ABSTRACT
BACKGROUND
Some herbal supplements may contain lead.
OBJECTIVE
To examine whether use of specific herbal dietary supplements during the last 30 days is associated with blood lead levels in US men and women.
DESIGN
Cross-sectional analysis.
STUDY POPULATION
NHANES participants from 1999–2004, a representative sample of the civilian non-institutionalized US population.
MEASUREMENTS
Lead was measured in blood. Associations between lead and self-reported supplement use were estimated using multivariable regression weighted to account for NHANES sampling. Herbal supplements investigated were those previously reported to contain high heavy metal content: Ayurvedic or traditional Chinese medicine herbs, echinacea, ginkgo, ginseng, St. John’s wort, and “other” herbs (specifically, kava, valerian, black cohosh, bee pollen, and nettle).
MAIN RESULTS
Among 6,712 women ≥20 years, those using herbal supplements had lead levels that were 10% higher than non-users (95% CI 3%–17%, p = 0.005). Women using Ayurvedic or traditional Chinese medicine herbs, St. John’s wort, and “other” herbs had lead levels 24% (95% CI 5%–45%, p = 0.01), 23% (95% CI 4%–46%), p = 0.02), and 21% (95% CI 2%–44%, p = 0.03) higher, respectively, than non-users. No significant associations were observed between herb use and lead levels among men (n = 6,095). Among reproductive-aged women (16–45 years), herbal supplement users had lead levels 20% higher than non-users (95% CI 5%–34%, p = 0.008). In contrast, garlic and other dietary supplements were not associated with higher lead levels.
CONCLUSION
Use of specific herbal supplements is associated with higher blood lead levels among women. Our data suggest testing guidelines for herbal supplements and regulations limiting lead in supplements are needed.
KEY WORDS: herbs, Phytotherapy, Lead/analysis, Dietary supplements, medicine, Ayurvedic
BACKGROUND
Since the 1970s the removal of lead from gasoline, food cans, and paint products has dramatically decreased lead exposure in the US.1 Nevertheless, reducing lead exposure remains a public health priority given accumulating research demonstrating associations between low levels of lead exposure and health risks, including increased hypertension, kidney disease, peripheral arterial disease, cardiovascular disease, cancer, and death from all causes,2–7 with significant associations for myocardial infarction and stroke mortality evident at blood lead levels >2 mcg/dl.4 Among adults, the potential implications of low-level lead exposure are most relevant to women of child-bearing age, as lead is especially harmful to developing nervous systems of fetuses and children8 and passes through the placenta and breast milk.9 Blood lead levels mainly estimate recent exposures, but also reflect past exposures, as lead accumulates in bones and is mobilized into circulation during periods of increased bone turnover (particularly in women during periods of pregnancy, lactation, and menopause).10 Calcium deficiency increases lead absorption and lead retention,11–14 and is a risk factor for increased maternal lead transfer.15–16
The risk of lead exposure from dietary supplements has been recognized for several years.17 Lead in calcium supplements has been most publicized, especially supplements derived from natural sources, such as bone meal and dolomite.18 However, by 1996, reductions in environmental lead and processing improvements resulted in substantial declines in lead content in natural calcium supplements.19 Despite awareness that supplements could pose an avoidable lead exposure risk, they are subject to limited governmental regulation in the US, and the Dietary Supplement and Health Education Act (DSHEA) of 1994 does not require supplements be approved by the FDA as safe and efficacious prior to marketing.20 Of concern, recent studies have identified heavy metal contaminants in several commercially available herbal supplements, with lead the most common to be present in potentially harmful concentrations.21–24 In 2001, 50% of 38 Asian herbal remedies purchased in the US and >80% of 16 purchased in Southeast Asia and China were identified to have excess lead.23 In 2003 and 2005, one in five Ayurvedic herbal supplements purchased in Boston24 or over the Internet25 had excess heavy metal content. Third-party evaluations of several other herbal supplements sold throughout the US have also revealed the presence of high lead content.26
Given evidence that some herbs may contain high lead content, we sought to determine the effect of herbal supplement use on blood lead levels of adult US women and men. Using data from the National Health and Nutrition Examination Survey (NHANES), a representative sample of the civilian non-institutionalized US population, we investigated use of herbal supplements that had previously been found to contain high lead content. We defined high lead content to be >1.5 mcg/serving, based on the 1999 California criteria for acceptable lead levels of <1.5 mcg/serving of natural calcium supplement.27 We stratified the analysis by sex, given different potential health concerns related to low-level lead exposure and because men and women typically use different types of herbs,28 have different lead exposures,29 and may metabolize lead differently.29
METHODS
Study Population
From 1999–2004, 13,504 US adults ≥20 years participated in NHANES interviews, examinations, and had blood lead assessed. We excluded 697 with missing values in variables of interest, leaving 12,807 in the primary analysis. These analyses were approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigation.
Supplement Use
Supplement use was queried during household interviews. We examined any herbal supplements found to contain excess lead on prior studies or product testing available to the public, or that had been implicated in heavy metal poisonings.21,23–26,30–34 Specifically, the following were included: Ayurvedic and/or traditional Chinese medicine (TCM) herbs used as individual herbs (astralagus, dong quai, glycyrrhiza, green tea extract, guggul, medicago saliva, trigonella foenum-graecum, and turmeric) or as combination herb formulations (e.g., Sheng mai, a combination of 18 herbs and Ayurvedic Blood Sugar Formula, a combination of 12 herbs); ginkgo; ginseng (including Asian ginseng, American ginseng, and eleuthero, formerly called Siberian ginseng and frequently combined with other ginsengs); echinacea (with or without goldenseal); St. John’s wort; and “other” herbal supplements, specifically, kava, valerian, black cohosh, bee pollen, and nettle. We dichotomized use or nonuse of any of these herbal supplements during the last month and examined exposure by specific supplement type. To adjust for possible effects of calcium on lead levels, we included use of calcium (yes/no) taken as a dietary supplement or antacid.
To assess potential confounding by unmeasured factors related to general use of supplements, we performed secondary analyses testing whether dietary supplement use overall was associated with lead levels. We also explored associations between lead levels and use of garlic supplements (used by 2%), which have not been found to contain excess lead.26,34
Blood Lead Levels
Lead was measured in whole blood at the CDC by graphite furnace atomic absorption spectrophotometry using a PerkinElmer Model SIMAA 6000 simultaneous multi-element atomic absorption spectrometer with Zeeman background correction. Results below the lower detection limit (<0.3 mcg/dl) were replaced with a value equal to the detection limit divided by √2.
Other Variables
Age, sex, race/ethnicity, education, country of birth, income, housing built before 1978 (when lead-based paint was banned from US housing), smoking, health status, activity level, and using home water treatment devices were based on self-report. Race/ethnicity was classified as non-Hispanic white, non-Hispanic black, Mexican American, or other. Participants were classified as never, former, or current smokers. Body mass index (BMI) was calculated as the measured weight in kilograms/the square of the measured height in meters. For kidney function, we corrected serum creatinine values in NHANES 1999–2000 (creatinine = 0.147 + 1.013 × NHANES 1999–2000 uncalibrated creatinine) to account for differences between NHANES 1999–2000 and the 2001–2002 and 2003–2004 surveys.35 Glomerular filtration rate (eGFR) was estimated using the abbreviated Modification of Diet in Renal Disease Study formula based on creatinine, age, and race.36 We defined kidney function as normal, mildly decreased, and low according to eGFR >90 ml/min, 60–90 ml/min, and <60 ml/min, respectively.37 Folate was measured in serum using the Bio-Rad Laboratories “Quantaphase II Folate/vitamin B12”″ radioassay kit.
Statistical Analyses
Analyses were performed using SAS 9.1.3 (SAS Institute, Inc., Cary, NC) and SUDAAN 9.0 (Research Triangle Park, NC). Estimates were weighted to account for the unequal probabilities of selection resulting from the complex sample design, non-response, and planned over-sampling of selected populations.
Lead levels were skewed and were log transformed to improve normality. We performed sex-stratified linear regression to evaluate independent relations of herbal supplement use with the logarithmic blood lead levels. For these analyses we fit separate models for men and women using the same variables in each model. Based on a priori assumptions, we included the potential confounders: age, race/ethnicity, education, country of birth, income, home built before 1978, smoking status, activity level, general health status, BMI, renal function (eGFR), calcium supplement use, and water treatment devise use. Race/ethnicity, education, income, and country of birth were included in all models to control as thoroughly as possible for socioeconomic status. Because some did not report incomes or know when their home was constructed, we created missing categories for income [n = 889 (6.4%)] and year home was built [n = 2,778 (15.8%)]. Given particular concerns about prenatal lead exposure, we created additional models to assess herbal supplement use and blood levels among women of reproductive age (ages 16–45). The percent differences in lead levels were estimated as [(eβ) − 1] × 100%, where β is the estimated regression coefficient.
To assess modifying effects of herbal supplement use by sex on blood lead levels, we fit a model including both men and women in a single regression and used an interaction term [sex*(supplement)] to assess for statistical interaction. We fit separate regression models to examine interactions between supplement use and other factors (age, race/ethnicity, BMI, and renal function) on blood lead levels, incorporating interaction terms [(factor)*(supplement)] into the original models for men and women. In post hoc analyses, we also examined whether blood folate or iron levels influenced the results. The level of statistical significance for all results was considered p < 0.05.
RESULTS
Use of the specific herbal supplements was more common among those with greater education and income and participants who were non-Hispanic white, born in the US, 40–59 years, never/former smokers, more active, using a home-water-treatment device, and taking calcium supplements (Table 1). Blood lead levels >10 mcg/dl were rare (0.6% of the total sample).
Table 1.
Characteristics of Participants According to Use or Non-Use of the Specific Herbal Supplementsa Evaluated in this Study. Adults ≥20 Years, NHANES 1999–2004
| Users (N = 455)% | Non-users (N = 12.352)% | P-value | |
|---|---|---|---|
| Age, years | <0.001 | ||
| 20–39 | 28 | 40 | |
| 40–59 | 46 | 38 | |
| ≥60 | 26 | 22 | |
| Male | 49 | 48 | 0.89 |
| Education | <0.001 | ||
| < High school | 12 | 21 | |
| High school graduate | 20 | 26 | |
| > High school | 68 | 53 | |
| Race/ethnicity | <0.001 | ||
| Non-Hispanic white | 84 | 72 | |
| Non-Hispanic black | 6 | 10 | |
| Mexican American | 4 | 8 | |
| Other | 5 | 11 | |
| Smoking | 0.01 | ||
| Never | 48 | 50 | |
| Former | 33 | 25 | |
| Current | 18 | 25 | |
| Country of birth | <0.001 | ||
| US | 95 | 85 | |
| Mexico | 2 | 5 | |
| Other | 3 | 10 | |
| Income | <0.001 | ||
| <$20,000 | 15 | 24 | |
| $20,000 to <$45,000 | 30 | 28 | |
| ≥$45,000 | 48 | 42 | |
| Refused or unknown | 7 | 6 | |
| Year home built | <0.001 | ||
| 1978 or later | 38 | 35 | |
| Before 1978 | 55 | 50 | |
| Unknown | 8 | 15 | |
| Health status | 0.48 | ||
| Excellent/very good | 57 | 53 | |
| Good | 27 | 30 | |
| Fair/poor | 16 | 17 | |
| Physical activity | <0.001 | ||
| Sedentary | 24 | 37 | |
| Moderate | 34 | 29 | |
| Vigorous | 42 | 34 | |
| Estimated GFRb | 0.02 | ||
| <60 ml/min | 5 | 5 | |
| 60–90 ml/min | 50 | 43 | |
| >90 ml/min | 45 | 52 | |
| Home water treatment | 40 | 29 | <0.001 |
| Use of calcium supplement | 39 | 24 | <0.001 |
| Blood lead level | 0.49 | ||
| <2 mcg/dl | 62 | 64 | |
| 2 to <10 mcg/dl | 37 | 36 | |
| >10 mcg/dl | 0.8 | 0.6 |
aAyurvedic herbs, bee pollen, black cohosh, echinacea, ginkgo, ginseng, kava, nettle, Saint John’s wort, traditional Chinese medicine herbs, and/or valerian
bTo convert values from conventional units to SI units, multiply by the conversion factor: 0.0483 µmol/l. GFR, glomerular filtration rate
Table 2 shows overall 4.3% of the population (representing 7.5 million adults) used at least one of the specific herbal supplements included during the last month. Although overall prevalence of any specific herbal supplement use was similar in women and men, the types of supplements used differed substantially by sex. Among herbal supplement users, 28% of women and 31% of men had used >2 of the specific herbal supplements included in this study during the last month.
Table 2.
Prevalence (%) Use of Specific Herbal Supplements and Any Dietary Supplements During the Last Month Among Adult Participants ≥20 Years of Age. NHANES 1999–2004
| Supplement | Total % | Female % | Male % |
|---|---|---|---|
| Any specific herbal supplement | 4.3 | 4.2 | 4.3 |
| Ginkgo | 1.8 | 1.6 | 2.0 |
| Echinacea | 1.1 | 1.1 | 1.0 |
| Ginsenga | 0.9 | 0.6 | 1.3 |
| Saint John’s wort | 0.6 | 0.6 | 0.6 |
| Traditional Chinese medicine | 0.5 | 0.6 | 0.3 |
| and/or Ayurvedic herbs | |||
| “Other herbs”b | 0.6 | 0.8 | 0.4 |
| Any dietary supplementc | 54 | 60 | 47 |
aAsian ginseng, American ginseng and/or eleuthero (formerly called Siberian ginseng)
bKava, valerian, black cohosh, bee pollen and/or nettle
c Vitamins, minerals, herbs, and non-vitamin/non-mineral supplements
Table 3 shows geometric means of blood lead levels by participant characteristics. The highest lead levels were found among men, older subjects, current/former smokers, individuals born in Mexico, and those with lower education, lower or missing income, poorer kidney function, and poorer health.
Table 3.
Blood Lead Levels by Participant Characteristics. Adults ≥20 Years, NHANES 1999–2004
| Covariatesb | n | Total (n = 12,807) lead, mcg/dla | Female (n = 6,712) lead, mcg/dla | Male (n = 6,095) lead, mcg/dla | |||
|---|---|---|---|---|---|---|---|
| Geometric mean | SE | Geometric mean | SE | Geometric mean | SE | ||
| Overall | 12,807 | 1.61 | 0.02 | 1.31 | 0.02 | 2.00 | 0.03 |
| Age, years | |||||||
| 20–39 | 4,620 | 1.26 | 0.02 | 0.98 | 0.03 | 1.63 | 0.03 |
| 40–59 | 3,828 | 1.76 | 0.03 | 1.42 | 0.03 | 2.19 | 0.04 |
| ≥60 | 4,359 | 2.15 | 0.03 | 1.86 | 0.04 | 2.59 | 0.04 |
| Education | |||||||
| < High school | 4,080 | 2.02 | 0.03 | 1.62 | 0.04 | 2.56 | 0.04 |
| High school graduate | 3,044 | 1.67 | 0.03 | 1.35 | 0.03 | 2.10 | 0.05 |
| > High school | 5,683 | 1.45 | 0.02 | 1.20 | 0.02 | 1.79 | 0.03 |
| Race/ethnicity | |||||||
| Non-Hispanic white | 6,488 | 1.58 | 0.02 | 1.29 | 0.02 | 1.96 | 0.03 |
| Non-Hispanic black | 2,347 | 1.76 | 0.04 | 1.49 | 0.05 | 2.20 | 0.07 |
| Mexican American | 2,942 | 1.75 | 0.05 | 1.33 | 0.04 | 2.24 | 0.06 |
| Other | 1,030 | 1.59 | 0.05 | 1.31 | 0.05 | 1.99 | 0.06 |
| Smoking | |||||||
| Never | 6,577 | 1.36 | 0.02 | 1.18 | 0.03 | 1.67 | 0.03 |
| Former | 3,426 | 1.82 | 0.03 | 1.42 | 0.04 | 2.21 | 0.04 |
| Current | 2,804 | 2.00 | 0.04 | 1.60 | 0.04 | 2.39 | 0.05 |
| Country of birth | |||||||
| US | 9,905 | 1.57 | 0.02 | 1.29 | 0.02 | 1.96 | 0.03 |
| Mexico | 1,702 | 2.10 | 0.06 | 1.59 | 0.05 | 2.62 | 0.07 |
| Other | 1,200 | 1.74 | 0.05 | 1.45 | 0.04 | 2.12 | 0.07 |
| Income | |||||||
| <$20,000 | 3,997 | 1.76 | 0.04 | 1.49 | 0.04 | 2.24 | 0.05 |
| $20,000 to <$45,000 | 3,808 | 1.64 | 0.03 | 1.30 | 0.03 | 2.09 | 0.04 |
| ≥$45,000 | 4,175 | 1.48 | 0.02 | 1.18 | 0.02 | 1.82 | 0.02 |
| Refused or unknown | 827 | 1.88 | 0.05 | 1.56 | 0.05 | 2.33 | 0.11 |
| Year home built | |||||||
| 1978 or later | 3,685 | 1.46 | 0.03 | 1.19 | 0.03 | 1.83 | 0.04 |
| Before 1978 | 6,344 | 1.69 | 0.03 | 1.38 | 0.03 | 2.09 | 0.04 |
| Unknown | 2,657 | 1.71 | 0.04 | 1.40 | 0.04 | 2.15 | 0.05 |
| Health status | |||||||
| Excellent/very good | 5,871 | 1.52 | 0.03 | 1.23 | 0.03 | 1.89 | 0.03 |
| Good | 4,061 | 1.63 | 0.03 | 1.32 | 0.03 | 2.06 | 0.04 |
| Fair/poor | 2867 | 1.89 | 0.04 | 1.58 | 0.04 | 2.35 | 0.05 |
| Estimated GFR | |||||||
| <60 ml/min | 1,015 | 2.19 | 0.05 | 2.00 | 0.06 | 2.58 | 0.10 |
| 60–90 ml/min | 4,935 | 1.72 | 0.03 | 1.42 | 0.03 | 2.09 | 0.03 |
| >90 ml/min | 6,857 | 1.47 | 0.03 | 1.16 | 0.03 | 1.89 | 0.03 |
| Home water treatment | |||||||
| Yes | 3,182 | 1.50 | 0.03 | 1.22 | 0.03 | 1.85 | 0.04 |
| No | 9,625 | 1.66 | 0.02 | 1.35 | 0.03 | 2.07 | 0.03 |
| Use of calcium supplement | |||||||
| Yes | 2,793 | 1.51 | 0.03 | 1.31 | 0.03 | 1.95 | 0.04 |
| No | 10,014 | 1.64 | 0.02 | 1.31 | 0.02 | 2.02 | 0.03 |
GFR, glomerular filtration rate. SE, standard error
aTo convert values from conventional units to SI units, multiply by the conversion factor: 0.0483 µmol/l
bAll covariates examined in relationship to lead level were statistically significant at a p-value <0.05, with the exception of calcium supplement use among men and women
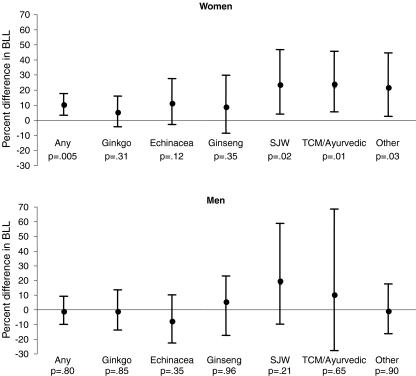
Figure 1 shows the estimated difference in blood lead level and 95% CIs associated with use of any specific herbal supplement included and with the types of herbal supplements used among women and men. Lead levels among women reporting any use of these herbal supplements were 10% (95% CI 4%–18%) higher compared to women non-users (p = 0.005). Statistically significant associations with higher lead levels were observed for use of St. John’s wort, TCM/Ayurvedic herbs, and “other” herbal supplements. Although similar patterns of association were seen among men, no statistically significant associations were observed, effects were less, and formal tests of interaction indicated a statistically significant difference in the estimated effect of herbal supplement use on lead levels in men compared to women (interaction term p-value = 0.045).
Figure 1.
Adjusted estimated difference and 95% CI in blood lead levels (BLL) among women and men ≥20 years by use of specific herbal supplements, ginkgo, echinacea, ginseng (Asian, American, or eleuthero), St. John’s wort (SJW), traditional Chinese medicine herbs (TCM) or Ayurvedic herbs, or “other” (kava, valerian, black cohosh, bee pollen, and nettle). Adjusted for age, race/ethnicity, educational levels, country of birth, income, year home was built, smoking status, BMI, renal function (estimated GFR), calcium supplement use, and use of a water treatment device.
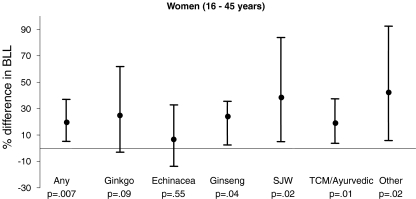
Figure 2 shows regression model results for reproductive-aged women (16–45 years). Among this group, women who used any of these herbal supplements had lead levels 20% (95% CI 5%–34%, p = 0.008) higher than women who did not.
Figure 2.
Adjusted estimated difference and 95% CI in blood lead levels (BLL) among women of child-bearing age by use of specific herbal supplements, ginkgo, echinacea, ginseng (Asian, American, or eleuthero), St. John’s wort (SJW), traditional Chinese medicine herbs (TCM) and/or Ayurvedic herbs, or “other” (kava, valerian, black cohosh, bee pollen, and nettle). Adjusted for age, race/ethnicity, educational levels, country of birth, income, year home built, smoking status, BMI, renal function (estimated GFR), calcium supplement use, and use of a water-treatment device.
We observed inverse relationships between calcium supplement use and lead levels. Among women, 31% used calcium supplements, and users had lead levels 7% lower than non-users (95% CI –10% – −3%, p = 0.0005); among men, 18% used calcium supplements and had lead levels 3% lower than non-users (95% CI –7% – 1%, p = 0.10).
Further adjustment for folate and iron levels did not materially alter the results in men or women. There were no statistically significant differences in the association with herbal supplements on lead levels across strata of age, race/ethnicity, renal function, or BMI.
When we examined use of other supplements, we found overall use of any dietary supplement was inversely associated with lead levels for women and men; however, the associations became non-significant when calcium was excluded. We found no significant association between lead levels and garlic supplement use.
DISCUSSION
To our knowledge, this study is the first showing associations between herbal supplement use and blood lead levels in US women. After controlling for multiple factors, lead levels of women users of specific herbal supplements were 10% higher than women nonusers. When we examined herbal supplement use among reproductive age women, the relationship with lead levels was even stronger, with lead levels 20% higher overall, up to 40% higher among users of select herbal supplements compared to non-users. These findings were not observed for other dietary supplements and were restricted to types of herbal supplements that have been implicated in heavy metal contamination.
Consistent with recent reports among US adults,38 mean blood lead levels were generally low for women (1.3 mcg/dl) and men (2.0 mcg/dl). Diet is the main lead exposure for most US adults and is estimated to be ~10 mcg/day.39 Consuming one supplement containing 1.5 mcg of lead represents 15% of this amount. The contribution would be greater for supplements containing more lead and when using multiple supplements (reported by 30%). Because adults absorb about 10% of ingested lead,40 among women, regular use of a supplement containing 1.5 mcg of lead/serving would be expected to increase lead levels by ~11%, which approximates the mean difference found in our study.
Our findings are similar to epidemiological studies in Taiwan,22,41 showing elevated blood lead levels in those who use TCM herbs compared to nonusers and a study in China showing increased lead in breast milk of mothers using Chinese medicine herbs compared with non-users.42 In contrast to prior studies, we observed no statistically significant associations in men. This should be interpreted with caution, as among men, use of some supplements was very low, and we observed wide confidence intervals around many effect estimates. Although we controlled for several factors, we lacked data on occupation; thus, some degree of unadjusted confounding is probable, particularly in men who are most apt to be employed in occupations with high lead exposure. Alternatively, differences in the impact of lead exposure could be explained by women having greater susceptibility. For example, compared to men and older women, young women appear to retain lead more avidly29 and have periods of increased nutrient demands (e.g., pregnancy and lactation). Increased lead absorption has been associated with lower iron stores and may occur before frank deficiency.43 Although we did not see an association between lead and circulating iron (measured by serum iron), inclusion of ferritin (a better measure of iron stores) in future studies would be useful to explore if higher iron requirements of childbearing women might explain observed differences between women of childbearing age and older women or men. Finally, growing literature suggests that genetic polymorphisms that may modify lead kinetics 44,45 play a greater role in women compared to men.46
We adjusted for calcium supplement use both because some calcium products may contain excess lead47 and prior studies suggest an inverse correlation between calcium and lead levels.16,48–50 Our findings support clinical trial evidence demonstrating a protective effect of calcium supplements on lead levels in lactating women49 and suggests the effect is not limited to this group alone. It is hypothesized that this effect is likely due to suppression of lead absorption in the gut and suppression of bone turnover.15
We considered whether higher lead levels might be associated with herbs not included in our study or with less well-defined factors related to using any dietary supplement. We did not observe higher lead levels among garlic supplement users or among dietary supplement users in general, lending support to the specificity of the association between specific herbal supplement use and blood lead levels.
Lead is readily taken up from soil by plants.51,52 Soil and air pollution influences herb lead content.53 It is possible that excess lead found in some herbs may, in part, be due to herbs grown in less-regulated countries, such as China and India, which are major exporters of raw plant products for the supplement industry.54 Alternatively, uncontaminated herbs could acquire lead during manufacturing due to contaminated water, equipment, pipes, or storage.19 Saper et al. found US-manufactured Ayurvedic supplements contained a median lead content of 7.5 mcg/g compared to 11.0 mcg/g for Indian-manufactured supplements,25 demonstrating that choosing only US-manufactured supplements may decrease, but not assure a lead-free supplement, suggesting the problem is likely due to multiple factors.
The study does not allow determination of causality, many variables were self-reported, and the use of some herbal supplements was low, which consequently limited our power to detect associations among specific herbal supplements, particularly among men who infrequently used some herbs showing the strongest associations among women. Differences in use of “Ayurvedic/TCM” and “Other” herbs could be because of higher use among women for menstrual and menopausal symptoms.55
Unfortunately, we lacked information on frequency and total duration of supplement use. This information and additional information on occupational and non-occupational lead exposures, such as urban living, home renovations, and hobbies, would be of interest for future studies. In this study, we restricted our examination of herbal supplements to those that had previously been reported to contain high levels of lead. However, not all supplements have been analyzed for lead content, and it is possible that some with excess lead are missing from our study. Some forms of herbs may have been missed as participants were queried about “dietary supplement” use and may not have reported herbs purchased as raw herb mixtures, whole herbs, or teas, which are not labeled as dietary supplements and may be regarded as traditional foods or medicine.
We lacked sufficient information to assess supplements according to source and lacked power to assess them according to different brands, which is of interest for future studies, as the quality of a supplement may vary according to herb source and manufacturer. Finally, the NHANES is conducted only in English and Spanish and may not represent recent immigrants from other countries living in the US, who may be disproportionably affected both by higher lead levels56 and by lead poisonings due to use of folk herbal remedies.57
Our observations suggest that among women, including women of reproductive age, specific herbal supplement use is a significant contributor to circulating lead. At present, there are limited data supporting the clinical efficacy of herbal supplements included in this study.58–62 Unless health benefits can be clearly demonstrated by well-designed, well-executed studies, our findings suggest that the potential for harm from use of some herbal supplements may exceed the benefit. Unfortunately, US consumers are often misinformed about the regulation, safety, and effectiveness of dietary supplements.63 Moreover, 37% of physicians surveyed across the US were unaware that supplements do not require FDA pre-market approval for safety and efficacy,64 indicating increased training and CME on this topic are needed. It is hoped that the FDA’s current good manufacturing practices (CGMPs), which by 2010 require all manufacturers to evaluate supplement identity, purity, strength, and composition, will lead to safer supplements. However, CGMPs are based primarily on industry self-regulation and do not address premarket proof of safety or efficacy. Furthermore, without increased resources, the FDA is unlikely to have the capacity to widely inspect or enforce these rules.
Acknowledgments
This work was presented in part at the Society of General Internal Medicine 31st Annual Meeting April 9–12, 2008. No internal or external funding supported this research project.
Conflict of Interests None of the authors have conflicts of interest, financial interests, or relationships or affiliations relevant to the subject matter or materials discussed in this manuscript.
References
- 1.Pirkle JL, Brody DJ, Gunter EW, et al. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA. 1994;272(4):284–91. [DOI] [PubMed]
- 2.Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int. 2006;70(12):2074–84. [DOI] [PubMed]
- 3.Jain NB, Potula V, Schwartz J, et al. Lead levels and ischemic heart disease in a prospective study of middle-aged and elderly men: the VA Normative Aging Study. Environ Health Perspect. 2007;115(6):871–5. [DOI] [PMC free article] [PubMed]
- 4.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/l (10 microg/dl) and mortality among US adults. Circulation. 2006;114(13):1388–94. [DOI] [PubMed]
- 5.Muntner P, He J, Vupputuri S, Coresh J, Batuman V. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003;63(3):1044–50. [DOI] [PubMed]
- 6.Nash D, Magder L, Lustberg M, et al. Blood lead, blood pressure, and hypertension in perimenopausal and postmenopausal women. JAMA. 2003;289(12):1523–32. [DOI] [PubMed]
- 7.Park SK, Schwartz J, Weisskopf M, et al. Low-level lead exposure, metabolic syndrome, and heart rate variability: the VA Normative Aging Study. Environ Health Perspect. 2006;114(11):1718–24. [DOI] [PMC free article] [PubMed]
- 8.Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20(2):172–7. [DOI] [PubMed]
- 9.Carrington CD, Bolger PM. An assessment of the hazards of lead in food. Regul Toxicol Pharmacol. 1992;16(3):265–72. [DOI] [PubMed]
- 10.Gomaa A, Hu H, Bellinger D, et al. Maternal bone lead as an independent risk factor for fetal neurotoxicity: a prospective study. Pediatrics. 2002;110(1 Pt 1):110–8. [DOI] [PubMed]
- 11.DeMichele S. Nutrition of lead. Comp Biochem Physiol A Comp Physiol. 1984;78(3):401–8. [DOI] [PubMed]
- 12.Heard MJ, Chamberlain AC. Effect of minerals and food on uptake of lead from the gastrointestinal tract in humans. Hum Toxicol. 1982;1(4):411–5. [DOI] [PubMed]
- 13.Six KM, Goyer RA. Experimental enhancement of lead toxicity by low dietary calcium. J Lab Clin Med. 1970;76(6):933–42. [PubMed]
- 14.Cheng Y, Willett WC, Schwartz J, Sparrow D, Weiss S, Hu H. Relation of nutrition to bone lead and blood lead levels in middle-aged to elderly men. The Normative Aging Study. Am J Epidemiol. 1998;147(12):1162–74. [DOI] [PubMed]
- 15.Ettinger AS, Hu H, Hernandez-Avila M. Dietary calcium supplementation to lower blood lead levels in pregnancy and lactation. J Nutr Biochem. 2007;18(3):172–8. [DOI] [PMC free article] [PubMed]
- 16.Hernandez-Avila M, Gonzalez-Cossio T, Palazuelos E, et al. Dietary and environmental determinants of blood and bone lead levels in lactating postpartum women living in Mexico City. Environ Health Perspect. 1996;104(10):1076–82. [DOI] [PMC free article] [PubMed]
- 17.Crosby WH. Lead-contaminated health food: the tip of an iceberg. JAMA. 1977;238(14):1544. [DOI] [PubMed]
- 18.Roberts HJ. Potential toxicity due to dolomite and bonemeal. South Med J. 1983;76(5):556–9. [DOI] [PubMed]
- 19.Scelfo GM, Flegal AR. Lead in calcium supplements. Environ Health Perspect. 2000;108(4):309–19. [DOI] [PMC free article] [PubMed]
- 20.Dietary Supplement Health and Education Act of 1994. Public Law; 1994:103–417.
- 21.Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci. 2002;23:136–9. [DOI] [PubMed]
- 22.Ernst E. Risks of herbal medicinal products. Pharmacoepidemiol Drug Saf. 2004;13(11):767–771. [DOI] [PubMed]
- 23.Garvey GJ, Hahn G, Lee RV, Harbison RD. Heavy metal hazards of Asian traditional remedies. Int J Environ Health Res. 2001;11(1):63–71. [DOI] [PubMed]
- 24.Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, Phillips RS. Heavy metal content of ayurvedic herbal medicine products. JAMA. 2004;292(23):2868–73. [DOI] [PubMed]
- 25.Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. 2008;300(8):915–23. [DOI] [PMC free article] [PubMed]
- 26.ConsumerLabs.com. Product Tests. Available at http://www.consumerlab.com/results/index.asp (date accessed 6/5/2009).
- 27.California Attorney General’s Office. Superior Court Settlement No. 984503. San Francisco, CA, 15 May 1997.
- 28.Kelly JP, Kaufman DW, Kelley K, Rosenberg L, Anderson TE, Mitchell AA. Recent trends in use of herbal and other natural products. Arch Intern Med. 2005;165(3):281–6. [DOI] [PubMed]
- 29.Popovic M, McNeill FE, Chettle DR, Webber CE, Lee CV, Kaye WE. Impact of occupational exposure on lead levels in women. Environ Health Perspect. 2005;113(4):478–84. [DOI] [PMC free article] [PubMed]
- 30.FDA Enforcement Report. Recalls and Field Corrections: Foods - Class II (Nutritional supplements containing propolis, Bee Pollen). 94 52.: Food and Drug Administration; 1994. Available at http://www.fda.gov/bbs/topics/ENFORChttp://74.125.95.132/search?q=cache:TzCtjo1SMGYJ:www.fda.gov/bbs/topics/ENFORCE/ENF00357.html+bee+pollen+and+fda+recall&cd=3&hl=en&ct=clnk&gl=us&client=firefox-a (date accessed 6/5/2009).
- 31.Dolan SP, Nortrup DA, Bolger MP, Capar SG. Analysis of dietary supplements for arsenic, cadmium, mercury, and lead using inductively coupled plasma mass spectrometry. J Agric Food Chem. 2003;51:1307–1312. [DOI] [PubMed]
- 32.Ernst E, Thompson Coon J. Heavy metals in traditional Chinese medicines: a systematic review. Clin Pharmacol Ther. 2001;70(6):497–504. [DOI] [PubMed]
- 33.Ernst E. Ayurvedic medicines. Pharmacoepidemiol Drug Saf. 2002;11(6):455–6. [DOI] [PubMed]
- 34.Raman P, Patino L, Nair M. Evaluation of metal and microbial contamination in botanical supplements. J Agric Food Chem. 2004;52:7822–7. [DOI] [PubMed]
- 35.NHANES 1999–2000 Data Documentation (Revised August 2006). Available at http://www.cdc.gov/nchs/data/nhanes/frequency/lab18_doc.pdf (date accessed 6/5/2009).
- 36.Levey AS, Coresh J, Greene T, et al. Expressing the MDRD study equation for estimating GFR with IDMS traceable (gold standard) serum creatinine values. J Am Soc Nephrol. 2005;16:69A.
- 37.Levey AS, Coresh J, Balk E, National Kidney Association, et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed]
- 38.Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165(18):2155–61. [DOI] [PubMed]
- 39.Review of Adult Lead Models: Evaluation of Models for Assessing Human Health Risks Associated with Lead Exposures at Non-Residential Areas of Superfund and Other Hazardous Waste Sites. Adult Lead Risk Assessment Committee of the Technical Review Workgroup for Lead; 2001. Available at www.epa.gov/superfund/lead/products/adultreview.pdf (date accessed 6/5/2009).
- 40.Toxicological profile for lead. Agency for Toxic Substances and Disease Registry, Department of Health and Human Services. 2005a. Available at www.atsdr.cdc.gov/toxprofiles/tp13.pdf (date accessed 6/5/2009).
- 41.Chu NF, Liou SH, Wu TN, Ko KN, Chang PY. Risk factors for high blood lead levels among the general population in Taiwan. Eur J Epidemiol. 1998;14:775–781. [DOI] [PubMed]
- 42.Chien LC, Yeh CY, Lee HC, Jasmine Chao H, Shieh MJ, Han BC. Effect of the mother’s consumption of traditional Chinese herbs on estimated infant daily intake of lead from breast milk. Sci Total Environ. 2006;354(2–3): 120–6. [DOI] [PubMed]
- 43.Levander OA. Lead toxicity and nutritional deficiencies. Environ Health Perspect. 1979;29:115–125. [DOI] [PMC free article] [PubMed]
- 44.Schwartz BS, Stewart WF, Kelsey KT, et al. Associations of tibial lead levels with BsmI polymorphisms in the vitamin D receptor in former organolead manufacturing workers. Environ Health Perspect. 2000;108(3):199–203. [DOI] [PMC free article] [PubMed]
- 45.Wetmur JG. Influence of the common human delta-aminolevulinate dehydratase polymorphism on lead body burden. Environ Health Perspect. 1994;102(Suppl 3):215–9. [DOI] [PMC free article] [PubMed]
- 46.Bjorkman L, Vahter M, Pedersen NL. Both the environment and genes are important for concentrations of cadmium and lead in blood. Environ Health Perspect. 2000;108(8):719–22. [DOI] [PMC free article] [PubMed]
- 47.Heaney RP. Lead in calcium supplements: cause for alarm or celebration? JAMA. 2000;284(11):1432–3. [DOI] [PubMed]
- 48.Gulson BL, Mizon KJ, Palmer JM, Korsch MJ, Taylor AJ, Mahaffey KR. Blood lead changes during pregnancy and postpartum with calcium supplementation. Environ Health Perspect. 2004;112(15):1499–507. [DOI] [PMC free article] [PubMed]
- 49.Hernandez-Avila M, Gonzalez-Cossio T, Hernandez-Avila JE, et al. Dietary calcium supplements to lower blood lead levels in lactating women: a randomized placebo-controlled trial. Epidemiology. 2003;14(2):206–12. [DOI] [PubMed]
- 50.Mahaffey KR, Gartside PS, Glueck CJ. Blood lead levels and dietary calcium intake in 1- to 11-year-old children: the Second National Health and Nutrition Examination Survey, 1976 to 1980. Pediatrics. 1986;78(2):257–62. [PubMed]
- 51.Depieri LA, Buckley WT, Kowalenko CG. Cadmium and lead concentrations of commercially grown vegetables of soils in the Lower Fraser Valley of British-Columbia. Can J Soil Sci. 1997;77:51–7.
- 52.Zurera G, Estrada F, Rincon F, Pozo R. Lead and cadmium contamination levels in edible vegetables. Bull Environ Contam Toxicol. 1987;38:805–812. [DOI] [PubMed]
- 53.Baranowska I, Srogi K, Wlochowicz A, Szczepanik K. Determination of heavy metal contents in samples of medicinal herbs. Pol J Environ Stud. 2002;11(5):467–471.
- 54.Marley WF, Thomas EG. The plant-derived chemicals marketplace. Business Economics. 1999;34(4):63–67.
- 55.Dennehy CE. The use of herbs and dietary supplements in gynecology: an evidence-based review. J Midwifery Womens Health. 2006 Nov–Dec;51(6):402–9. [DOI] [PubMed]
- 56.Trepka MJ. Using surveillance data to develop and disseminate local childhood lead poisoning screening recommendations: Miami-Dade County’s experience. Am J Public Health. 2005;95(4):556–8. [DOI] [PMC free article] [PubMed]
- 57.Lead poisoning associated with ayurvedic medications-five states, 2000–2003. MMWR Morb Mortal Wkly Rep. Jul 9, 2004 ed. Vol. 53: Center for Disease Control and Prevention:582–584. [PMC free article] [PubMed]
- 58.Linde K, Mulrow CD, Berner M, Egger M. St John’s wort for depression. Cochrane Database Syst Rev. 2005(2):CD000448. [DOI] [PubMed]
- 59.Melchart D, Wagner H, Hager S, Saller R, Ernest E. Quality assurance and evaluation of Chinese medicinal drugs in a hospital of traiditional Chinese medicine in Germany: a 5-year report. Altern Ther. 2001;7:S24.
- 60.Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2007(2):CD003120. [DOI] [PubMed]
- 61.Buettner C, Yeh GY, Phillips RS, Mittleman MA, Kaptchuk TJ. Systematic review of the effects of ginseng on cardiovascular risk factors. Ann Pharmacother. 2006;40(1):83–95. [DOI] [PubMed]
- 62.Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med. 2005;2(4):465–73. [DOI] [PMC free article] [PubMed]
- 63.New York State Task Force on Life and the Law. Dietary Supplements - Balancing Consumer Choice and Safety. October, 2005. http://www.health.state.ny.us/regulations/task_force/docs/dietary_supplement_safety.pdf (date accessed 6/5/2009).
- 64.Ashar BH, Rice TN, Sisson SD. Physicians’ understanding of the regulation of dietary supplements. Arch Intern Med. 2007;167(9):966–9. [DOI] [PubMed]