696. doi: 10.1016/j.neuroimage.2003.07.028. [DOI] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J. Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J. Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J. Neurosci. 2007;27:13413–13419. doi: 10.1523/JNEUROSCI.2570-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag CC, Théoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Théoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2003;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Lu X, Ashe J. Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron. 2005;45:967–973. doi: 10.1016/j.neuron.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Traversa R, Cicinelli P, Filippi MM, Pasqualetti P, Tomaiuolo F, Caltagirone C. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain. 1999;122:1731–1739. doi: 10.1093/brain/122.9.1731. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabré A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J. Neurosci. 2006;26:12466–12470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Rossini PM, Feige B, Ulivelli M, Glocker FX, Battistini N, Lucking CH, Kristeva-Feige R. Effects of repetitive transcranial magnetic stimulation on movement-related cortical activity in humans. Cereb. Cortex. 2000;10:802–808. doi: 10.1093/cercor/10.8.802. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S. Clinical applications of motor evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1998;106:180–194. doi: 10.1016/s0013-4694(97)00097-7. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Payne BR, Rushmore R, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity in the cat. Exp. Brain Res. 2005;163:1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]


