Abstract
Nonalcoholic steatohepatitis (NASH) can develop into end-stage disease such as cryptogenic cirrhosis and hepatocellular carcinoma. Hence, it is important to understand the pathogenesis of NASH. In general, the “two-hit theory” has prevailed as a pathogenic mechanism of NASH. According to this theory, lipopolysaccharides (LPS) contained in normal portal blood are the “second hit,” but their role is not completely understood. Based on this theory, we evaluated the role of LPS in NASH pathogenesis. For the first hit to develop metabolic abnormalities, a synthetic diet rich in disaccharide (synthetic diet: 12.1 cal% disaccharide) was fed to Zucker (fa/fa) rats for 12 weeks. For the second hit, 100 µg/kg LPS was injected intraperitoneally once daily for 2 weeks. Synthetic diet-fed rats treated with LPS showed an increase in the triglyceride content and higher expression of profibrogenic mRNAs in the liver. Plasma alanine aminotransferase levels were significantly elevated using this protocol. Furthermore, histological examination demonstrated that this protocol induced mild hepatic fibrosis and focal necrosis in the livers of all rats. Synthetic diet-fed Zucker (fa/fa) rats treated with LPS could be useful for understanding the development of hepatic fibrosis in the two-hit theory.
Keywords: Nonalcoholic steatohepatitis (NASH), two-hit theory, synthetic diet, lipopolysaccharides (LPS), hepatic fibrosis
Introduction
Currently, obesity and its associated diseases are among the most prevalent and challenging conditions confronting the medical profession. Nonalcoholic fatty liver disease (NAFLD) is a clinical and pathological term that encompasses a disease spectrum ranging from simple triglyceride accumulation in hepatocytes to hepatic steatosis with inflammation, fibrosis, and cirrhosis, and is commonly observed in obese patients [1]. Nonalcoholic steatohepatitis (NASH) is a type of NAFLD, and is currently the most frequent cause of chronic liver disease in developed countries. It can lead to cirrhosis and increased risk of hepatocellular carcinoma [2–4]. The pathogenesis of NASH is yet to be clearly defined, but a recent major accepted theory is “two-hit theory” [5, 6]. The first hit refers to factors that promote hepatic steatosis [7, 8], and the second hit refers to factors that aggravate hepatic steatosis to steatohepatitis [9–11]. Till date, lipopolysaccrides (LPS) have been proposed to be the second hit [10]. Leptin- or leptin receptor-deficient animals that are genetically obese are highly susceptible to low-dose LPS for short-term (30 min, 1, 6, or 24 h) and develop steatohepatitis [8]. In addition, probiotics diminish NASH in leptin-deficient ob/ob mice, suggesting a relationship between enteric flora and liver injury [12, 13]. However, the exact role of LPS in the pathogenesis of NASH is not completely understood.
In this study, we attempted to evaluate the role of LPS in NASH development based on the two-hit theory. Since NASH is often observed in obesity and type 2 diabetes, we used obese diabetic Zucker (fa/fa) rats. For the first hit, we employed a synthetic diet rich in disaccharides (synthetic diet: 12.1 cal% disaccharide) to induce hepatic steatosis and metabolic abnormalities in rats. Following this, for the second hit, the rats were treated with LPS, which is reported to accelerate inflammation via hepatic production of proinflammatory cytokines such as tumor necrosis factor (TNF)-α [13–15]. We evaluated biochemical and morphological analysis of synthetic diet-fed Zucker (fa/fa) rats treated with LPS to study the role of LPS in NASH development.
Material and Methods
Animals
Six-week-old male Zucker (fa/fa) and control (fa/−) rats were purchased from Nippon SLC Co. Ltd. (Shizuoka, Japan). Rats were housed in plastic cages for 24 weeks on a 12-h light/12-h dark cycle under controlled temperature (22 ± 1°C) and humidity (50 ± 10%), with ad libitum access to food and tap water.
Experimental designs
Rats were fed either a regular diet (Funahashi Farm, Chiba, Japan) or a synthetic diet rich in disaccharides (sucrose and lactose; synthetic diet; CLEA Japan Inc., Tokyo, Japan) for 12 weeks (Table 1). The calories per gram were identical in the two diets and the only difference between the two diets was the content of disaccharides. It has been reported that feeding Zucker (fa/fa) rats with a diet rich in disaccharides for 1 week or 3 weeks induces hepatic steatosis with no fibrosis or infiltration of inflammatory cells [16]. Our dietary contents induced a gradual hepatic steatosis compared with the diet reported by Novikoff [16]. Rats were divided into three experimental groups: (i) regular diet-fed rats treated with LPS (n = 3), (ii) synthetic diet-fed rats treated with LPS (n = 3), and (iii) synthetic diet-fed rats treated with saline (n = 3). These rats were intraperitoneally (i.p.) injected with LPS (Sigma Chemical Co., St. Louis, MO) at 100 µg/kg once daily for 2 weeks. All rats were fed for 12 weeks and treated with LPS for the last 2 weeks under the same conditions. After feeding the respective diet for 12 weeks, the rats were fasted overnight, and blood and liver samples were collected for further experiments. The 12-week feeding and the last 2-week treatment were carried out three times.
Table 1.
Diet composition
| Regular diet (cal%) | Synthetic diet (cal%) | |
|---|---|---|
| Protein | 20.7 | 20.7 |
| Fat | 12.5 | 9.8 |
| Nitric-free extract | 66.8 | 69.5 |
| Disaccharides | 0.1 | 12.1 |
All surgical and experimental procedures were performed according to the guidelines for the care and use of animals approved by Osaka Medical College.
Measurement of plasma biochemical parameters
Levels of plasma alanine aminotransferase (ALT), insulin, glucose, leptin, and concentrations of LPS were measured by a local laboratory for clinical examinations (SRL Co. Ltd., Tokyo, Japan).
Measurement of hepatic biochemical parameters
Hepatic tissues were homogenized with a Janke & Kunkel Polytron homogenizer (ULTRA-TURRAX TP18/1051; IKA-Labortechnik, Staufeni, Germany) in a buffer (pH 7.4) containing 20 mM Tris HCl, 1 mM EGTA, 2 mM EDTA, and a protease inhibitor (2 µg/mL leupeptin cocktail). Hepatic tissue triglyceride (TG) levels were measured by SRL Co. Ltd.
Histology
Livers were formalin fixed, paraffin embedded, and processed for hematoxylin and eosin (H&E) or Azan staining.
Measurement of mRNA levels in the liver
Total RNA was extracted using trizol reagent (QIAGEN, Tokyo, Japan) according to the manufacturer’s protocol. cDNAs were synthesized from 1 µg of isolated RNA using a first-strand cDNA Synthesis Kit for real-time quantitative polymerase chain reaction (RT-PCR) (AMV)+ (Roche Diagnostics, Mannheim, Germany). RT-PCR was performed for quantitative assessment of mRNA using a Lightcycler (Roche Diagnostics) according to the manufacturer’s protocol. The sequences of the primers used were: Transforming growth factor (TGF)-β1 (TGF-F2, 5'-TGCTTCAGCTCCACAGAGAA-3'; TGF-R2, 5'-TACTGTGTGTCCAGGCTCCA-3'), type 1 collagen α1 (Col-F1, 5'-GAGAGCATGACCGATGGATT-3'; Col-R1, 5'-TTGAGGTTGCCAGTCTGTTG-3'), α-smooth muscle actin (α-SMA) (SMA-F1, 5'-TGTGCTGGACTCTGGAGATG-3'; SMA-R1, 5'-TCCAGAGCGACATAGCACAG-3').
Relative levels of target mRNAs were normalized to the corresponding level of GADPH mRNA (GADPH-F, 5'-TGAACGGGAAGCTCACTGG-3'; GADPH-R, 5'-TCCACCACCCTGTTGCTGTA-3') in the same cDNA sample using a standard curve method recommended in the Lightcycler Software Ver. 3.5 (Roche Diagnostics). PCR reactions and analyses were performed according to the manufacturer’s protocols (Roche Diagnostics) by UNITECH, Co. Ltd. (Chiba, Japan).
Statistical analysis
Data are mean ± SD. The significance of differences among values was analyzed by unpaired Student’s t test. A p value of <0.01 was considered significant.
Results
According to our hypothesis that LPS plays a role in hepatic fibrosis and in NASH development, we evaluated the mechanism of progressive hepatic fibrosis on NASH.
Each of the Zucker (fa/fa) rats groups were fed a regular or synthetic diet for 12 weeks, and treated with LPS or saline for the last 2 weeks. The three groups were abbreviated as follows: regular diet with LPS, regular diet-fed Zucker (fa/fa) rats treated with LPS group (n = 3); synthetic diet with LPS, synthetic diet-fed Zucker (fa/fa) rats treated with LPS group (n = 3); synthetic diet with saline, synthetic diet-fed Zucker (fa/fa) rats treated with saline group (n = 3).
Body weight and liver/body weight ratio
Body weight at the end of the experimental period increased significantly in the synthetic diet with LPS group compared to the other groups (531 ± 11.5, 584.3 ± 2.1, and 551 ± 3.6 g; in regular diet with LPS, synthetic diet with LPS, synthetic diet with saline, respectively) (Fig. 1). Liver/body weight ratio increased significantly in the synthetic diet with LPS group compared to the other groups (p<0.01) (Table 2).
Fig. 1.
Development of obesity in Zucker (fa/fa) rats fed a synthetic diet or regular diet. Data are mean ± SD *p<0.01 vs Regular diet with LPS, **p<0.01 vs Synthetic diet with saline.
Table 2.
Plasma, hepatic biochemical parameters, and liver weight/body weight ratios of Zucker (fa/fa) rats fed a regular diet or a synthetic diet, and injected with LPS or saline (i.p.)
| Regular diet + LPS |
Synthetic diet + LPS |
Synthetic diet + saline |
|||
|---|---|---|---|---|---|
| (n = 3) | (n = 3) | (n = 3) | |||
| Liver/body weight ratio (%) | 3.2 ± 0.12 | 4.3 ± 0.2*,** | 3.5 ± 0.1 | ||
| Glucose (mg/dL) | 233.46 ± 4.64 | 268.08 ± 4.35*,** | 239.97 ± 3.2 | ||
| Insulin (ng/mL) | 10.85 ± 0.39 | 24 ± 1.16*,** | 16.76 ± 0.34* | ||
| ALT (IU/L) | 87.06 ± 3.23** | 172.52 ± 31.59*,** | 63.26 ± 5.63 | ||
| Leptin (ng/mL) | 108.25 ± 4.47 | 259.8 ± 6.39*,** | 143.38 ± 7.35* | ||
| Hepatic TG (mg/dL) | 94.53 ± 5.47 | 227.72 ± 11.36*,** | 169.47 ± 3.59* |
Data are mean ± SD. *p<0.01 vs regular diet + LPS. **p<0.01 vs synthetic diet + saline.
Plasma and hepatic biochemical parameters
Synthetic diet with LPS showed significantly higher levels of plasma glucose and insulin among all groups (p<0.01) (Table 2).
To examine whether synthetic diet with LPS induced liver damage and steatosis, we quantified plasma ALT levels and hepatic TG accumulations. Plasma ALT levels in the synthetic diet with LPS group were about 2–3 times significantly higher than those in the other groups (p<0.01) (Table 2). Hepatic TG levels were compared among the groups and were found to be about 1.3–2.4 times significantly higher in the synthetic diet with LPS group than those in the other groups (p<0.01) (Table 2).
Zucker (fa/fa) rats, which are homozygous for leptin receptor mutation, have a deficiency in leptin signaling, and exhibit hyperphagia, obesity, and type 2 diabetes. We evaluated plasma leptin levels since it contributes to hepatic fibrosis to some extent in NAFLD development [17–19]. Remarkably high levels of leptin were observed in the synthetic diet with LPS group as compared to other groups (p<0.01) (Table 2).
Plasma LPS concentrations did not show a significant difference among all groups (data not shown).
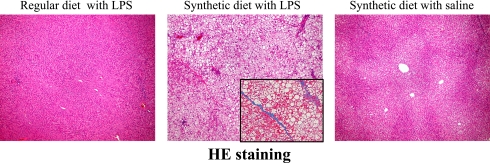
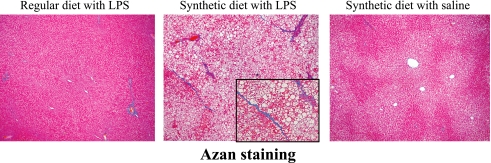
Histological analysis
Several fatty droplets and focal necrosis were observed in the synthetic diet with LPS group (Fig. 2). This finding coincided with biochemical examinations of blood and liver. Moreover, the livers of rats in the synthetic diet with LPS group showed mild pericellular fibrosis (Fig. 3).
Fig. 2.
Effects of a synthetic diet and LPS treatment on the livers of Zucker (fa/fa) rats. Various fatty droplets and focal necrosis are observed in the liver of synthetic diet-fed Zucker (fa/fa) rats treated with LPS. H&E staining (original magnification, 200×).
Fig. 3.
Effects of a synthetic diet and LPS treatment on the livers of Zucker (fa/fa) rats. Mild fibrosis is observed in the liver of synthetic diet-fed Zucker (fa/fa) rats treated with LPS. Azan staining (original magnification, 200×).
Hepatic mRNA expressions in rats
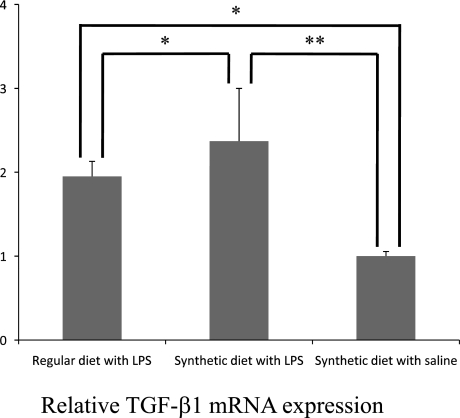
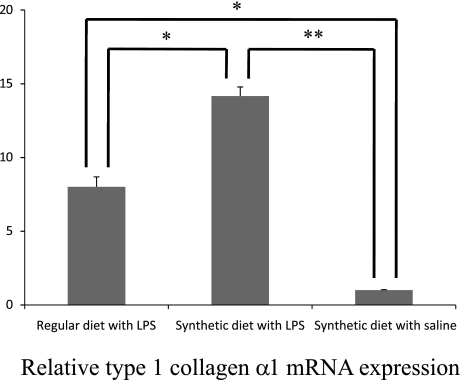
Observed histological findings suggested expressions of cytokines involved in inflammation and fibrosis. TGF-β1 produced from Kupffer cells and infiltrating inflammatory cells activates hepatic stellate cells (HSCs) that play a role in fibrogenesis in the liver, and result in accumulation of type 1 collagen [20–22]. Since TGF-β1, α-SMA; a marker of activated HSCs, and type 1 collagen are associated with hepatic fibrosis, their mRNA expressions were examined and found to be relatively higher in the synthetic diet with LPS group (Figs. 4, 5, 6) [23].
Fig. 4.
Expression of TGF-β1 mRNA in the liver of synthetic diet-fed Zucker (fa/fa) rats treated with LPS. TGF-β1 mRNA expressions are significantly increased in rat livers as evaluated by quantitative RT-PCR. Data are mean ± SD (*,**: p<0.01).
Fig. 5.
Expression of α-SMA mRNA in the liver of synthetic diet-fed Zucker (fa/fa) rats treated with LPS. α-SMA mRNA expressions are significantly increased in rat livers as evaluated by quantitative RT-PCR. Data are mean ± SD (*,**: p<0.01).
Fig. 6.
Expression of type 1 collagen α1 mRNA in the liver of synthetic diet-fed Zucker (fa/fa) rats treated with LPS. Type 1 collagen α1 mRNA expressions are significantly increased in rat livers as evaluated by quantitative RT-PCR. Data are mean ± SD (*,**: p<0.01).
Discussion
To evaluate the current role of LPS in NASH development using the two-hit theory, we employed Zucker (fa/fa) rats who developed obesity and type 2 diabetes. We found that 12 weeks of exposure to a synthetic diet and subsequent 2-week treatment with low-dose LPS led to steatosis and focal necrosis accompanied by fibrosis in the livers of Zucker (fa/fa) rats as confirmed by histological findings. Elevation of plasma ALT levels and increase in accumulation of hepatic TG supported the development of steatosis with liver damage. We showed that subsequent 2-week treatment with LPS stimulated expressions of profibrogenic mRNAs such as TGF-β1, α-SMA, and type 1 collagen α1 in the livers of synthetic diet-fed rats. The increase in these hepatic mRNA expressions corresponded to the histological findings in the livers of synthetic diet-fed rats treated with LPS.
Zucker (fa/fa) rats are highly susceptible to low-dose LPS for short term and develop steatohepatitis, but fibrosis development in those rats was not confirmed [8]. The combination of 12-week exposure to a synthetic diet and subsequent 2-week treatment with LPS induced hepatic steatosis and fibrosis. Hepatic fibrosis was not induced in regular diet-fed rats, and was therefore induced by the combination of exposure to a synthetic diet and LPS treatment.
Another interesting result was that the synthetic diet-fed Zucker (fa/fa) rats treated with LPS showed an increase in body weight and elevation of liver/body weight ratio. This may be because the subsequent 2-week treatment with LPS synergistically enhanced accumulation of hepatic TG into the hepatocytes of synthetic diet-fed rats, which corresponded with the histological findings. It has also been suggested that induction of insulin resistance due to LPS treatment can promote adipose accumulation in rats [24].
The histological findings and biochemical examinations in the present study may highlight a relationship between LPS treatment and plasma leptin levels on the development of hepatic fibrosis. We confirmed that LPS treatment induced a significant increase in plasma leptin levels in synthetic diet-fed Zucker (fa/fa) rats, which was independent of liver damage, hyperglycemia, and hyperinsulinemia through TNF-α mediated by LPS [25]. Leptin is produced from adipocytes [17]. It activates HSCs and contributes to the development of hepatic fibrosis to some extent without leptin signaling [18, 19]. Increase in plasma leptin levels in synthetic diet-fed rats observed in the present study may be induced by treatment with LPS itself. This relationship led us to the hypothesis that LPS may be a risk factor for hepatic fibrosis.
Hepatic steatosis in humans gradually progresses with daily consumption of food. Excessive intake of disaccharides induces accumulation of triglycerides in hepatocytes. Zucker (fa/fa) rats develop hepatic steatosis by short-term feeding of a diet containing a high content of disaccharides such as sucrose (85.7 cal% to carbohydrate) [16]. We confirmed that our diet containing a low content of disaccharides such as sucrose and lactose (17.4 cal% to carbohydrate) compared with the diet induced gradual hepatic steatosis in Zucker (fa/fa) rats. Therefore, it was important that hepatic steatosis in Zucker (fa/fa) rats was gradually induced by 12-week feeding of the diet used in the present study because it was the “first hit” of the two-hit theory.
NASH is commonly observed in patients with obesity and in type 2 diabetes [1]. Zucker (fa/fa) rats lack leptin signals. Although the deficiency is rarely observed in obese patients, these rats reflect metabolic abnormalities in humans [26]. We think that the combination of Zucker (fa/fa) rats and LPS contained in normal portal blood is significant. Compared with the result by treatment with probiotics, our results suggested that LPS treatment was associated with the development of hepatic fibrosis [12, 13]. Histological findings in the livers of synthetic diet-fed Zucker (fa/fa) rats treated with LPS did not demonstrate typical steatohepatitis, but this result suggested that LPS had a role in the pathogenesis of hepatic fibrosis in the two-hit theory as the “second hit.” We suggest that our protocol may be useful for studying the role of LPS during the development of hepatic fibrosis in the two-hit theory.
References
- 1.Neuschwander-Teri B.A., Caldwell S.H. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell S.H., Crespo D.M. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J. Hepatol. 2004;40:578–584. doi: 10.1016/j.jhep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell S.H., Crespo D.M., Kang H.S., Al-Osaimi A.M. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki D., Hashimoto E., Kaneda K., Tokushige K., Shiratori K. Liver failure caused by non-alcoholic steatohepatitis in an obese young male. J. Gastroenterol. Hepatol. 2005;20:327–329. doi: 10.1111/j.1440-1746.2005.03724.x. [DOI] [PubMed] [Google Scholar]
- 5.Day C.P., James O.F. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 6.Reid A.E. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710–723. doi: 10.1053/gast.2001.27126. [DOI] [PubMed] [Google Scholar]
- 7.Feldstein A.E., Canbay A., Guicciardi M.E., Higuchi H., Bronk S.F., Gores G.J. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J. Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 8.Yang S.Q., Lin H.Z., Lane M.D., Clemens M., Diehl A.M. Obesity increases sensitivity to endotoxin liver injury: implication for the pathogenesis of steatohepatitis. Proc. Natl. Acad. Sci. USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George D.K., Goldwurm S., MacDonald G.A., Cowley L.L., Walker N.I., Ward P.J., Jazwinska E.C., Powell L.W. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–318. doi: 10.1016/s0016-5085(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 10.Harrison S.A., Kadakia S., Lang K.A., Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am. J. Gastroenterol. 2002;97:2714–2724. doi: 10.1111/j.1572-0241.2002.07069.x. [DOI] [PubMed] [Google Scholar]
- 11.Letteron P., Fromenty B., Terris B., Degott C., Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J. Hepatol. 1996;24:200–208. doi: 10.1016/s0168-8278(96)80030-4. [DOI] [PubMed] [Google Scholar]
- 12.Li Z., Yang S., Lin H., Huang J., Watkins P.A., Moser A.B., DeSimone C., Song X.Y., Diehl A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 13.Solga S.F., Diehl A.M. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J. Hepatol. 2003;38:681–687. doi: 10.1016/s0168-8278(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 14.Portincasa P., Grattagliano I., Palmieri V.O., Palasciano G. Nonalcoholic steatohepatitis: recent advances from experimental model to clinical management. Clin. Biochem. 2005;38:203–217. doi: 10.1016/j.clinbiochem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui H. TNF signaling as pleiotropic gates in the liver. Hepatol. Res. 2005;31:121–123. doi: 10.1016/j.hepres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Novikoff P.M. Fatty liver induced in Zucker “fatty” (ff) rats by a semisynthetic diet rich in sucrose. Proc. Natl. Acad. Sci. USA. 1977;74:3038–3042. doi: 10.1073/pnas.74.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 18.Potter J.J., Womack L., Mezey E., Anania F.A. Transdifferentitation of rat hepatic stellate cells results in leptin expression. Biochem. Biophys. Res. Commun. 1998;244:178–182. doi: 10.1006/bbrc.1997.8193. [DOI] [PubMed] [Google Scholar]
- 19.Saxena N.K., Ikeda K., Rockey D.C., Friedman S.L., Anania F.A. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka M., Tsukamoto H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor β: implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology. 1990;11:599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- 21.Friedman S.L. The cellular basis of hepatic fibrosis. Mechanism and treatment strategies. N. Engl. J. Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt-Gräff A., Krüger S., Bochard F., Gabbiani G., Denk H. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am. J. Pathol. 1991;138:1233–1242. [PMC free article] [PubMed] [Google Scholar]
- 23.Enzan H., Himeno H., Iwamura S., Saibara T., Onishi S., Yamamoto Y., Hara H. Immunohistochemical identification of Ito cells and their myofibroblastic transformation in adult human liver. Virchows. Arch. 1994;424:249–256. doi: 10.1007/BF00194608. [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil G.S., Budavari A., Murray D., Spiegelman B.M. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin. Invest. 1994;94:1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J., Sakaida I., Uchida K., Terai S., Okita K. Leptin enhances TNF-alpha production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502–1515. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Paracchini V., Pedotti P., Taioli E. Genetics of leptin and obesity: a HuGE review. Am. J. Epidemiol. 2005;162:101–114. doi: 10.1093/aje/kwi174. [DOI] [PubMed] [Google Scholar]