Abstract
Myristic acid (MyA), which is a saturated fatty acid (C14:0) and a side chain of phorbol 12-myristate 13-acetate (PMA), was examined if MyA stimulates human polymorphonuclear leukocytes (PMNs) to release oxygen radicals comparable to PMA by applying electron paramagnetic resonance (EPR)-spin-trapping method. When MyA was added to isolated human PMNs, spin adducts of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO)-OH and DMPO-OOH were time-dependently observed. The amounts of these spin adducts were larger than those of PMNs stimulated by PMA. These results clearly show that MyA is more potent agent to prime human PMNs than PMA, in a point of view of not only O2·− but also ·OH production. This fact calls attention that too much intake of MyA that is known to be contained vegetable oils can lead to crippling effect through uncontrolled production of reactive oxygen species.
Keywords: polymorphonuclear leukocyte, myristic acid, phorbol 12-myristate 13-acetate, superoxide, EPR spin-trapping
Introduction
Polymorphonuclear leukocytes (PMNs) play an important role in host defense against microbial infections. The microbicidal mechanism consists of phagocytosis of pathogens, production and subsequent release of reactive oxygen species (ROS) and bactericidal proteins to phagosomes. The process involves activation of NADPH oxidase (NOX), which catalyzes the reduction of oxygen molecular to O2·− at the expense of NADPH in phagocytes [1–3]. NOXs are a group of plasma membrane-associated multicomponent enzymes consisting of at least two components bound to plasma membrane (gp91phox and p22phox that together form the flavocytochrome b558), and three cytosolic components (p47phox, p67phox, and p40phox), and a small GTPase Rac [4]. In resting PMNs, NOXs are dormant and their components separately exist in the membrane and in the cytosol. Once PMNs are primed by appropriate stimuli, NOXs are activated to produce O2·− by association of these cytosolic components with the plasma or phagosome membrane components [5–8]. Besides the bactericidal action as a beneficial effect, the uncontrolled production of ROS by phagocytic granulocytes may lead to crippling disorders such as shock and inflammation [9].
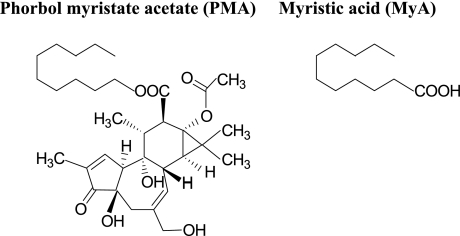
Phorbol 12-myristate 13-acetate (PMA, Fig. 1), a potent activator of protein kinase C (PKC), induces the phosphorylation of p47phox and subsequent O2·− production by the assembled NOX components [10]. More in detail, phosphorylation of proteins plays an essential role in the regulation of NOX activity, and the activation of PKC with PMA promotes the phosphorylation of serine and threonine residues of the p47phox and its translocation to the plasma membrane [11–13]. The respiratory burst is accompanied by tyrosine phosphorylation in cytosolic and membrane proteins [14], and it is also well known that tyrosine phosphorylation of proteins occurs during priming of PMNs [15]. As shown in Fig. 1, a side chain group of PMA is myristic acid (MyA), a 14-carbon, straight chain saturated fatty acid. MyA is mainly contained in vegetable oils such as coconut oil and palm oil, and likely affects lipid metabolism such as hypercholesterolemia [16–18], i.e. MyA causes high LDL cholesterol and apoB levels and low HDL to LDL ratios in healthy men and women [16], a positive regression between the plasma cholesterol level and the dietary level of MyA was observed in hamsters [17], and incorporation of trimyristin in the oil resulted in marked rise in serum cholesterol of rats [18]. In term of oxidative metabolism of PMNs, it was reported that MyA has an ability to stimulate the generation of O2·− from human neutrophils [19]. It was reported that MyA induces the hydrolysis of phosphatidylcholine leading to generation of arachidonic acid (AA) [19]. AA when added with membrane protein separated from bovine PMNs can activate NOX of bovine PMNs [20]. In addition, it was reported that apocynin (a specific inhibitor of NADPH oxidase) suppressed this stimuli (including MyA) -dependent ROS generation of undifferentiated HL-60 cells [21]. Thus, MyA may prime PMNs through two routes, one is NOX activated by AA from phosphatidylcholine and the other is direct effect on NOX. Thus, to assess the effect of MyA, when absorbed into the circulation, on ROS generation, we need to estimate the potency of AA to generate ROS in addition to the potency of MyA itself. These findings tempted us to compare the stimulative effect of MyA to release ROS with that of PMA, and to examine the stimulative effect of AA as an indirect effect of MyA. In this study, we examined stimuli-dependent ROS release from human PMNs by applying electron paramagnetic resonance (EPR)-spin-trapping method.
Fig. 1.
Chemical structures of phorbol 12-myristate 13-acetate; PMA and myristic acid; MyA.
Materials and Methods
Reagents
Reagents were purchased from the following sources: dextran, PMA, MyA and diethylenetriamine-N,N,N',N'',N''-pentaacetic acid (DTPA) from Wako Pure Chemical Industries (Osaka, Japan), AA from ICN Biomedicals, Inc. (Costa Mesa, CA), superoxide dismutase (SOD from bovine erythrocytes) and 4-hydroxyl-2,2,6,6-tetrametylpiperidin-1-oxyl (TEMPOL) from Sigma-Aldrich (St. Lous, MO), Ficoll-Paque Plus from Amersham Biosciences (Buckinghamshire, UK) and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) from Labotec Inc. (Tokyo, Japan). All other reagents used were of analytical grade.
Isolation of human PMNs
Heparinized venous blood was obtained from human healthy volunteers. PMNs were prepared from the blood by density gradient centrifugation using a Ficoll-Paque Plus (d = 1.077) following dextran sedimentation as previously described [22]. After the pellet of PMNs were subjected to rapid hypotonic lysis of erythrocyte, the cells were suspended in Hank’s balanced salt solution (HBSS) to be 1.0 × 106 cell/ml.
EPR-spin trapping determination of oxygen free radicals generated from human PMNs
PMA and MyA were dissolved in dimethyl sulfoxide (DMSO) to be 0.80 to 4.0 mM. AA was dissolved in DMSO to be 3 and 30 mM. DTPA was dissolved in 0.1 M phosphate buffer solution (PB, pH 7.4) to be 20 mM, SOD was to be 5000 U/ml in PB, and DMPO was to be 2.25 M in HBSS. One hundred and twenty µl of cell suspension was mixed with 16 µl of DMPO and 16 µl of DTPA solution. After addition of 8 µl of PMA, MyA or AA, the resultant mixture was transferred to a quartz flat EPR cell within 40 s. EPR spectra of DMPO spin adducts were recorded at given times. When necessary, 8 µl of SOD solution was added to the mixture. The measurement conditions for EPR (JES-FA100, JEOL, Tokyo, Japan) were as follows: scan rate, 10 mT/min; scan field, 335.5 ± 5 mT; modulation width, 0.1 mT; modulation frequency, 100 KHz; time constant, 0.03 s; microwave power, 8 mW; and microwave frequency, 9.5 GHz. Peak areas of EPR spectra of DMPO spin adducts, which are compensated by the peak area of Mn2+, were compared with those of the given concentrations of TEMPOL standard.
Statistical analysis
Each data is expressed as the mean ± standard error. Statistical significances between the two mean values of each time point were assessed by Student’s t test or Welch t test following analysis of variance. Values of p<0.05 were considered to be significant.
Results and Discussion
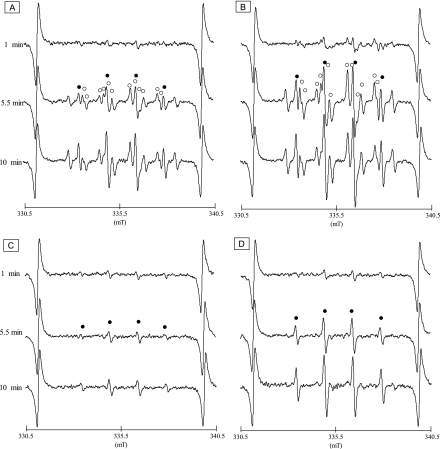
Fig. 2 shows the typical EPR spectra of DMPO spin adducts of oxygen radicals generated from human PMNs stimulated by PMA or MyA. As shown in Fig. 2A, multiple-lines of EPR spectra of human PMNs primed by PMA were detected. The hyperfine coupling constants (hfcc) of aN = 1.48 and aH = 1.48 mT (closed circle) at the four-line spectrum were typical DMPO spin adducts of ·OH (DMPO-OH) [22, 23], and those of aN = 1.425, aH = 1.145 and aH = 0.124 (open circle) were DMPO spin aducts of O2·− (DMPO-OOH) [21, 22]. Fig. 2B shows the representative EPR spectra obtained from human PMNs primed by MyA. As is the case with Fig. 2A, spin adducts of DMPO-OH and DMPO-OOH were observed, suggesting that MyA can stimulate human PMNs to release O2·− and ·OH. To confirm that the DMPO-OOH was derived from O2·−, the PMNs were treated with SOD, a potent O2·− scavenger, prior to PMA and MyA stimulation. Fig. 2C and D show characteristic EPR spectra of DMPO-OH obtained from PMNs primed with PMA and MyA following SOD treatment, respectively. Since the twelve-line spectrum with the hfcc of aN = 1.425, aH = 1.145 and aH = 0.124 (open circle) was completely disappeared in either of Fig. 2C and D, these spectra was derived from spin adduct of DMPO and O2·−.
Fig. 2.
EPR spectra of DMPO spin adducts of oxygen free radicals generated from human PMNs 1, 5.5 and 10 min after PMA- and MyA-stimulation. A and B: EPR spectra of DMPO spin adducts from human PMNs with 81 µM PMA- and 81 µM MyA-stimulation, respectively. Closed circles and open circles indicate DMPO spin adducts of ·OH (DMPO-OH) and O2·− (DMPO-OOH), respectively. C and D: EPR spectra of the DMPO spin adducts from PMA- and MyA-activetaed human PMNs pretreated with SOD, respectively. Closed circles indicate DMPO-OH.
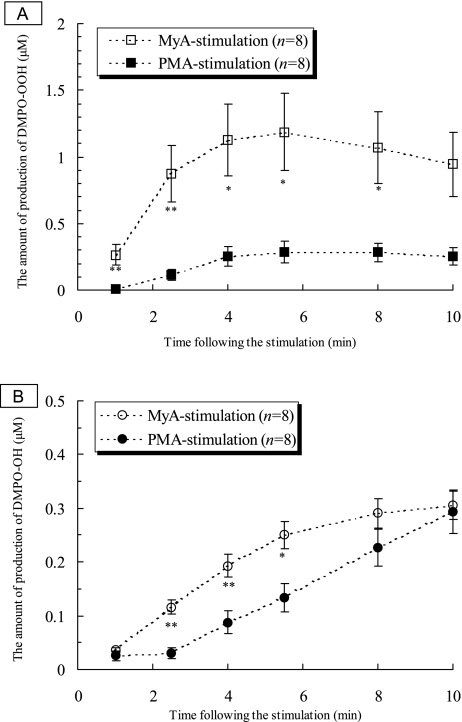
Fig. 3A shows time course changes in DMPO-OOH production after the addition of PMA, and of MyA to be a concentration of 81 µM. Apparently MyA stimulated PMNs to produce O2·− more strongly than did PMA when the same concentrations were added. Fig. 3B shows time course changes in DMPO-OH production after the addition of PMA, and MyA to be a concentration of 81 µM, indicating that ·OH production was time-dependently increased by either PMA or MyA. Similar to the case of O2·−, MyA stimulated PMNs to produce ·OH more strongly than did PMA when the same concentrations were added. These results clearly showed that MyA has an ability to prime human PMNs to produce oxygen radicals such as O2·− more potently than PMA. It was reported that MyA induces chemiluminescence of human promyelocytic leukemia cells with a luminol analogue L-012 [24]. However, qualitative analysis of ROS has not been conducted yet because chemiluminescence is induced by various ROS [25]. On the other hands, the EPR spin-trapping method could detect O2·− and ·OH simultaneously as DMPO-spin adducts as shown in our study.
Fig. 3.
Time course changes in productions of DMPO spin adducts of oxygen radicals by human PMNs treated with 81 µM PMA and MyA. A: DMPO-OOH productions after PMA- and MyA stimulations. Each value represents the mean ± SEM of 8 determinations. Statistical significances are shown by *; p<0.01 and **; p<0.005. B: DMPO-OH productions after PMA- and MyA stimulations. Each value represents the mean ± SEM of 8 determinations. Statistical significances are shown by *; p<0.01 and **; p<0.005.
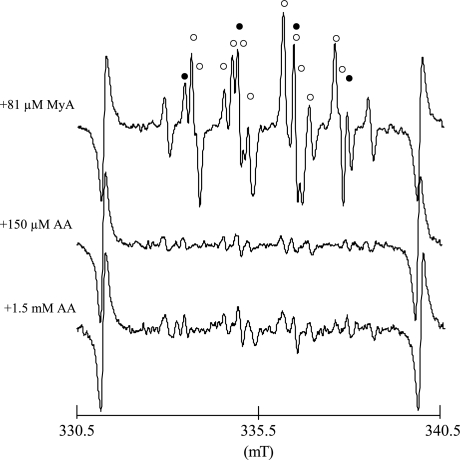
Fig. 4 shows the typical EPR spectra of DMPO-OOH and DMPO-OH by human PMNs with 81 µM MyA, 150 µM AA, and 1.5 mM AA. The EPR spectra were observed at 8 min after additions of MyA or AA. These findings show that AA can also prime human PMNs to relerase O2·− and ·OH, even though the potency is weaker than that of MyA. As for the mechanism by which AA primes PMNs, it was reported that AA activates NOX and proton channels of dormant neutrophils [26].
Fig. 4.
EPR spectra of DMPO spin adducts of oxygen free radicals generated from human PMNs 8 min after the addition of MyA and AA. Closed circles and open circles indicate DMPO spin adducts of ·OH (DMPO-OH) and O2·− (DMPO-OOH), respectively. In the top figure, closed circles and open circles indicate DMPO spin adducts of ·OH (DMPO-OH) and O2·− (DMPO-OOH), respectively. The signal other than DMPO-OH and DMPO-OOH is DMPO-CR that is an adduct of carbon-center radical derived from DMSO used as a solvent of MyA. DMPO-CR, which is produced by a chemical reaction between DMSO and ·OH, has six-line spectrum with the hfcc of aN = 1.64, aH = 2.24 [27].
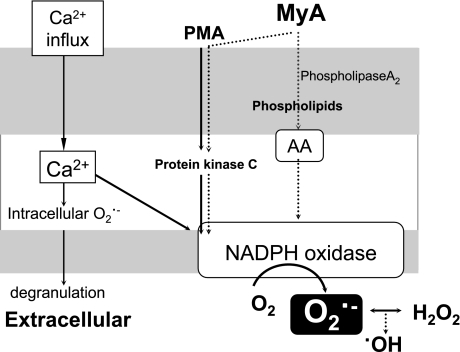
The proposed mechanisms by which MyA primes human PMNs are summarized in Fig. 5. Unlike C5a and N-formyl-L-methionyl-L-leucyl-L-phenylalanine, both of which can stimulate PMNs to produce O2·− by inducing calcium (Ca) influx, MyA-functions, like PMA-functions, bypass Ca influx since it has been reported that MyA induces O2·− production from human neutrophils in the absence of Ca2+ and magnesium [19, 26]. Although the potency of AA to stimulate the release of ROS is much weaker than that of MyA, the possibility of involvement of AA to the effect of MyA when absorbed into the circulation should be considered. Our study shows that MyA is a more potent activator of PMNs than PMA, and this fact calls attention that too much intake of MyA can lead to crippling effect through uncontrolled production of ROS.
Fig. 5.
Schematic illustration of the proposed mechanism of ·OH and O2·− generations from human PMNs by MyA.
Conclusions
We found that MyA is a stronger inducer of O2·− and ·OH generation by PMNs than PMA. MyA is mainly contained in vegetable oils and likely affects lipid metabolism [16–18]. Too much intake of MyA-rich vegetable oil may work as an inducer of PMNs’ activation, which may lead to undesirable conditions of the body. In the future study, these points should be elucidated.
Acknowledgments
The authors are grateful to healthy volunteers in NICHe of Tohoku University.
Abbreviations
- MyA
myristic acid
- PMA
phorbol 12-myristate 13-acetate
- PMNs
human polymorphonuclear leukocytes
- EPR
electron paramagnetic resonance
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- NOX
NADPH oxidase
- AA
arachidonic acid
- SOD
superoxide dismutase
- TEMPOL
4-hydroxyl-2,2,6,6-tetrametylpiperidin-1-oxyl
- DMPO-OH
DMPO spin adducts of ·OH
- DMPO-OOH
DMPO spin aducts of O2·−
References
- 1.Cros A.R., Jones O.T.G. Enzymic mechanisms of superoxide production. Biochim. Biophys. Acta. 1991;1057:281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- 2.Babior B.M. The respiratory burst oxidase. Adv. Enzymol. Relat. Areas Mol. Biol. 1992;65:49–95. doi: 10.1002/9780470123119.ch2. [DOI] [PubMed] [Google Scholar]
- 3.Chanock S.J., Benna J.E., Smith R.M., Babior B.M. The respiratory burst oxidase. J. Biol. Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- 4.Babior B.M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 5.Inanami O., Johnson J.L., Babior B.M. The leukocyte NADPH oxidase subunit p47phox: the role of the cysteine residues. Arch. Biochem. Biophys. 1998;350:36–40. doi: 10.1006/abbi.1997.0484. [DOI] [PubMed] [Google Scholar]
- 6.Inanami O., Johnson J.L., McAdara J.K., El Benna J., Faust L.R., Newburger P.E., Babior B.M. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47phox on serine 303 or 304. J. Biol. Chem. 1998;273:9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- 7.Johnson J.L., Park J.W., El Benna J., Faust L.P., Inanami O., Babior B.M. Activation of p47phox, a cytosolic subunit of the leukocyte NADPH oxidase. J. Biol. Chem. 1998;273:35147–35152. doi: 10.1074/jbc.273.52.35147. [DOI] [PubMed] [Google Scholar]
- 8.Inanami O., Yamamori T., Takahashi T.A., Nagahata H., Kuwabara M. ESR detection of intraphagosomal superoxide in polymorphonuclear leukocytes using 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide. Free Radic. Res. 2001;34:81–92. doi: 10.1080/10715760100300081. [DOI] [PubMed] [Google Scholar]
- 9.Cuzzocrea S., Riley D.P., Caputi A.P., Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 10.Combadiere C., Hakim J., Giroud J.P., Perianin A. Staurosporine, a protein kinase inhibitor, up-regulates the stimulation of human neutrophil respiratory burst by n-formyl peptides and platelet activating factor. Biochem. Biophys. Res. Commun. 1990;168:65–70. doi: 10.1016/0006-291x(90)91675-i. [DOI] [PubMed] [Google Scholar]
- 11.Clark R.A., Volpp B.D., Leidal K.G., Nauseef W.M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J. Clin. Invest. 1990;85:714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauseef W.M., Volpp B.D., McCormick S., Leidal K.G., Clark R.A. Assembly of the neutrophil respiratory burst oxidase. J. Biol. Chem. 1991;266:5911–5917. [PubMed] [Google Scholar]
- 13.EI Benna J., Ruedi J.M., Babior B.M. The phosphorylation of the respiratory bust oxidase component p47phox during neutrophil activation. J. Biol. Chem. 1994;269:6729–6734. [PubMed] [Google Scholar]
- 14.Gomez-Cambronero J., Huang C.K., Bonak V.A., Wang E., Casnellie J.E., Shiraishi T., Sha’afi R.I. Tyrosine phosphorylation in human neutrophil. Biochem. Biophys. Res. Commun. 1989;162:1478–1485. doi: 10.1016/0006-291x(89)90841-3. [DOI] [PubMed] [Google Scholar]
- 15.Lloyds D., Brindle N.P.J., Hallett M.B. Tyrosine phosphorylation of proteins during ‘priming’ of neutrophils. Biochem. Soc. Trans. 1993;21:436S. doi: 10.1042/bst021436s. [DOI] [PubMed] [Google Scholar]
- 16.Zock P.L., De Vries J.H.M., Katan M.B. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler. Thromb. 1994;14:567–575. doi: 10.1161/01.atv.14.4.567. [DOI] [PubMed] [Google Scholar]
- 17.Loison C., Mendy F., Serougne C., Lutton C. Increasing amounts of dietary myristic acid modify the plasma cholesterol level and hepatic mass of scavenger receptor BI without affecting bile acid biosynthesis in hamsters. Reprod. Nutr. Dev. 2002;42:101–114. doi: 10.1051/rnd:2002010. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S., Dutta R., Bandyopadhyay C. The influence of myristic acid of dietary fats on serum cholesterol. J. Atheroscler. Res. 1969;10:51–54. doi: 10.1016/s0368-1319(69)80081-5. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y., Ponnappan U., Lipschitz D.A. Excess formation of lysophosphatidic acid with age inhibits myristic acid-induced superoxide anion generation in intact human neutrophils. FEBS Letters. 1996;394:149–152. doi: 10.1016/0014-5793(96)00937-4. [DOI] [PubMed] [Google Scholar]
- 20.Ligeti E., Doussiere J., Vignais P.V. Activation of the O2·−-generating oxidase in plasma membrane from bovine polymorphonuclear neutrophils by arachidonic acid, a cytosolic factor of protein nature, and nonhydrolyzable analogues of GTP. Biochemistry. 1988;27:193–200. doi: 10.1021/bi00401a029. [DOI] [PubMed] [Google Scholar]
- 21.Muranaka S., Fujika H., Fujiwara T., Odino T., Sato E.F., Akiyama J., Imada I., Inoue M., Utsumi K. Mechanism and characteristics of stimuli-dependent ROS generation in undifferentiated HL-60 Cells. Antioxid. Redox signaling. 2005;7:1367–1376. doi: 10.1089/ars.2005.7.1367. [DOI] [PubMed] [Google Scholar]
- 22.Black C.D.V., Samuni A., Cook J.A., Krishna C.M. Kinetics of superoxide production by stimulated neutrophils. Arch. Biochem. biophys. 1991;286:126–131. doi: 10.1016/0003-9861(91)90017-d. [DOI] [PubMed] [Google Scholar]
- 23.Headlam H.A., Davies M.J. Cell-mediated reduction of protein and peptide hydroperoxides to reactive free radicals. Free. Rad. Biol. Med. 2003;34:44–88. doi: 10.1016/s0891-5849(02)01181-4. [DOI] [PubMed] [Google Scholar]
- 24.Kohno M., Sato E., Yaekashiwa N., Mokudai T., Niwano Y. Proposed mechanisms for HOOOH formation in two typical enzyme reactions responsible for superoxide anion production in biological systems. Chem. Lett. (in press) [Google Scholar]
- 25.Foubert T.R., Burritt J.B., Taylor R.M., Jesaitis A.J. Structural changes are induced in human neutrophil cytochrome b by NADPH oxidase activators, LDS, SDS, and arachidonate: intermolecular resonance energy transfer between trisulfopyrenyl-wheat germ agglutinin and cytochrome b558. Biochim. Biophys. Acta. 2002;1567:221–231. doi: 10.1016/s0005-2736(02)00619-3. [DOI] [PubMed] [Google Scholar]
- 26.Suda T., Suzuki Y., Matsui T., Inoue T., Niide O., Yoshimaru T., Suzuki H., Ra C., Ochiai T. Dapsone suppresses human neutrophil superoxide production and elastase release in a calcium-dependent manner. Br. J. Dermatol. 2005;152:887–895. doi: 10.1111/j.1365-2133.2005.06559.x. [DOI] [PubMed] [Google Scholar]
- 27.Kohno M., Minuta Y., Kusai M., Masumizu T., Makino K. Measurements of superoxide anion radical and superoxide anion scavenging activity by electron spin resonance spectroscopy coupled with DMPO spin trapping. Bull. Chem. Soc. Jpn. 1994;67:1085–1090. [Google Scholar]