Abstract
Iron may induce oxidative stress via production of reactive oxygen species, facilitating mammary carcinogenesis. This study investigated the role of iron in relation to oxidative stress as a potential risk factor in the development of breast cancer (BC). BC patients (n = 121) and healthy age-matched controls (n = 149) were entered into the study. Iron and antioxidant vitamins intakes were estimated using a quantitative food frequency questionnaire. Thirty one subjects from each group provided blood samples for measurement of serum iron, plasma malondialdehyde (MDA) and ferric reducing ability of plasma (FRAP). Total and non-heme iron intake of BC patients were lower than those of the controls. However, the serum iron level was significantly higher in BC patients. Plasma MDA levels were also significantly higher in BC patients whereas no significant difference in FRAP values were observed between the two groups. Log-transformed serum iron concentration showed no significant correlation with MDA or FRAP. These results suggest that serum iron overload may be a breast cancer risk factor possibly due to increased oxidative stress.
Keywords: breast cancer, iron, oxidative stress, MDA
Introduction
Breast cancer (BC) is the most prevalent form of cancer among women worldwide. The rate of breast cancer is increasing rapidly in East Asian countries where the incidence had previously been low [1, 2]. Risk factors for BC include family history, circulating levels of estrogen, obesity, and certain diets. The increased consumption of meat has been speculated to be a contributing factor to excess iron intake which may facilitate cancer development [3]. Iron is an essential micronutrient and the most abundant transition metal in the human body. It plays a diversity of important physiological functions to include oxygen transport, energy production, and DNA synthesis [4, 5]. However, as a transition metal, iron’s loosely bound electrons in its outer shell facilitates the production of reactive oxygen species (ROS), resulting in oxidative stress, DNA single breaks, and oncogene activation [6, 7]. Interaction between free radicals and polyunsaturated fatty acids in cell membranes also induces lipid peroxidation, which may damage the cell structure and function [8].
The accumulation of molecular damage has been linked to various pathological conditions including carcinogenesis [9]. Elevated DNA adducts as well as DNA strand breaks have been found in aggressive breast tumors [10]. It has been proposed that the conversion of estradiol to catechol estrogen and semi-quinones may be a key process in breast carcinogenesis [11]. Iron may reinforce estrogen-induced carcinogenesis via accelerated production of ROS [12].
There has been research correlating the intake of heme iron with BC risk [13]. Cade et al. reported that dietary iron intake was inversely associated with breast cancer risk [14]. Others have found no association between dietary iron intake and breast cancer [15–17]. Serum iron levels of female who died of cancer have been shown to be significantly higher than the level in control group [18]. No significant association between serum iron level and breast cancer risk was found in prior reports [19–22]. This study investigated the role of iron in relation to oxidative stress as a possible risk factor in the development of breast cancer.
Materials and Methods
Subjects
The study involved 270 women aged 20–80 years. Breast cancer patients (n = 121) and their age-matched controls (n = 149) were recruited from Bundang Jesaeng General Hospital or St. Vincent’s Hospital, Gyeonggi-do, South Korea between 2006–2007. Breast cancer patients prior to receiving any chemotherapy or radiotherapy were allowed participation in the study. The number of patients classified as stage 0, I, II, III and IV were 20, 41, 41, 15 and 4, respectively (Table 1). Subjects with any history of liver diseases, diabetes mellitus, respiratory disorders or cardiovascular diseases were not included in the study. The study was approved by the Institutional Review Board of the Bundang Jesaeng General Hospital and St. Vincent’s Hospital in South Korea.
Table 1.
Anthropometric and lifestyle characteristics of the study subjects
| Control | Cancer | t test (p value) | X2-test (p value) | |
|---|---|---|---|---|
| Age (yrs) | 49.27 ± 8.63 | 50.07 ± 9.30 | 0.466 | — |
| Height (cm) | 157.79 ± 4.86 | 156.71 ± 4.74 | 0.066 | — |
| Weight (kg) | 56.78 ± 6.83 | 58.28 ± 7.49 | 0.09 | — |
| BMI (kg/m2) | 22.83 ± 2.73 | 23.76 ± 2.92 | p<0.01 | — |
| Smoking | ||||
| Current (%) | 2.01 | 10.71 | X2 = 9.7751 | |
| Former (%) | 0.67 | 1.79 | — | (df = 2) |
| Never (%) | 97.32 | 87.5 | 0.007 | |
| Drinking | ||||
| Current (%) | 44.3 | 48.21 | X2 = 1.2110 | |
| Former (%) | 0.67 | 1.79 | — | (df = 2) |
| Never (%) | 55.03 | 50 | 0.545 | |
| Clinical Stage | ||||
| 0 | 20* | |||
| I | 41 | |||
| II | 41 | |||
| III | 15 | |||
| IV | 4 |
Each value represents the Mean ± SD. BMI indicated body mass index. *Number of patients according to the stage.
Dietary assessment
Dietary intakes were assessed using a 100-item semi-quantified food frequency questionnaire. Subjects completed the interviewer-administered questionnaire on the average frequency (daily, weekly, monthly, yearly or never) and consumption (small- a half of the median size, median, and large-1.5 times of the median size) of each food item. A color photograph of the median sized food items were used to improve the accuracy of portion size estimates. The food frequency questionnaire was coded and analyzed by a computer aided nutrient analysis program for professionals (CAN-Pro 3.0, APAC Intelligence, Seoul, South Korea). The intake of heme iron is calculated as 40% of the iron supplied from MFP (meat, fish, poultry), while the remaining was calculated as non-heme iron [23, 24].
Blood analysis
Blood samples were taken from 31 age-matched members of each group who agreed to donate. The samples were left at room temperature for 30 min, centrifuged for 15 min at 2,500 rpm to separate the serum and plasma, and then stored at −70°C. Serum iron concentrations were determined by ICP-Atomic Emission Spectrometer (Vista-PRO, Varian, Australia) after dissolution with nitric acid and H2O2 using a microwave (Ethos touch control, Milestone Inc., Italy). MDA (malondialdehyde) was measured in plasma based on the method described by Yagi et al. [25]. FRAP (ferric reducing ability of plasma) assay was carried out based on the method described by Benzie and Strain [26].
Statistical analysis
All analyses were performed using the SASTM (version 9.1, SAS Inc., Cary, NC). The data were expressed as means ± standard deviations (SD) and the significance of differences between the patient and control group was analyzed using the Student t test. Comparisons for general characteristics and serum iron levels were analyzed using the x2 test. The level of serum iron did not show a normal distribution, and the log-transformed values were used for statistical analysis. The associations between serum iron and oxidative stress markers were determined by Pearson’s correlation. The level of significance was set at p<0.05 unless otherwise stated.
Results
Anthropometric and lifestyle measures of the studysubjects are shown in Table 1. The average age, height and weight of BC patients were not significantly different from those of the control subjects. However, the mean BMI of cancer patients was significantly higher than that of the control subjects (p<0.01). The patient group had a larger proportion of subjects who were current smokers (p<0.01) compared to their controls. No difference was observed in alcohol intake habit.
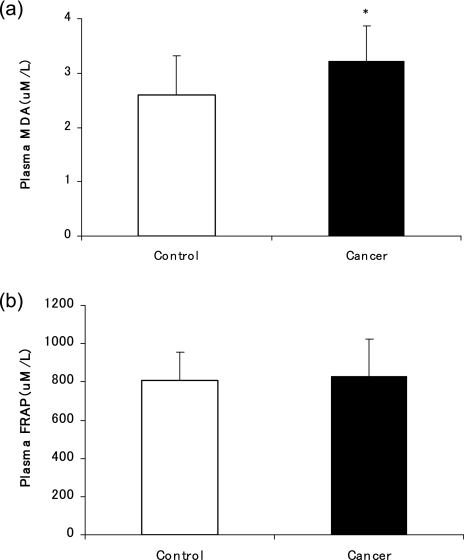
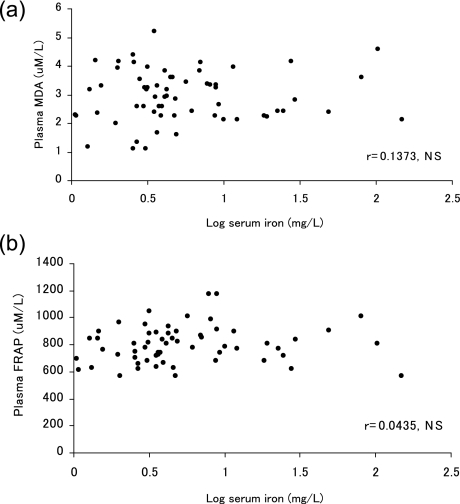
Energy and antioxidant vitamins intakes are shown in Table 2. The energy intake did not differ between the two groups. The intakes of vitamin A (p<0.001), β-carotene (p<0.001), vitamin C (p<0.001) and vitamin E (p<0.01) in the BC patients were significantly lower than those of the control subjects. Table 3 compares dietary iron intake and serum iron levels between BC patients and their counterparts. The breast cancer group consumed significantly less total iron (p<0.001) and non-heme iron (p<0.001) compared to the control group. No difference was observed with heme iron intake. However, the serum iron level and the proportion of iron overloaded subjects were significantly higher in BC patients compared to those of the control group (p<0.001 and p<0.05, respectively). The levels of plasma oxidative stress markers are shown in Fig. 1. Plasma MDA level was significantly higher in BC patients compared to that of the control subjects (p<0.05). There was no significant difference in plasma levels of FRAP between the BC and control group. Log-transformed serum iron concentrations showed no significant correlation with either MDA or FRAP after the values were adjusted for BMI and smoking status (Fig. 2).
Table 2.
Energy and antioxidant vitamin intakes of the breast cancer and control group
| Control | Cancer | t test (p value) | |
|---|---|---|---|
| Energy (kcal) | 1898.00 ± 427.66 | 1911.58 ± 447.38 | 0.799 |
| Vitamin A (ug RE) | 924.77 ± 569.25 | 674.37 ± 318.05 | p<0.001 |
| Retinol | 93.27 ± 56.29 | 100.13 ± 63.64 | 0.924 |
| β-carotene | 4564.86 ± 2805.73 | 3166.32 ± 1686.16 | p<0.001 |
| Vitamin C (mg) | 159.76 ± 72.84 | 128.56 ± 61.91 | p<0.001 |
| Vitamin E (mg) | 15.48 ± 6.59 | 13.20 ± 6.26 | p<0.01 |
Each value represents the Mean ± SD.
Table 3.
Dietary iron intake and the level of serum iron of the breast cancer and control group
| Control | Cancer | t test (p value) | X2-test (p value) | |
|---|---|---|---|---|
| Iron intake (mg) | 15.22 ± 4.70 | 13.27 ± 4.14 | p<0.001 | — |
| Heme iron | 0.45 ± 0.26 | 0.46 ± 0.30 | 0.591 | — |
| Non-heme iron | 14.84 ± 4.64 | 12.8 ± 4.02 | p<0.001 | — |
| Serum iron (mg/L) | 1.71 ± 0.43 | 3.03 ± 1.87 | p<0.001 | |
| Iron overload (%) | 45.16 | 70.97 | — | X2 = 4.2393 |
| Normal (%) | 54.84 | 29.03 | (df = 1) 0.039 |
Each value represents the Mean ± SD.
Fig. 1.
The level of plasma MDA (a) and FRAP (b) of the breast cancer and control group. *p<0.05; Significance between cancer and control as determined by Student’s t test.
Fig. 2.
Correlations between log-transformed serum iron and plasma MDA (a) and FRAP (b). NS; Not significant.
Discussion
To investigate iron as a possible risk factor in the development of breast cancer in relation to excess oxidative stress, we measured dietary intake and serum levels of iron, along with blood oxidative stress markers in BC patients and their healthy counterparts. Iron is an essential micronutrient to maintain normal cell function. However, oxidative injuries as a consequence of excess local iron accumulation within the breast have been postulated as a risk factor in breast carcinogenesis [27]. Excessive intake of iron may predispose to mammary carcinogenesis due to the fact that free iron works as a catalyst for ROS generation [12]. However, study results on the relationship between dietary iron intake and breast cancer are not consistent. Epidemiological studies have reported that there is no association between dietary iron intake and BC risk [16, 17]. Lee et al. also found no overall association between dietary intake of total iron or heme iron with breast cancer [28]. However, a positive association of both iron and heme iron intake with breast cancer risk among women who consumed greater than 20 grams of alcohol per day was found indicating iron as a possible confounding risk factor of breast cancer [28]. A large scale study conducted in 3,452 Chinese female BC patients reported that the intake of heme iron increased the risk of breast cancer [13].
In the present study, the intake of total and non-heme iron was significantly lower in BC patients compared to the control subjects, while the level of serum iron in BC patients was higher than that of the control subjects. The efficiency of iron absorption is influenced by various factors including the dietary source of iron and body iron storage. The intake of non-heme iron consisted more than 95% of total iron intake in both groups. Due to the low bioavailability of non-heme iron, it is thought that the difference of non-heme iron intake between the breast cancer patients and control subjects did not largely affect the level of serum iron.
Results from this study also indicated that a significantly higher proportion of the cancer patients (71.0%) were iron overloaded compared to the control group (45.2%). NHANES II data showed that the average serum iron level of 3,244 BC patients was significantly higher than that of their control [18]. The authors suggested that the relative risk of developing cancer increases by 66% as the concentration of iron increases by 100 µg/dl. Iron-induced oxidative stress may leads to one of two consequences: (1) redox regulation failure that results into lipid peroxidation and oxidative DNA damage, or (2) redox regulation that facilitates a variety of reducing and oxidative stress-protective mechanisms through signal transduction. Both consequences appear to be essential for iron-induced carcinogenesis. Iron may facilitate estrogen-induced carcinogenesis via accelerated production of ROS and induce oxidative stress from redox regulation failure [12].
The most common parameters of oxidative stress include blood levels of antioxidants, lipid peroxides and other oxidized biomolecules [29–31]. We measured plasma MDA concentrations as a representative for lipid peroxides and FRAP as a marker of oxidative stress levels in plasma. Results indicated that MDA levels were significantly higher in BC patients. Although the FRAP value was slightly higher in cancer patients, no statistical significance was observed.
Previous studies have found that breast cancer patients have higher levels of plasma MDA compared to their healthy controls [19, 32, 33]. Women with mammographic dysplasia also displayed increased levels of urinary MDA [34]. The increased circulating level of oxidized lipid products such as MDA may derive from the lower intakes of antioxidant nutrients such as vitamin A, β-carotene, vitamin C, vitamin E, and/or higher level of oxidative stress such as serum iron overload as observed in this study. The elevated level of iron may serve as a stimulator to produce lipid peroxides [35–37]. Iron has been implicated as having a major role in the development of cardiovascular complications by acting as a catalyst to produce oxidized lipid molecules including oxLDL [35]. Renal lipid peroxidation was also observed in animals treated with ferric nitorilotriacetate, a known renal carcinogen [36, 37].
Iron has been indicated as a tumor associated factor in breast carcinogenesis [12]. Ferritin, the iron storage protein, has been shown to be increased in breast tumor tissues [38] indicating there is an increased need for iron availability in breast cancer development. Iron was also found significantly higher in breast tumor tissue compared to normal tissue although it is not clear whether iron overload is a cause or a consequence of carcinogenesis [39]. The authors suggested that pathological accumulation of iron in breast tissues may be closely related to the development of breast cancer. Mechanistic studies showed that circulating estrogen facilitated iron release from ferritin storage, which contributes to cancer initiation [40]. Based on these results, it is speculated that higher serum levels of iron may be partly associated with oxidative stresses found in breast cancer patients. Although iron overload-induced increases in oxidative stress has been explained to facilitate tumor formation by regulating cell signaling processes that control proliferation and apoptosis [41], there is no evidence to show carcinogenesis itself can accumulate more iron in breast tissue or women who tends to accumulate more iron in their body have higher risk of breast cancer. No significant associations between serum iron and oxidative stress markers were found. The level of oxidative stress is related to the imbalance between the production of ROS and the antioxidant capability, and iron is one of many factors regulating oxidation-reduction homeostasis. Therefore, in addition to iron, other factors are involved in the maintenance of the oxidation-reduction balance. Our small sample size and limited number of markers may have decreased the power to detect a significant association between serum iron and oxidative stress markers. In conclusion, serum iron overload may be related to breast cancer risk possibly secondary to increased oxidative stress. A lack of consistency among different markers advocates for further studies to elucidate factors that contribute to oxidative stress and breast tumor development.
Acknowledgment
This work was supported by the SRC Research Center for Women’s Diseases of Sookmyung Women’s University and by Korea Research Foundation Grant (KRF-2005-042-C00194).
Abbreviations
- BC
breast cancer
- ROS
reactive oxygen species
- MDA
malondialdehyde
- FRAP
ferric reducing ability of plasma
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA. Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Korea National Cancer Center, author. Report on the statistics for national cancer incidence 1999–2001; Korea National Cancer Center; 2006. [Google Scholar]
- 3.Tappel A. Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med. Hypotheses. 2007;68:562–564. doi: 10.1016/j.mehy.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Aisen P., Enns C., Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 5.Lieu P.T., Heiskala M., Peterson P.A., Yang Y. The roles of iron in health and disease. Mol. Aspects. Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 6.McCord J.M. Iron, free radicals, and oxidative injury. Semin. Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- 7.Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat. Res. 2003;533:153–171. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Khanzode S.S., Muddeshwar M.G., Khanzode S.D., Dakhale G.N. Antioxidant enzymes and lipid peroxidation in different stages of breast cancer. Free Radic. Res. 2004;38:81–85. doi: 10.1080/01411590310001637066. [DOI] [PubMed] [Google Scholar]
- 9.Klaunig J.E., Xu Y., Isenberg J.S., Bachowski S., Kolaja K.L., Jiang J., Stevenson D.E., Walborg E.F. Jr. The role of oxidative stress in chemical carcinogenesis. Environ. Health Perspect. 1998;106:289–295. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malins D.C., Polissar N.L., Gunselman S.J. Progression of human breast cancers to the metastatic state is linked to hydroxyl radical-induced DNA damage. Proc. Natl. Acad. Sci. USA. 1996;93:2557–2563. doi: 10.1073/pnas.93.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalieri E., Chakravarti D., Guttenplan J., Hart E., Ingle J., Jankowiak R., Muti P., Rogan E., Russo J., Santen R., Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Liehr J.G., Jones J.S. Role of iron in estrogen-induced cancer. Curr. Med. Chem. 2001;8:839–849. doi: 10.2174/0929867013372931. [DOI] [PubMed] [Google Scholar]
- 13.Kallianpur A.R., Lee S.A., Gao Y.T., Lu W., Zheng Y., Ruan Z.X., Dai Q., Gu K., Shu X.O., Zheng W. Dietary animal-derived iron and fat intake and breast cancer risk in the Shanghai Breast Cancer Study. Breast Cancer Res. Treat. 2008;107:123–132. doi: 10.1007/s10549-007-9538-3. [DOI] [PubMed] [Google Scholar]
- 14.Cade J., Thomas E., Vail A. Case-control study of breast cancer in south east England: nutritional factors. Epidemiol. Community Health. 1998;52:105–110. doi: 10.1136/jech.52.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Böing H., Martinez L., Frentzel-Beyme R., Oltersdorf U. Regional nutritional pattern and cancer mortality in the Federal Republic of Germany. Nutr. Cancer. 1985;7:121–130. doi: 10.1080/01635588509513847. [DOI] [PubMed] [Google Scholar]
- 16.Adzersen K.H., Jess P., Freivogel K.W., Gerhard I., Bastert G. Raw and cooked vegetables, fruits, selected micronutrients, and breast cancer risk: a case-control study in Germany. Nutr. Cancer. 2003;46:131–137. doi: 10.1207/S15327914NC4602_05. [DOI] [PubMed] [Google Scholar]
- 17.Kabat G.C., Miller A.B., Jain M., Rohan T.E. Dietary iron and heme iron intake and risk of breast cancer: a prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 2007;16:1306–1308. doi: 10.1158/1055-9965.EPI-07-0086. [DOI] [PubMed] [Google Scholar]
- 18.Wu T., Sempos C.T., Freudenheim J.L., Muti P., Smit E. Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann. Epidemiol. 2004;14:195–201. doi: 10.1016/S1047-2797(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y.L., Sheu J.Y., Lin T.H. Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin. Biochem. 1999;32:131–136. doi: 10.1016/s0009-9120(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Geissler C., Parpia B., Li J., Campbell T.C. Antioxidant status and cancer mortality in China. Int. J. Epidemiol. 1992;21:625–635. doi: 10.1093/ije/21.4.625. [DOI] [PubMed] [Google Scholar]
- 21.Knekt P., Reunanen A., Takkunen H., Aromaa A., Heliövaara M., Hakulinen T. Body iron stores and risk of cancer. Int. J. Cancer. 1994;56:379–382. doi: 10.1002/ijc.2910560315. [DOI] [PubMed] [Google Scholar]
- 22.Ulbrich E.J., Lebrecht A., Schneider I., Ludwig E., Koelbl H., Hefler L.A. Serum parameters of iron metabolism in patients with breast cancer. Anticancer Res. 2003;23:5107–5109. [PubMed] [Google Scholar]
- 23.Cook J.D., Monsen E.R. Food iron absorption in human subjects. III. Comparison of the effect of animal proteins on nonheme iron absorption. Am. J. Clin. Nutr. 1976;29:859–867. doi: 10.1093/ajcn/29.8.859. [DOI] [PubMed] [Google Scholar]
- 24.Monsen E.R., Hallberg L., Layrisse M., Hegsted D.M., Cook J.D., Mertz W., Finch C.A. Estimation of available dietary iron. Am. J. Clin. Nutr. 1978;31:134–141. doi: 10.1093/ajcn/31.1.134. [DOI] [PubMed] [Google Scholar]
- 25.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 26.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Kallianpur A.R., Hall L.D., Yadav M., Christman B.W., Dittus R.S., Haines J.L., Parl F.F., Summar M.L. Increased prevalence of the HFE C282Y hemochromatosis allele in women with breast cancer. Cancer Epidemiol. Biomarkers Prev. 2004;13:205–212. doi: 10.1158/1055-9965.epi-03-0188. [DOI] [PubMed] [Google Scholar]
- 28.Lee D.H., Anderson K.E., Harnack L.J., Jacobs D.R. Jr. Dietary iron intake and breast cancer: The Iowa Women’s Health Study. Proc. Am. Assoc. Cancer Res. 2004;45:A2319. [Google Scholar]
- 29.Itabe H., Yamamoto H., Imanaka T., Shimamura K., Uchiyama H., Kimura J., Sanaka T., Hata Y., Takano T. Sensitive detection of oxidatively modified low density lipoprotein using a monoclonal antibody. J. Lipid Res. 1996;37:45–53. [PubMed] [Google Scholar]
- 30.Schnitzer E., Pinchuk I., Bor A., Fainaru M., Samuni A.M., Lichtenberg D. Lipid oxidation in unfractionated serum and plasma. Chem. Phys. Lipids. 1998;92:151–170. doi: 10.1016/s0009-3084(98)00021-8. [DOI] [PubMed] [Google Scholar]
- 31.Dotan Y., Lichtenberg D., Pinchuk I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog. Lipid Res. 2004;43:200–227. doi: 10.1016/j.plipres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Polat M.F., Taysi S., Gul M., Cikman O., Yilmaz I., Bakan E., Erdogan F. Oxidant/antioxidant status in blood of patients with malignant breast tumour and benign breast disease. Cell Biochem. Funct. 2002;20:327–331. doi: 10.1002/cbf.980. [DOI] [PubMed] [Google Scholar]
- 33.Sener D.E., Gönenç A., Akinci M., Torun M. Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem. Funct. 2007;25:377–382. doi: 10.1002/cbf.1308. [DOI] [PubMed] [Google Scholar]
- 34.Boyd N.F., McGuire V. The possible role of lipid peroxidation in breast cancer risk. Free Radic. Biol. Med. 1991;10:185–190. doi: 10.1016/0891-5849(91)90074-d. [DOI] [PubMed] [Google Scholar]
- 35.de Valk B., Marx J.J. Iron, atherosclerosis, and ischemic heart disease. Arch. Intern. Med. 1999;159:1542–1548. doi: 10.1001/archinte.159.14.1542. [DOI] [PubMed] [Google Scholar]
- 36.Toyokuni S., Okada S., Hamazaki S., Minamiyama Y., Yamada Y., Liang P., Fukunaga Y., Midorikawa O. Combined histochemical and biochemical analysis of sex hormone dependence of ferric nitriolotriacetate-induced renal lipid peroxidation in ddY mice. Cancer Res. 1990;50:5574–5580. [PubMed] [Google Scholar]
- 37.Toyokuni S., Uchida K., Okamoto K., Hattori-Nakakuki Y., Hiai H., Stadtman E.R. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc. Natl. Acad. Sci. USA. 1994;91:2616–2620. doi: 10.1073/pnas.91.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott R.L., Elliott M.C., Wang F., Head J.F. Breast carcinoma and the role of iron metabolism. A cytochemical, tissue culture, and ultrastructural study. Ann. NY Acad. Sci. 1993;698:159–166. doi: 10.1111/j.1749-6632.1993.tb17204.x. [DOI] [PubMed] [Google Scholar]
- 39.Ionescu J.G., Novotny J., Stejskal V., Lätsch A., Blaurock-Busch E., Eisenmann-Klein M. Increased levels of transition metals in breast cancer tissue. Neuro. Endocrinol. Lett. 2006;27:36–39. [PubMed] [Google Scholar]
- 40.Wyllie S., Liehr J.G. Release of iron from ferritin storage by redox cycling of stilbene and steroid estrogen metabolites: a mechanism of induction of free radical damage by estrogen. Arch. Biochem. Biophys. 1997;346:180–186. doi: 10.1006/abbi.1997.0306. [DOI] [PubMed] [Google Scholar]
- 41.Galaris D., Skiada V., Barbouti A. Redox signaling and cancer: the role of “labile” iron. Cancer Lett. 2008;266:21–29. doi: 10.1016/j.canlet.2008.02.038. [DOI] [PubMed] [Google Scholar]