Abstract
We treated patients with C-viral chronic hepatitis (CH) and liver cirrhosis (LC) with polaprezinc and determined prospectively the effect on long-term outcome. 62 patients were enrolled. Of these, 32 were administered 1.0 g polaprezinc and the remainder were not administered polaprezinc. We measured the serum zinc concentrations using conventional atomic absorption spectrometry and conducted a prospective study to determine the long-term outcome of the polaprezinc therapy. Changes of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in the polaprezinc administration group were significantly lower than those of the untreated group. The decrease in platelet count was clearly less than that of the untreated group. The factors that inhibited increases in serum zinc concentrations following administration of polaprezinc included low serum zinc concentration states. Furthermore, the reductions of AST and ALT levels in the low zinc group were significantly greater than those of the high zinc group. When the patients who were administered polaprezinc were divided into two groups whose zinc concentrations increased (zinc responders) or remained stable or decreased (zinc non-responders), the zinc responders had a clearly lower cumulative incidence of HCC than the zinc non-responders. We conclude zinc supplementation improved the long-term outcome in C-viral CH and LC patients.
Keywords: polaprezinc, zinc supplementation, chronic hepatitis C, liver cirrhosis, hepatocellular carcinoma (HCC)
Introduction
Zinc is an essential trace element in the human body, with approximately two grams in healthy adults. The daily amount of zinc required by an adult is 10–15 mg and this is absorbed primarily from the upper gastrointestinal tract, especially the small intestine [1, 2]. Zinc is involved in the activation of approximately 300 different metallo-enzymes and metal-activated enzymes in vivo and is regarded as essential for the metabolism of nucleic acids and proteins [3, 4]. Therefore, it has been determined that zinc deficiency causes various pathological conditions in humans. Among these, it is known that, in patients with C-viral chronic liver disease, the blood zinc concentration decreases with progression of the disease from chronic hepatitis (CH) to compensated liver cirrhosis (LC), to decompensated LC, to hepatocellular carcinoma (HCC) [5–9]. It is also known that patients with liver failure or HCC are in an especially severe state of zinc deficiency and the liver damage is found to improve with zinc supplementation [10, 11]. Recently, it was reported that the hepatitis C virus (HCV) NS5A protein is a zinc metalloprotein and that zinc is closely involved in the activation of the NS5A protein [12]. Also, it has been reported that the rate of HCV eradication is higher when interferon (IFN) therapy for C-viral CH is combined with zinc supplementation, compared to IFN therapy alone [5, 13]. Thus, it would appear that zinc supplementation has a clear influence on the clinical profiles of C-viral CH or LC. However, there has been no report to date as to what influence zinc supplementation has on the long-term outcome of C-viral CH or LC. We gave polaprezinc (orally administered, 150 mg/day) to patients with C-viral CH or LC and studied prospectively the influence of zinc supplementation on the long-term outcome.
Patients and Methods
Patients
A total of 70 Japanese patients with CH or LC, who were examined by the first author at Nihon University Itabashi Hospital from September 1999 through January 2001, gave informed consent to their participation in this study. All of the patients were positive for serum HCV RNA (Amplicor HCV Monitor, Roche Diagnostic K.K., Tokyo, Japan) and were observed for more than three years. All were negative for serum hepatitis B surface antigen (HBsAg, enzyme-linked immunosorbent assay, EIA, Dinabot, Tokyo, Japan), LE cells, anti-smooth muscle antibody (fluorescence antibody method, FA), and anti-mitochondria antibody (FA). No heavy drinkers (more than 30 g ethanol intake per day) were included in the study. Patients whose blood alanine aminotransferase (ALT) levels remained persistently in the abnormal range (>40 international units; IU/L) for more than 6 months were enrolled in the study. The criteria for diagnosing patients as having LC were as follows: continuation of abnormal blood ALT levels for more than 6 months, ICGRs of more than 10% at 15 min, platelet counts below 100,000/mm3, the presence of esophageal varices, and the presence of LC pattern and splenomegaly on abdominal diagnostic imaging. Blood samples were obtained only from patients who gave informed consent. The follow-up schedule was as follows: the patients underwent abdominal ultrasonography every 3 or 6 months, and abdominal CT examination every 6 to 12 months, for the detection of HCC. When space occupying lesions (SOL) were detected in the livers of patients with CH or LC by dynamic CT during the time periods stated above, enhancement of SOL was observed at the early phase of dynamic CT and the disappearance of SOL staining was observed at the late phase. A precise diagnosis was made by abdominal angiography. When SOL in the liver were not enhanced in the early phase of dynamic CT, or if a precise diagnosis by abdominal angiography could not be made, tumor biopsy was carried out and a precise diagnosis was made on the basis of the pathological findings. Blood samples were collected on this occasion and stored frozen at −80°C.
Study design
According to the χ2 test, when the event occurrence rate of the control group is set to 30% and that of the treated group is set to 10%, the number of cases required to obtain a significant difference between the event occurrence rates is 62. Therefore, we planned to study 35 cases in the treated group and 35 cases in the untreated group. The HCV RNA positive patients who visited first author’s outpatient clinic between September 1999 and January 2001 numbered 175. Among 85 of these patients, it was estimated that 5 were asymptomatic HCV carriers, 50 had achieved ALT levels continuously within the normal range following medication, 20 had been treated with IFN alpha, and 10 diagnosed as HCC. The remaining 90 patients (51.4%) were the target population for this study; they had declined to give their consent for IFN therapy or had been treated but achieved only low sustained virologic response (SVR) rates because of refractory chronic hepatitis C, or IFN therapy was contraindicated because of complications such as depression, interstitial pneumonia, renal dysfunction, cardiovascular disease, etc., or they did not wish to receive IFN administration because of the relatively high frequency of complications and side effects of IFN or ribavirin therapy. Furthermore, IFN therapy is not indicated by national insurance criteria for patients diagnosed with LC in Japan. The reason we selected a study population who had not undergone IFN therapy was as follows: because IFN therapy may lead to a reduction in serum zinc concentrations or may affect the absorption of zinc from the small intestine, it was thought possible that IFN therapy could influence the serum zinc concentration in treated patients.
The patients who were treated with polaprezinc gave their consent to administration of the drug after the benefits and potential risks had been explained to them. Furthermore, the benefits and potential risks of administration of Rebamipide (Mucosta; Otsuka pharmaceutical, Osaka, Japan) and Teprenone (Selbex; Eizai, Tokyo, Japan) also were explained to them. These patients were randomized following a complete explanation of the purpose of the study, namely to determine whether administration of polaprezinc or other drugs modified the clinical profiles in the subjects and other effects of zinc supplementation. The patients who agreed to take polaprezinc or the other drugs were permitted to choose which drug they preferred. Therefore, we administered polaprezinc to patients with CH or LC who opted for zinc supplementation after detailed explanation and we administered the other drugs to patients with CH or LC who chose not to take zinc supplementation. No consent to administration of these drugs was obtained from 10 patients. In the final analysis, there were 36 treated and 34 untreated cases. All 70 patients gave their consent to participate in the study. The study period was planned initially to continue for three years from when observations commenced. Among the 70 patients, three with CH and one with LC stopped visiting the clinic during the three year period. Furthermore, during the study period, IFN therapy was initiated in four patients at their request. Therefore, in the final analysis, there were 32 patients in the polaprezinc treated group and 30 in the untreated group. The clinical background factors of the patients are shown in Table 1a, which compares the clinical background factors between patients with CH and LC, and Table 1b, which compares the clinical background factors between patients with and without zinc supplementation. The primary end points were set as aspartate aminotransferase (AST), ALT and HCV RNA levels, platelet counts, and the occurrence of HCC. For the C-viral CH and LC patients, the initial collection of blood was made on the day of registration. The second collection of blood was made one year later and the third, within the following two years. We measured the zinc concentrations of these samples and investigated the changes in zinc concentrations from the day of registration. This study was in a accordance with the ethical standards as formulation in the Helsinki Declaration of 1975.
Table 1a.
Comparison of clinical characteristics between patients with chronic hepatitis and liver cirrhosis
(n = 62)
| Parameter | Chronic hepatitis | Liver Cirrhosis |
|---|---|---|
| Number | 48 (77.4%) | 14 (22.6%) |
| Gender (male) | 33 (68.8%) | 5 (35.7%) |
| Age | 59.0 (26–72) | 62.5 (45–74) |
| ASTa (U/L) | 54.0 (25–131) | 78.0 (46–138) |
| ALTb (U/L) | 73.0 (27–231) | 83.5 (36–201) |
| Platelet count (×10−4) | 17.3 (13–26.4) | 9.75 (6.3–12.6) |
| HCV genotype | ||
| 1b | 38 (79.2%) | 14 (100%) |
| 2a | 7 (14.6%) | 0 |
| 2b | 3 (6.3%) | 0 |
| HCV RNA level (kiuc/ml) | 480.5 (8.4–850) | 725.0 (7.4–850) |
| Observation period (years) | 5.04 (3.17–6.4) | 4.6 (3.32–5.43) |
* Median (range). a AST, Aspartate aminotransferase; the upper limit of the normal range is 38 IU per liter. b ALT, Alanine aminotaransferase; the upper limit of the normal range is 44 IU per liter. c Kilointernational unit.
Table 1b.
Comparison of clinical characteristics between patients with and without zinc supplementation.
(n = 62)
| Parameter | Promac administrations | Controls |
|---|---|---|
| Number | 30 | 32 |
| Chronic hepatitis | ||
| Liver cirrhosis | ||
| Gender (male) | 33 (68.8%) | 5 (35.7%) |
| Age | 55.9 (26–72) | 61.4 (46–74) |
| AST (U/L) | 61.0 (40–118) | 82.1 (46–138) |
| ALT (U/L) | 86.3 (41–231) | 93.39 (45–201) |
| Platelet count (×10−4) | 18.2 (13–26.4) | 8.9 (6.3–10.0) |
| HCV | ||
| 1b | 38 (79.1%) | 14 (100%) |
| 2a | 7 (14.6%) | 0 |
| 2b | 3 (6.3%) | 0 |
| HCV RNA level (kiu/ml) | 499.6 (8.4–850) | 576.0 (7.4–850) |
| Observation period (years) | 5.04 (3.12–5.46) | 4.59 (3.05–5.43) |
Administration of polaprezinc
1.0 g Promac® (ZERIA Pharmaceutical Co., Ltd., Tokyo, Japan) containing 150 mg polaprezinc was administered orally twice daily: after breakfast and after dinner. Medications administered prior to the study were continued during the study. Patients whose serum zinc concentrations had increased by more than 5.0 µg/ml three years after the study began, compared to the start of the study, were classified as zinc responders, while those whose zinc concentrations remained within 5.0 µg/ml were zinc non-responders (stable), and those whose zinc concentrations decreased more than 5.0 µg/ml were zinc non-responders (decreased).
Measurement of serum zinc concentrations
Serum zinc concentrations were evaluated using conventional atomic absorption spectrometry using a Z-6100 polarized Zeeman Atomic Absorption Spectrophotometer (HITACHI, Tokyo, Japan) within 48 h of collection of blood [14, 15]. Silicone-coated syringes and needles were used for collecting the blood, which was transferred into zinc-free test tubes. Serum separation was done carefully to avoid haemolysis.
Haematological and biochemical examinations
Serum levels of AST, levels of ALT, platelet counts and HCV RNA concentrations were determined once every two months. Determination of HCV RNA concentrations was performed using the Amplicor monitor method (Amplicor HCV Monitor, Roche Diagnostic K.K.). The results of HCV RNA testing were classified into low and high groups according to whether the value was greater or less than 100 kilo international units (kiu)/ml. Determination of HCV genotype was performed by the method of Okamoto et al. [16] and the results were interpreted according to the classification of Simmonds et al. [17].
Long-term outcome of patients
We compared the long-term outcome of patients with CH or LC in terms of the cumulative probability of occurrence of HCC, according to whether they were treated with polaprezinc or untreated and according to changes in serum concentrations of zinc.
Statistical analysis
The serum concentrations of zinc were compared using the chi-square test for independence. Cumulative incidence curves were determined with the Kaplan-Meier method and the differences between groups were assessed using the log-rank test. The remaining parameters were compared using analysis of variance and Fisher’s Protected Least Significant difference post hoc test with Statview 4.5 software (Abacus Concepts, Berkeley, CA). A p value of less than 0.05 was considered significant.
Multivariate regression analysis
Other variables among the 32 cases were investigated as risk factors for occurrence of HCC. Independent factors of sex, age, CH or LC, ALT level, AST level, platelet count, HCV RNA concentration (more than 100 kiu/ml vs less than 100 kiu/ml), HCV genotype (genotype 1 vs genotype 2), and changes in zinc concentration were identified by logistic regression analysis using a stepwise method of analysis of factors for the risk of developing HCC using SPSS 11.0 software (SPSS Inc., Chicago, IL). Furthermore, we investigated the factors at the start of the study that contributed to whether patients administered polaprezinc become zinc responders. Independent factors of sex, age, CH or LC, ALT level, AST level, platelet count, HCV RNA concentration (more than 100 kiu/ml vs less than 100 kiu/ml), HCV genotype (genotype 1 vs genotype 2), and serum zinc concentration were identified by the Cox proportional hazard model using a stepwise method of analysis of factors for the risk of developing HCC using SPSS11.0 software (SPSS Inc.).
Results
Evaluation of zinc concentrations in C-viral chronic liver disease
In patients with C-viral chronic liver diseases, the median zinc concentration was 65.0 µg/dl (range; 50–109) in the CH group and 53.0 µg/dl (29–91) in the LC group. The median zinc concentration in the LC group was significantly lower than that in the CH group (p = 0.0006). The median zinc concentration of the polaprezinc administration group was 64.0 µg/ml (range; 41–88) and of the untreated group was 53.5 µg/dl (range; 35–68).
Efficacy of administration of polaprezinc
We examined changes in serum zinc concentrations in patients divided into the polaprezinc administration group and untreated group. In the polaprezinc administration group, compared to the initial examination, 15/32 (46.9%) patients were zinc responders, 12/32 (37.5%) were zinc non-responders whose serum zinc concentrations were stable and 5/32 (15.6%) were zinc non-responders whose serum zinc concentrations decreased. On the other hand, in the untreated group compared to the initial examination, the serum zinc concentrations of 1/30 (3.3%) patients had increased at three years, in 14/30 (46.7%) they remained stable and in 15/30 (50%) they decreased. In this analysis, the proportion of patients whose serum zinc concentrations increased was significantly greater in the polaprezinc administration group than the untreated group (p<0.0001), and the proportion of patients whose serum zinc concentrations decreased in the untreated group was significantly greater than in the polaprezinc administration group (p<0.0001).
Next, when the polaprezinc administration group was divided into two groupsaccording to the median zinc concentration at start of the study, a high zinc group (≥64 µg/dl, n = 17) and a low zinc group (<64 µg/dl, n = 15), the efficacy of polaprezinc administration in the low zinc group was 11/15 (73.4%) for zinc responders, 4/15 (13.3%) for zinc non-responders whose serum zinc concentrations were stable and 2/15 (13.3%) for zinc non-responders whose serum zinc concentrations decreased. On the contrary, the efficacy of polaprezinc administration in the high zinc group was 4/17 (23.5%) for zinc responders, 10/17 (58.8%) for zinc non-responders whose serum zinc concentrations were stable and 3/17 (17.7%) for zinc non-responders whose serum zinc concentrations decreased.
Comparison of changes of zinc concentrations among patients with or without polaprezinc administration
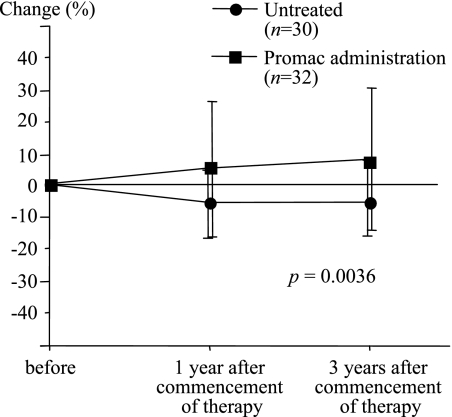
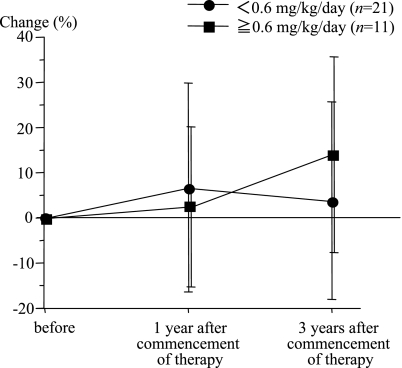
The changes of serum zinc concentrations in the polaprezinc administration group and the untreated group were compared. The results showed that in the polaprezinc administration group three years after the study started, the serum zinc concentrations had increased by about 10% compared to the level at the start of the study. However, in the untreated group the serum zinc concentrations had decreased by about 5% three years after the start of the study, compared to the level at the start of the study (Fig. 1). The polaprezinc administration group showed a significant increase in serum zinc concentrations compared to the untreated group (p = 0.0036). Then, the changes in the serum zinc concentrations relative to the daily dose of zinc administered per kg body weight were compared. In the group of patients for whom the daily dose of zinc administered was less than 0.6 mg/kg, the serum zinc concentrations increased during the early period of administration from the level prior to administration but there was a tendency to decrease later. However, in the group for whom the daily dose of zinc was more than 0.6 mg/kg, the serum zinc concentrations showed a tendency to increase from one year after the start of administration (Fig. 2).
Fig. 1.
Changes in serum zinc concentrations in patients with C-viral chronic hepatitis (CH) and liver cirrhosis (LC), with and without zinc administration. The median serum zinc concentration in the group of patients who had been administered polaprezinc was significantly higher than that of the untreated group (p = 0.0036).
Fig. 2.
The changes in the serum zinc levels were compared according to the quantity of polaprezinc administered per kg body weight. In the group administered less than 0.6 mg/kg zinc, the serum zinc level increased above that before the start of administration during the early stage of administration, but decreased later. However, in the group administered more than 0.6 mg/kg, the serum zinc level increased continuously from one year after the start of administration.
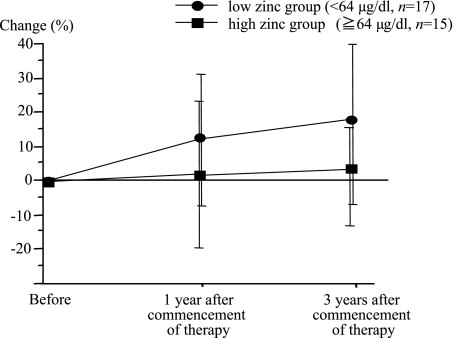
Next, we compared the changes in serum zinc concentrations between two groups of patients who were administered polaprezinc, those with low (low zinc group <64 µg/dl) and high (high zinc group; ≥64 µg/dl) serum zinc concentrations at the start of the study. The serum zinc concentrations in the low zinc group at one year after the start of administration had increased by 12.1 ± 19.3%, and at three years had increased by 18.3 ± 24.8%, compared to the level at the start of administration. On the other hand, that of the high zinc group at one year after the start of administration had decreased by 1.4 ± 21.3%, and at three years had decreased by 2.3 ± 14.2%, compared to the level at the start of administration. The changes in serum zinc concentrations in the low zinc group were significantly greater than those of the high zinc group (Fig. 3, p = 0.0002).
Fig. 3.
Comparison of changes of serum zinc concentrations in the polaprezinc administration group between the low zinc group, whose serum zinc concentrations were below 64 µg/dl, and the high zinc group, whose serum zinc concentrations were above 64 µg/dl. The change of serum zinc concentrations in the low zinc group was significantly greater than that of the high zinc group (p = 0.0002).
Comparison between the polaprezinc administration and untreated groups in terms of changes of blood and biochemical data
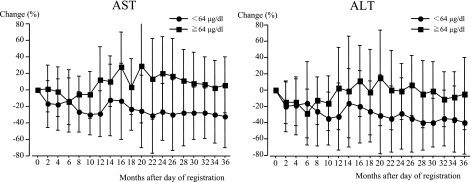
When the reductions of AST or ALT levels, compared to the levels after the start of the study, were compared between the polaprezinc administration and untreated groups, the reductions of ALT or AST levels in the polaprezinc administration group were significantly greater than in the untreated group (Table 2, p = 0.0019, p = 0.0069). Furthermore, the change of platelet counts in the polaprezinc administration group was significantly less than in the untreated group (p = 0.0001). However, the changes in HCV RNA levels did not differ between the two groups (p = 0.6736). Next, when the polaprezinc administration group was divided into high and low zinc groups, the reductions of AST levels and ALT levels in the low zinc group were significantly greater than those of the high zinc group (Fig. 4; p = 0.0008, p = 0.0193). Although a significant correlation was not found, the reductions of HCV RNA levels in the low zinc group tended to be greater than those of the high zinc group (p = 0.0506). However, the change of platelet counts did not differ between the low and high zinc groups (p = 0.3169). Although the achievement of reduction of AST or ALT levels was significantly greater for the zinc responders than the zinc non-responders in the low group (p = 0.0274), there was no such difference in the high group (p = 0.5349).
Table 2.
Comparison of the rate of changes of AST levels, ALT levels, platelet counts and HCV RNA levels between the polaprezinc administrationsgroup and untreated group, after the day of registration
| Months | AST* |
ALT¶ |
Platelet count‡ |
HCV RNA |
||||
|---|---|---|---|---|---|---|---|---|
| Polaprezinc | Untreated | Polaprezinc | Untreated | Polaprezinc | Untreated | Polaprezinc | Untreated | |
| +2 Months | −12.4 ± 23.6 | 8.9 ± 29.1 | −15.7 ± 29.0 | 4.7 ± 30.0 | 0.6 ± 8.2 | −7.5 ± 7.4 | −19.4 ± 38.0 | −9.2 ± 33.3 |
| +4 Months | 17.2 ± 29.3 | 9.8 ± 49.0 | −20.8 ± 31.6 | 9.5 ± 64.6 | 0.2 ± 8.1 | −5.8 ± 10.1 | −22.9 ± 42.5 | −4.1 ± 29.6 |
| +6 Months | −21.2 ± 30.9 | 16.5 ± 65.9 | −26.5 ± 41.0 | 7.6 ± 116.3 | 0.9 ± 10.1 | −5.7 ± 8.8 | −17.2 ± 43.9 | 3.1 ± 32.6 |
| +8 Months | −23.1 ± 21.7 | 11.6 ± 54.6 | −23.8 ± 34.7 | 11.6 ± 84.6 | 0.3 ± 6.2 | −8.3 ± 7.2 | −17.9 ± 41.9 | −5.9 ± 50.5 |
| +10 Months | −24.7 ± 25.0 | 9.7 ± 49.3 | −29.9 ± 31.3 | 5.2 ± 58.8 | 0.7 ± 11.2 | −8.7 ± 7.3 | −16.1 ± 51.9 | −17.6 ± 49.9 |
| +12 Months | −15.6 ± 36.4 | 10.6 ± 57.8 | −22.0 ± 37.8 | 5.6 ± 81.1 | 0.1 ± 7.9 | −8.3 ± 10.4 | −11.8 ± 49.5 | −3.4 ± 65.5 |
| +14 Months | −9.1 ± 36.1 | 10.5 ± 55.5 | −17.4 ± 36.6 | 7.3 ± 67.2 | 2.7 ± 13.5 | −6.7 ± 13.0 | −13.4 ± 53.4 | 6.0 ± 70.1 |
| +16 Months | −9.2 ± 44.3 | 16.9 ± 58.1 | −9.7 ± 44.1 | 11.0 ± 69.9 | 3.9 ± 18.1 | −7.2 ± 9.5 | −21.0 ± 45.4 | 3.9 ± 58.4 |
| +18 Months | −16.9 ± 29.2 | 16.8 ± 49.5 | −21.3 ± 32.5 | 11.4 ± 60.8 | 1.0 ± 11.1 | −8.8 ± 12.9 | 23.1 ± 184.0 | 2.9 ± 57.2 |
| +20 Months | −8.6 ± 51.7 | 9.1 ± 41.3 | −15.8 ± 43.4 | 13.6 ± 74.0 | 1.3 ± 10.7 | −10.7 ± 11.8 | 2.4 ± 77.8 | 10.5 ± 64.7 |
| +22 Months | −15.9 ± 50.0 | 6.2 ± 38.8 | −22.2 ± 45.6 | 8.4 ± 51.9 | 1.4 ± 11.3 | 9.8 ± 12.1 | −17.6 ± 51.7 | 10.1 ± 61.8 |
| +24 Months | −12.8 ± 34.2 | 14.7 ± 49.9 | −18.7 ± 41.0 | 8.3 ± 46.0 | 0.8 ± 13.0 | −8.8 ± 10.7 | 6.9 ± 86.6 | 10.5 ± 59.8 |
| +26 Months | −15.3 ± 42.5 | 9.1 ± 52.0 | −18.8 ± 40.8 | 7.9 ± 56.4 | −2.5 ± 13.0 | −8.1 ± 12.6 | −16.2 ± 60.3 | 6.1 ± 70.1 |
| +28Months | −15.9 ± 39.7 | 13.8 ± 54.6 | −25.9 ± 40.6 | 10.0 ± 74.5 | −1.5 ± 15.0 | 9.4 ± 15.1 | 7.4 ± 100.6 | 12.5 ± 69.1 |
| +30Months | −18.0 ± 32.9 | 13.0 ± 50.4 | −24.2 ± 37.7 | 13.0 ± 54.4 | −4.5 ± 11.9 | −11.6 ± 14.3 | 1.3 ± 78.4 | 5.4 ± 61.2 |
| +32 Months | −18.0 ± 33.4 | 17.6 ± 77.6 | −26.2 ± 34.7 | 16.4 ± 91.0 | −4.9 ± 15.4 | −11.4 ± 12.8 | −4.1 ± 76.2 | 9.8 ± 66.9 |
| +34 Months | −21.4 ± 3.19 | 19.9 ± 66.6 | −25.5 ± 36.9 | 17.7 ± 73.6 | −5.5 ± 16.2 | −10.8 ± 14.1 | −5.2 ± 66.5 | 4.0 ± 52.8 |
| +36 Months | −20.5 ± 37.1 | 22.2 ± 69.9 | −26.2 ± 42.9 | 18.5 ± 70.0 | −5.6 ± 14.7 | −5.2 ± 19.9 | −10.1 ± 65.4 | 12.2 ± 51.6 |
Mean (%) ± standard deviation (S.D.) are shown. AST: aspartate aminotransferase; ALT: alanine aminotransferase. *p = 0.0019 by the Repeated measure ANOVA for the comparison between Polaprezinc and Untreated groups, ¶p = 0.0069 by the Repeated measure ANOVA for the comparison between Polaprezinc and Untreated groups, ‡p = 0.0001 by the Repeated measure ANOVA for the comparison between Polaprezinc and Untreated groups.
Fig. 4.
When the polaprezinc administration group was divided into two, the high zinc group and the low zinc group, the reductions of AST levels and ALT levels in the low zinc group were significantly greater than those of the zinc high group (p = 0.0008, p = 0.0193).
The cumulative incidence of HCC in patients with C-viral CH or LC
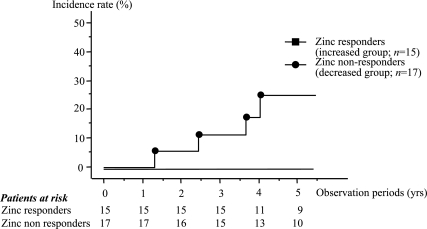
The incidence of HCC development after the initial examination in patients with C-viral liver disease was 4/48 (3.8%) for CH and 4/14 (28.6%) for LC, up to March 2005. The cumulative incidence of HCC, the assessment of which was started prospectively after the initial measurement of the zinc concentration, was compared between the polaprezinc administration group (median observation period, 4.877 years, range; 1.315–5.466) and untreated group (median observation period, 5.167 years, range; 1.271–5.436). HCC occurred in four patients (12.5%) in the polaprezinc administration group and in four patients (12.1%) in the untreated group. The cumulative incidence of HCC over three years was 13.1% in the polaprezinc administration group and 13.3% in the untreated group. The difference between the two groups was not statistically significant. However, considering the patients who were administered polaprezinc according to whether they were zinc responders (15 patients; median observation period, 4.718 years, range; 1.575–5.419) or zinc non-responders, (17 patients; mean observation period, 5.036 years, range; 1.271–5.466), the incidence of HCC after the initial examination was 4/17 (17.6%) for the zinc non-responders and 0/15 for the zinc responders. The incidence of HCC after the initial examination was 4/5 (80%) for the zinc non-responders whose serum zinc concentrations decreased and 0/12 for the zinc non-responders whose serum zinc concentrations were stable. The cumulative incidence of HCC was compared between these groups. The cumulative incidence of HCC over five years was 26.1% in the zinc non-responders and 0% in the zinc responders (Fig. 5).
Fig. 5.
The cumulative incidence of HCC was compared between the responders and the non-responders, based on changes in serum zinc concentrations between the initial and final examinations. The cumulative incidence of HCC over four years was 24.9% in the zinc non-responders and 0% in the zinc responders. The cumulative incidence of HCC over four years in the zinc responders was clearly less than that in the zinc non-responders.
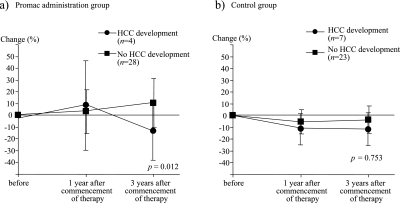
Then we compared the changes of serum zinc concentration with or without HCC development in patients with polaprezinc administration group and the control group. The serum zinc concentrations in patients who developed HCC decreased significantly compared to those who did not, however, the changes of serum zinc concentrations in the control group did not differ significantly between those who developed HCC and those who did not (Fig. 6a, b).
Fig. 6.
Changes of serum zinc concentration in patients with or without HCC development in the polaprezinc administration group and control group. The changes of serum zinc concentrations were significantly lower in the patients who developed HCC than those who did not (a). However, the changes of serum zinc concentrations in the control group did not differ significantly between those who developed HCC and those who did not (b).
Multivariate analysis
In order to identify risk factors for the development of HCC in patients administered polaprezinc, univariate analyses were made using logistic regression analysis (Table 3). The analyses revealed that zinc non-response was a significant risk factor (risk ratio; 0.048, 95% confidence interval (CI); 0.003–0.712, p = 0.0274), although a low platelet count also was a significant risk factor (risk ratio; 0.684, 95% CI; 0.475–0.984, p = 0.0408). Then, we examined the risk factors for the development of HCC in patients administered polaprezinc by multivariate analysis using logistic regression analysis. The analyses revealed that no significant risk factor was observed. Furthermore, in order to identify factors contributing to zinc non-responsiveness to polaprezinc administration, multivariate analyses were made using the Cox proportional hazard model. The analyses revealed that liver cirrhosis (risk ratio; 0.001, 95% CI; 0.000246–0.512) and high serum zinc concentration at the start of the study (risk ratio; 0.734, 95% CI; 0.556–0.968) were contributory factors.
Table 3.
Clinical characteristics of the patients with or without occurrence of HCC at the beginning of the study
| Paramater | Hepatocellular carcinoma |
p value | |
|---|---|---|---|
| occurrence | absence | ||
| Number | 4 | 28 | |
| Gender (male) | 3 (75%) | 18 (64.8%) | 0.6659 |
| Age | 65.2 (59.7–67.7) | 55.3 (26.5–70.1) | 0.0890 |
| AST (U/L) | 73.0 (51–138) | 53.5 (40–116) | 0.2320 |
| ALT (U/L) | 88.5 (65–201) | 89.5 (40–231) | 0.7954 |
| Platelet count (×10−4) | 9.6 (8.3–17.2) | 16.7 (7.4–25.7) | 0.0213 |
| Zinc concentration | 51.0 (41–73) | 64.5 (45–88) | 0.1.09 |
| HCV genotype | |||
| 1b | 4 (100%) | 24 (85.8%) | 0.7214 |
| 2a | 0 | 2 (7.1%) | |
| 2b | 0 | 2 (7.1%) | |
| HCV RNA level (kiu/ml) | 435.0 (140–710) | 600.0 (7.4–850) | 0.4665 |
| Zinc supplementation | 0.0493 | ||
| Zinc responders | 0 | 15 (53.6%) | |
| Zinc non responders | 4 (100%) | 13 (46.4%) | |
* Median (range). Gender, Genotype, Efficacy of supplementation were analyzed by chi-square test for independence, other factors by student’s t test.
Discussion
Polaprezinc is composed of zinc and L-carnocin. In Japan, it has been used to treat gastric ulcers in general clinical practice since 1994. The medicine we used in this study was Promac granules, 1.0 g/day. Promac contains 150 mg of polaprezinc per gram and also contains D-mannitol, cornstarch, calmerose calcium, polyvinyl pyrrolidone K, and aminoalkylacrylate polymer E as additives. Promac was administered orally twice a day, after breakfast and after dinner, to a total of 1.0 g. Therefore, the daily administration of zinc was 33.9 mg. In this study, we compared the long-term outcome between the Promac (polaprezinc) administration group and the untreated group. The results showed that, in the polaprezinc administration group, the average serum zinc concentrations had increased by 9.8% three years after the treatment started compared to the level before the start of treatment. However, in the untreated group, the serum zinc concentrations had decreased by approximately 5% compared to the level at the commencement of therapy. There were fifteen cases (46.9%) in the polaprezinc administration group whose serum zinc concentrations increased, significantly more than in the untreated group, in which there was only one case (3.3%). On the contrary, the patients whose serum zinc concentrations decreased numbered only five (15.6%) in the polaprezinc administration group but 15 (50%) in the untreated group. Therefore, it was confirmed that the serum zinc concentrations increased following the administration of polaprezinc, even in patients with C-viral CH or LC.
In this study, polaprezinc was administered at a fixed amount, regardless of the height and/or weight of the patient. When the changes in serum zinc concentrations were compared according to the daily dose of zinc administered per kg body weight, the number of patients who became zinc responders was significantly higher in the group of patients of lower weight (data not shown). Therefore, it suggested that there were cases in which administration of the daily dose of 150 mg polaprezinc was insufficient. In fact, in the group that was administered a daily dose of zinc below 0.6 mg/kg, the serum zinc concentrations increased slightly immediately after the start of administration, but after that a tendency to decrease was observed. However, in the group that was administered a daily dose of zinc above 0.6 mg/kg, the serum zinc concentrations increased gradually after the start of administration and exceeded the levels in the group administered a daily dose of zinc less than 0.6 mg/kg. Therefore, when polaprezinc is used clinically, attention must be paid to the individual’s body weight.
Comparing the long-term outcomes, the polaprezinc administration group showed a clear reduction in ALT and AST levels, compared to the untreated group. Although the platelet counts decreased compared to the level at the start of administration, the reduction was clearly less than that of the untreated group. The ALT and AST levels also had a tendency to decrease in the group with increased zinc concentrations. Therefore, this prospective study confirmed that the serum zinc concentrations gradually reduced without zinc supplementation in patients with C-viral CH or LC. The serum zinc concentrations increased or remained unchanged in 84.3% of patients with C-viral chronic liver disease when zinc was administered, but 15.6% of patients showed a reduction in their zinc concentrations. It is known that serum zinc concentrations decreased in patients with liver disease in parallel with the development of disease stage because zinc absorption from the intestine decreases and the zinc content of the liver reduces due to the decrease in the number of functional hepatocytes [18–22]. However, our study suggests that the serum zinc concentrations can increase in the majority of patients if a sufficient quantity of zinc is administered.
When the patients administered polaprezinc were divided into two groups whose serum zinc concentrations were more than 64 µg/dl (high zinc group) and less than 64 µg/dl (low zinc group), the reduction of AST and ALT levels in the low zinc group was significantly greater than in the high zinc group. Therefore, zinc supplementation in patients with CH or LC with low serum zinc concentrations was more effective than in those with high serum zinc concentrations. Specifically, patients with low serum zinc concentrations seemed to achieve a greater reduction in serum AST or ALT levels following zinc supplementation, regardless whether the zinc concentration increased or decreased. On the other hand, patients with high serum zinc concentrations, whose serum zinc concentrations did not increase with the daily dose of zinc given, also did not achieve a reduction of ALT levels and perhaps require a greater degree of zinc supplementation. The significance of maintaining the serum zinc concentrations at a high level in C-viral liver disease is that, by achieving a continuous low level of ALT, it is possible to slow the progression of liver fibrosis and, further, to restrain the development of liver carcinogenesis. Also, in patients with a decreased reserve of liver function, such as those with decompensated cirrhosis, it is possible to prolong the survival period by increasing their zinc concentrations, leading to improvement in hyper-ammonia and recovery of liver function. Our study showed no significant difference in the cumulative incidence of HCC between the group administered polaprezinc and the untreated group. However, comparing the zinc responders and zinc non-responders, regardless of polaprezinc administration, the cumulative incidence of HCC was significantly lower in the former, suggesting that the influence of zinc supplementation on the long-term outcome of C-viral CH or LC is significant. Therefore, we report that zinc supplementation for C-viral CH or LC is clinically useful.
Multivariate analysis of the clinical background factors was performed on the zinc non-responders in the group administered polaprezinc. The results showed that there was no significant relationship between the age, body weight or BMI, platelet count, ALT level, AST level, or the HCV RNA level. However, when the changes in the serum zinc concentrations were evaluated according to the daily dose of zinc administered per kg body weight, in the polaprezinc administration group for whom the daily dose of zinc was more than 0.6 mg/kg the number of cases whose serum zinc concentrations increased compared to the level before the start of therapy was significantly high, whereas in the group with zinc administration at daily dose of zinc less than 0.6 mg/kg the number of cases whose serum zinc concentrations decreased was significantly high. Therefore, it seems necessary to consider the daily dose of zinc administered per kg body weight on an individual basis and to increase the daily dose of zinc administered per day for patients of greater body weight. In addition, the data suggest that, for the zinc non-responders in the polaprezinc administration group, increasing the daily dose of polaprezinc could lead to an improvement in the long-term outcome of the patient.
As for the reason why ALT and AST levels reduced with zinc administration, the following may be suggested: It is known that zinc has an anti-oxidant effect. It has an inhibitory action on iron-dependent radical reactions and on lipid peroxidation. It has been assumed that the state of zinc deficiency in chronic liver disease first leads to an increase in hepatic phospholipids, resulting in intensification of lipid peroxidation, and thereby causes hepatic cell injury [23, 24]. Therefore, it is assumed that zinc administration inhibits lipid peroxidation and subsequently alleviates hepatic cell injury and improves the AST and ALT levels. Zinc complexes with ferritin in hepatocytes. With zinc administration, complexing of zinc and ferritin also increases in the liver. This zinc-ferritin complex is readily used by apoenzymes that require zinc. Therefore, it is suggested that the ferritin level in hepatocytes may decrease with increasing zinc concentration, and zinc consequently enhances the chelation of iron in the liver. As a result, there should be a reduction in oxidative stress from iron in the hepatocytes and a decrease in aminotransferase levels [25]. In fact, it has been reported that the action of polaprezinc inhibiting gastric mucosal injury is due to the strong anti-oxidant action and membrane stabilizing action [26–28]. Therefore, in a similar manner in the liver, liver function improves and liver fibrosis is suppressed by the anti-oxidant action and membrane stabilization effect of polaprezinc. The reason the incidence of HCC in zinc responders may be reduced more than that of zinc non-responders may be as follows: Serum ALT or AST levels reduced through the anti-oxidant effect of zinc. Also, zinc deficiency is known to affect certain mediators of innate immunity, such as the function of neutrophils, NK cells and complement [29, 30]. Furthermore, the numbers and activity of NK cells are dependent on serum zinc concentrations [31]. Zinc supplementation results in significantly greater numbers of cytotoxic and helper T and NK cells than are seen in the control group [32, 33].
Finally, we also compared the changes of serum zinc concentrations, levels of AST and ALT, and the cumulative incidence of HCC development between the patients with CH and those with LC. The serum zinc concentrations increased and the serum levels of AST and ALT were reduced after three years of polaprezinc administration in both groups. Furthermore, in the CH group, the cumulative incidence of HCC development in the zinc responders was significantly lower than that in the zinc non-responders. However, the cumulative incidence of HCC development did not differ significantly between the zinc responders and non-responders in the LC group. We assume that liver cirrhosis constitutes a high carcinogenic state and the development of HCC could not be prevented simply by polaprezinc administration.
In conclusion, we performed zinc supplementation for patients with C-viral CH and LC and the following results were obtained: The serum zinc concentration increased in approximately half of the patients who received the zinc supplement. Compared to the untreated patients, the AST and ALT levels decreased significantly. Compared to the untreated patients, the reduction in the platelet counts was significantly lower. The factors that inhibited increases in serum zinc concentrations following administration of polaprezinc included low serum zinc concentration states such as liver cirrhosis. Furthermore, the reductions of AST and ALT levels in the low zinc group were significantly greater than those of the high zinc group. The zinc responders had a significantly lower cumulative incidence of HCC than the zinc non-responders.Thus, it was confirmed that zinc supplementation for patients with C-viral CH or LC improves both the degree of the liver damage and the long-term outcome.
Acknowledgments
The authors thank Professor Masao Omata and Haruhiko Yoshida, MD (Department of Gastroenterology, Graduate School of Medicine, University of Tokyo) for helpful advice regarding this article.
References
- 1.AMA Department of Foods and Nutrition, author. Guidline for essential trace element preparations for parental use. JAMA. 1979;241:2051–2054. [PubMed] [Google Scholar]
- 2.Evans G.W. In: Zinc absorption and transport. In trace elements in Human Health and Disease, in Zinc and Copper. Prassd A.S., editor. Vol. 1. Academic Press; New York: 1976. [Google Scholar]
- 3.Dibley M.J. Pres in Nutrition. Eighteen Edition. Kenpakusha; Tokyo: 2002. pp. 344–345. [Google Scholar]
- 4.Krebs N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000;130(5S Suppl):1374S–1377S. doi: 10.1093/jn/130.5.1374S. [DOI] [PubMed] [Google Scholar]
- 5.Takagi H., Nagamine T., Abe T., Takayama H., Sato K., Otsuka T., Kakizaki S., Hashimoto Y., Matsumoto T., Kojima A., Takezawa J., Suzuki K., Sato S., Mori M. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. J. Viral. Hepat. 2001;8:367–371. doi: 10.1046/j.1365-2893.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- 6.Ebara M., Fukuda H., Hatano R., Yoshikawa M., Sugiura N., Saisho H., Kondo F., Yukawa M. Metal contents in the liver of patients with chronic liver disease caused by hepatitis C virus. Reference to hepatocellular carcinoma. Oncology. 2003;65:323–330. doi: 10.1159/000074645. [DOI] [PubMed] [Google Scholar]
- 7.Riggio O., Merli M., Capocaccia L., Caschera M., Zullo A., Pinto G., Gaudio E., Franchitto A., Spagnoli R., D’Aquilino E., et al. Zinc supplementation reduce blood ammonia and increases liver ornithin transcarbamirase activity in experimental cirrhosis. Hepatology. 1992;16:785–789. doi: 10.1002/hep.1840160326. [DOI] [PubMed] [Google Scholar]
- 8.Nandi S.S., Chawla Y.K., Nath R., Dilawari J.B. Serum and urinary zinc in fulminant hepatic failure. J. Gastroenterol. Hepatol. 1989;4:209–213. doi: 10.1111/j.1440-1746.1989.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida Y., Higashi T., Nouso K., Nakatsukasa H., Nakamura S.I., Watanabe A., Tsuji T. Effects of zinc deficiency/zinc supplementation on ammonia metabolism in patients with decompensated liver cirrhosis. Acta. Med. Okayama. 2001;55:349–355. doi: 10.18926/AMO/32003. [DOI] [PubMed] [Google Scholar]
- 10.Grüngreiff K., Reinhold D. Liver cirrhosis and “liver” diabetes mellitus are linked by zinc deficiency. Med. Hypotheses. 2005;64:316–317. doi: 10.1016/j.mehy.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Marchesini G., Fabbri A., Bianchi G., Brizi M., Zoli M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology. 1996;23:1084–1092. doi: 10.1053/jhep.1996.v23.pm0008621138. [DOI] [PubMed] [Google Scholar]
- 12.Tellinghuisen T.L., Marcotrigiano J., Gorbalenya A.E., Rice C.M. The NS5A protein of hepatitis C virus is a zinc metalloprotein. J. Biol. Chem. 2004;279:48576–48587. doi: 10.1074/jbc.M407787200. [DOI] [PubMed] [Google Scholar]
- 13.Coon J.T., Ernst E. Complementary and alternative therapies in the treatment of chronic hepatitis C: a systematic review. J. Hepatol. 2004;40:491–500. doi: 10.1016/j.jhep.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Oka S., Ogino K., Matsuura S., Yoshimura S., Yamamoto K., Okazaki Y., Takemoto T., Kato N., Uda T. Human serum immuno-reactive copper, zinc-superoxide dismutase assayed with an enzyme monoclonal immunosorbent in patients with digestive cancer. Clin. Chim. Acta. 1989;182:209–219. doi: 10.1016/0009-8981(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 15.Muranaka H., Kato N. Detection of serum zinc by atomic absorption spectrometry. Rinsho Byori. 1969;17:559–562. (in Japanese) [PubMed] [Google Scholar]
- 16.Okamoto H., Sugiyama Y., Okada S., Kurai K., Akahane Y., Sugai Y., Tanaka T., Tanaka T., Sato K., Tsuda F., Miyakawa Y., Mayumi M. Typing of hepatitis C virus by PCR with type-specific primers: application to clinical surveys and tracing infectious sources. J. Gen. Virol. 1992;73:673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- 17.Simmonds P., Alberti A., Alter H.J., Bonino F., Bradley D.W., Brechot C., Brouwer J.T., Chan S.W., Chayama K., Chen D.S., Choo Q.L., Colombo M., Cuypers H.T.M., Date T., Dusheiko G.M., Esteban J.I., Fay O., Hadziyannis S.J., Han J., Hatzakis A., Holmes E.C., Hotta H., Houghton M., Irvine B., Kohara M., Kolberg J.A., Kuo G., Lau J.Y.N., Lelie P.N., Maertens G., McOmish F., Miyamura T., Mizokami M., Nomoto A., Prince A.M., Reesink H.W., Rice C., Roggendorf M., Schalm S.W., Shikata T., Shimotohno K., Stuyver L., Trepo C., Weiner A., Yap P.L., Urdea M.S. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology. 1994;19:1321–1324. [PubMed] [Google Scholar]
- 18.Boyett J.D., Sullivan J.F. Distribution of protein-bound zinc in normal and cirrhotic serum. Metabolism. 1970;19:148–157. [PubMed] [Google Scholar]
- 19.Boyett J.D., Sullivan J.F. Zinc and collagen content of cirrhotic liver. Am. J. Dig. Dis. 1970;15:797–802. doi: 10.1007/BF02236039. [DOI] [PubMed] [Google Scholar]
- 20.Scholmerich J., Becher M.S., Kottgen E., Rauch N., Haussinger D., Lohle E., Vuilleumier J.P., Gerok W. The influence of portosystemic shunting on zinc and vitamin A metabolism in liver cirrhosis. Hepatogastroenterology. 1983;30:143–147. [PubMed] [Google Scholar]
- 21.Wakiyama K., Arakawa Y., Suzuki T., Miyamoto M., Matsuo Y., Honda T., Tomioka E., Mano M., Kigoshi K., Sasaki A., Takeuchi S. A pathophysiological significance of trace metals in rats with experimental cirrhosis. Acta. Pathologica. Hepatica. 1986;140:1182–1183. (In Japanese) [Google Scholar]
- 22.Arakawa Y., Moriyama M., Tanaka N. Hepatic Diseases and Trace Elements. Gatsukai jimu center; Osaka, Japan,: 2003. pp. 1–30. (In Japanese) [Google Scholar]
- 23.Cabre M., Camps J., Paternain J.L., Ferre N., Joven J. Time-course of changes in hepatic lipid peroxidation and glutathione metabolism in rats with carbon tetrachloride-induced cirrhosis. Clin. Exp. Pharmacol. Physiol. 2000;27:694–699. doi: 10.1046/j.1440-1681.2000.03322.x. [DOI] [PubMed] [Google Scholar]
- 24.Camps J., Bargallo T., Gimenez A., Alie S., Caballeria J., Pares A., Joven J., Masana L., Rodes J. Relationship between hepatic lipid peroxidation and fibrogenesis in carbon tetrachloride-treated rats: effect of zinc administration. Clin. Sci. (Lond) 1992;83:695–700. doi: 10.1042/cs0830695. [DOI] [PubMed] [Google Scholar]
- 25.Bray T., Bettger W.J. The physiological role of zinc as an antioxidant. Free Radic. Biol. Med. 1990;8:281–291. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 26.Yoshikawa T., Naito Y., Tanigawa T., Yoneta T., Kondo M. The antioxidant properties of a novel zinc-carnocine chelate compound, N-(3-amino-propionyl)-L-histidine zinc. Biochim. Biophys. Acta. 1991;1115:15–22. doi: 10.1016/0304-4165(91)90005-2. [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa T. Role of oxygen radicals in the pathogenesis of gastric mucosal lesions induced by water-immersion restraint stress and burn stress in rats. J. Clin. Biochem. Nutr. 1990;8:227–234. [Google Scholar]
- 28.Yoshikawa T., Naito Y., Tanigawa T. Effect of zinc-carnosine chelate compound (Z-103), a novel antioxidant, on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Free Radic. Res. Commun. 1991;14:289–296. doi: 10.3109/10715769109088958. [DOI] [PubMed] [Google Scholar]
- 29.Shanker A.H., Prased A.S. Zinc and immune function: the biological basis of altered resistence to infection. Am. J. Clin. Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 30.Ibs K.H., Rink L. Zinc-altered immune function. J. Nutr. 2003;133:1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 31.Ravaglia G., Forti P., Maioli F., Bastagli L., Facchini A., Mariani E., Savarino L., Sassi S., Cucinotta D., Lenaz G. Effect of micronutrient status on natural killer immune function in healthy free-living subjects aged>/=90 y. Am. J. Clin. Nutr. 2000;71:590–598. doi: 10.1093/ajcn/71.2.590. [DOI] [PubMed] [Google Scholar]
- 32.Sazawal S., Jalla S., Mazumder S., Sinha A., Black R.E., Bhan M.K. Effect of zinc supplementation on cell-mediated immunity and lymphocyte subsets in preschool children. Indian Pediatr. 1997;34:589–597. [PubMed] [Google Scholar]
- 33.Chandra P.K. Effect of vitamin and trace-ellement supplementation on immune responses and infection in elderly subjects. Lancet. 1992;340:1124–1127. doi: 10.1016/0140-6736(92)93151-c. [DOI] [PubMed] [Google Scholar]