Abstract
Many researchers have hypothesized that differences in reactive oxygen species levels can trigger the cellular decision between hypertrophy and cell death in cardiomyocytes. In the present study, we examined the relationship between reactive oxygen species levels and hypertrophy or cell death in H9c2 cardiomyocytes after the addition of hydrogen peroxide. Following addition of hydrogen peroxide, we observed a slight increase in fluorescence intensity of 2',7'-dichlorofluorescein, a probe of intracellular reactive oxygen species, and cell hypertrophy in H9c2 cells (normal cells). In contrast, a dramatic increase in fluorescence intensity was followed by cell death in glutathione-depleted H9c2 cells. In the presence of the antioxidant Trolox or the iron chelator deferoxamine, both normal and glutathione-depleted cells developed hypertrophy without a concomitant increase in levels of reactive oxygen species. An inhibitor of p53, pifithrin-alpha, prevented cell death after the addition of hydrogen peroxide; instead a substantial increase in levels of reactive oxygen species and hypertrophy were observed. These results suggest that H9c2 cells exhibit differential sensitivity to intracellular reactive oxygen species generation with regard to their hypertrophic versus death responses to exogenously added hydrogen peroxide.
Keywords: H2O2, cardiomyocyte, hypertrophy, cell death, ROS
Introduction
Numerous studies have demonstrated that reactive oxygen species (ROS) may cause aging [1] and diseases such as malignant neoplasm [2], Alzheimer’s disease [3], cataracts [4] and cardiac dysfunction [5]. Hydrogen peroxide that can be converted to HO· is removed by intracellular enzymes such as catalase and glutathione peroxidase (GPx). GPx catalyzes the elimination reaction of H2O2 using reduced glutathione (GSH). Large quantities of GSH, an antioxidant, are found in organisms that eliminate ROS by enzymatic or non-enzymatic mechanisms. In contrast, the heart has low activity of GPx and catalase and an overall lower GSH content as compared with other organs [6], suggesting that the heart is more sensitive to ROS. Indeed, it has been demonstrated that ischemia-reperfusion leading to an increase in ROS levels results in cardiac contractile dysfunction [7, 8]. Administration of L-buthionine-sulfoximine (BSO) which blocks GSH biosynthesis to diabetic rats increases cardiac cell death [9]. These findings suggest that when the generation of ROS exceeds the elimination of it, excess ROS can impair normal biological functions.
There are numerous studies that various stimuli cause myocardial cell death and hypertrophy. Takemoto et al. [10] demonstrated that angiotensin II (Ang II) results in the generation of ROS through NADPH oxidase activation and the induction of hypertrophy in neonatal rat cardiomyocytes. Grishko et al. [11] observed that Ang II-induced apoptosis and the cell death are inhibited by an NADPH oxidase inhibitor in neonatal cardiomyocytes. In adult rat ventricular myocytes, β-adrenergic receptor stimulation by norepinephrin induces apoptosis mediated by the ROS/c-Jun N-terminal kinase (JNK)-dependent mitochondrial death pathway [12]. In addition, norepinephrin also induces hypertrophy in H9c2 cells, a cardiomyocyte cell line derived from rat ventricles [13]. These findings imply that ROS are associated with cell death and/or hypertrophy in cardiomyocytes. It is demonstrated that cyclic mechanical stretching of neonatal rat ventricular myocytes generates superoxide anion radical (O2·−) that induces hypertrophy and/or cell death depending on the stretch levels [14]. Chen et al. [15] demonstrated that a fraction of cells undergoes apoptosis at low concentrations of added H2O2 and the surviving cells develop hypertrophy, whereas high dose of H2O2 causes necrosis in H9c2 cells and primary cultured neonatal rat cardiomyocytes. These findings suggest the possibility that differences in intracellular ROS levels can trigger the decision between hypertrophy and cell death in cardiomyocytes.
In the present study, we investigated the relationship between ROS levels and hypertrophy or cell death in H9c2 cardiomyocytes after the addition of H2O2. Here, we propose that the development of hypertrophy might not be connected with the levels of intracellular ROS, but death is, after the addition of H2O2 to H9c2 cells.
Materials and Methods
Chemicals
BSO, deferoxamine (DFX), Dulbecco’s modified Eagle’s medium (DMEM), pifithrin-α (PFT) and Trolox were obtained from SIGMA Chemical Co. (St. Louis, MO). A florescent probe 2',7'-dichlorofluorescin diacetate (DCFH2-DA) was purchased from CALBIOCHEM (San Diego, CA). Trypsin-EDTA was obtained from Invitrogen (Grand Island, NY). Phosphate-buffered saline (PBS) was obtained from TAKARA BIO INC. (Tokyo, Japan). H2O2 was obtained from Kanto Chemical Co. Inc. (Tokyo, Japan). Fetal bovine serum (FBS) was purchased from BIOSOURCE Inc. (Camarillo, CA). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit was obtained from Promega (Madison, WI). All other chemicals used in this study were of the highest grade available from commercial suppliers.
Cell culture
Rat cardiomyocyte H9c2 cells were obtained from the American Type Culture Collection (Rockville, MD) and were grown in DMEM containing 10% heat-inactivated FBS in an incubator in an atmosphere of 5% CO2, 95% air at 37°C. The standard treatments for H9c2 cells were as follows: the cells were seeded onto 6-well plates at a density of 3 × 105 cells/well containing 3 ml culture medium. After an overnight preincubation with or without 0.1 mM BSO, we refer to BSO-untreated cells as normal cells, the cells were incubated with H2O2 for various time periods at the concentrations indicated in the figure legends. Cell number was measured using a hemocytometer (Erma, Tokyo, Japan). After removal of the medium, the cells were washed once in PBS and once in culture medium before trypsinization. The cells were resuspended with culture medium and washed twice with PBS by centrifugation at 300 × g for 5 min. The cell pellets were resuspended with PBS and the cell number was determined.
Cell viability
Cell viability was determined using an MTT assay kit. After incubation with H2O2, the medium was removed. For the MTT assay, fresh culture medium (1725 µl) containing a dye solution (225 µl) was added to each of the wells containing cells and incubated for 2 h at 37°C. A solubilization/stop solution (1500 µl) was then added for an additional overnight incubation. The absorbance at 540 nm (formation of formazan) and 690 nm (reference) were recorded with a microplate reader (Labsystems Multiskan Bichromatic, Helsinki, Finland). Viability was determined as compared to control cells (normal H9c2 cells incubated without H2O2).
Measurement of ROS levels
Intracellular ROS levels were assessed using DCFH2-DA [16]. Cells were preincubated with DCFH2-DA in the culture medium at a final concentration of 10 µM for 15 min. Following incubation with H2O2 for 15 min, the cells were trypsinized, collected and washed with PBS by centrifugation at 300 × g for 5 min. The cell pellets were resuspended with PBS (1 ml) and analyzed using a flow cytometer (Beckman Coulter, Fullerton, CA). The cells (5 × 103 cells) were screened and the fluorescence of DCF was detected by fluorescence channel 1. The relative intensity of DCF fluorescence was determined as compared to control cells.
Measurement of cell size
Relative change in cell size was observed using a flow cytometer. After incubation with H2O2 for 72 h, the medium was removed from the cultures and the cells were washed once in PBS and once in culture medium before trypsinization. The cells were collected and washed with PBS by centrifugation at 300 × g for 5 min. The cell pellets were resuspended with PBS (1–2 ml) and analyzed using a flow cytometer. The cells (1 × 104 cells) were screened and the forward light scatter (FS) and side light scatter (SS) were recorded for each cell to determine the relative size and density, respectively. Control cells were gated so that large sizes were presumed to represent 10% of the cell population (region hypertrophy).
ANP gene expression
After incubation with or without H2O2 for 72 h, total cellular RNA was extracted from H9c2 cells using an illustra RNAspin Mini RNA Isolation kit (GE Healthcare, Tokyo, Japan) according to the manufacturer’s instructions, and was quantified by absorbance at 260 nm. RNA (2.5 µg) was reverse transcribed into cDNA using a High-capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster, CA) according to the manufacturer’s instructions. TaqMan PCR primer and probe for target gene atrial natriuretic peptide (ANP) and internal standard gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Applied Biosystems. TaqMan PCR was performed with 1 µl of sample cDNA in a 50-µl reaction mixture containing TaqMan Universal PCR Master Mix using a TaqMan gene expression assay (Applied Biosystems). Amplification was performed using a 7500 real time PCR system (Applied Biosystems). Cycling conditions were 50°C for 2 min, 95°C for 10 min followed by a 50-cycle amplification at 95°C for 15 s and 60°C for 1 min. A threshold cycle number (Ct) at which each PCR amplification reaches a significant threshold level was calculated with the software equipped by the 7500 system. The threshold cycle number is proportional to the number of ANP and GAPDH mRNA copies existing in the reaction mixture. GAPDH was used to normalize mRNA expression. Data were calculated using the ΔΔCt method.
Statistical analysis
Data are represented as the means ± SD and were statistically analyzed by Student’s t test or Welch’s t test following the F test for pair data. The value p<0.05 was considered statistically significant.
Results
Effects of H2O2 on H9c2 cells pretreated with or without BSO
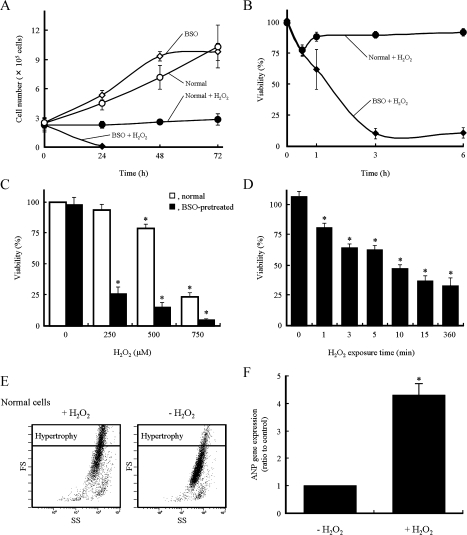
To evaluate oxidative stress, the H9c2 cells were treated with various concentrations of H2O2. As shown in Fig. 1A, the number of normal cells exposed to H2O2 remained constant during a 72-h incubation period. In BSO-pretreated H9c2 cells, treatment with H2O2 noticeably decreased the total cell number within 24 h. After the 72-h incubation period, the numbers of normal cells without H2O2 treatment and BSO-pretreated cells without H2O2 treatment were about 4.1 and 3.9 times compared with it at 0 h, respectively. The addition of H2O2 dramatically decreased cell viability after 3 h in H9c2 cells pretreated with BSO (Fig. 1B). When BSO-pretreated cells were exposed to H2O2 at 250 µM or higher for 6 h, the remarkable decrease in cell viability was observed (Fig. 1C). In normal cells, however, the decrease of cell viability was barely noticeable at 250 µM, slightly apparent at 500 µM, but remarkably observed at 750 µM of H2O2. To examine the effect of H2O2 exposure time on cell death in BSO-pretreated cells, we replaced the medium at various times after the addition of H2O2. When the medium containing H2O2 was removed at 5, 15 and 360 min after the addition of H2O2 (250 µM), cell viabilities were approximately 63, 37 and 33%, respectively (Fig. 1D), suggesting that a signal of the cell death developed within 15 min after the addition of H2O2. We measured the changes of cell size after the addition of H2O2 using a flow cytometer to assess hypertrophy. FS and SS were recorded to determine the relative size and density of cells, respectively. Normal H9c2 cells not exposed to H2O2 were gated so that larger cell sizes were 10% of the total cell population (hypertrophic cells). The population of cells that belong to a region of hypertrophy was approximately 30% at 72 h after the addition of H2O2 (Fig. 1E). The expression of ANP gene, a maker of cardiac hypertrophy [14], in normal cells treated with H2O2 for 72 h, was remarkably increased compared with normal cells without H2O2 (Fig. 1F). We were unable to detect the alteration of cell sizes and gene expression in the BSO-pretreated cells because the substantial decrease in cell viability was caused within 6 h after the addition of H2O2. These results indicate that H2O2 induces cell death in BSO-pretreated H9c2 cells and hypertrophy in normal cells.
Fig. 1.
Effects of H2O2 on H9c2 cells pretreated with or without BSO. H9c2 cells were pretreated with (open diamond and closed diamond) or without (open circle and closed circle) BSO. Cells (closed diamond and closed circle) were exposed to H2O2 (250 µM) and the number of cells (A) and viability (B) were determined at the times indicated in the figures by counting and an MTT assay, respectively. (C) Cell viability 6 h after exposure to different concentrations of H2O2. (D) Effect of H2O2 (250 µM) exposure time on viability of the BSO-pretreated H9c2 cells. These data represent the means ± SD of three independent experiments. (E) Size of cells was analyzed using a flow cytometer at 72 h after the addition of H2O2 (250 µM). FS and SS were recorded for each cell to determine the relative size and density, respectively. Similar results were obtained in two additional independent experiments using different cell preparations. (F) ANP gene expression in normal H9c2 cells at 72 h after the addition of H2O2 (250 µM). Data represent the means ± SD of three independent experiments. * p<0.05 compared with H2O2-untreated normal cells (C and F) and cells pretreated with BSO (D).
ROS levels and cell viability in H9c2 cells
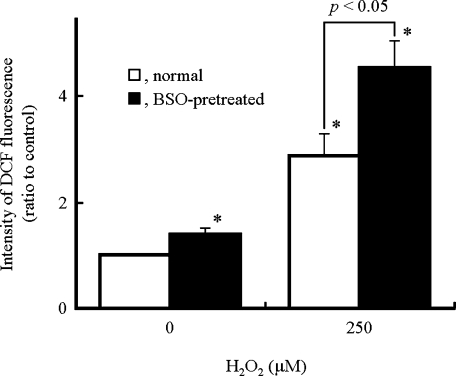
The levels of intracellular ROS in H9c2 cells were measured 15 min after the addition of H2O2 using a fluorescent probe, DCFH2-DA (Fig. 2). In normal cells exposed to H2O2, the intensity of DCF fluorescence was slightly increased. In BSO-pretreated H9c2 cells, addition of H2O2 significantly increased the intensity of DCF fluorescence compared to that obtained in the normal cells (Fig. 2), suggesting the increasing generation of ROS resulting from H2O2 in the GSH-depleted cells. The intensity of DCF fluorescence decreased 15 min or later after H2O2 treatment and returned to basal levels at 60 min.
Fig. 2.
Intracellular ROS levels by the addition of H2O2 in H9c2 cells. H9c2 cells pretreated with or without BSO were preincubated with DCFH2-DA (10 µM) for 15 min prior to the addition of H2O2. After incubation with H2O2 (250 µM) for 15 min, the intensity of DCF fluorescence was analyzed using a flow cytometer. Data represent the mean intensities of DCF fluorescence ± SD relative to the DCF fluorescence intensity of the control of seven independent experiments. * p<0.05 compared with normal cells untreated with H2O2.
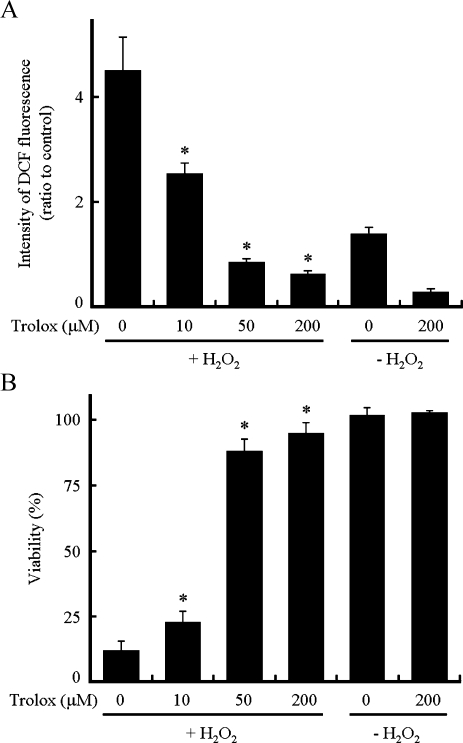
To determine whether ROS are associated with cell death, we examined the effect of the antioxidant Trolox on ROS levels and cell viability in H9c2 cells. As shown in Fig. 3A, Trolox prevented the increase in intensity of DCF fluorescence in a dose-dependent manner, and very little DCF fluorescence was observed at 50 µM or higher concentrations of Trolox. Trolox also protected H9c2 cells pretreated with BSO from cell death by the addition of H2O2 in a dose-dependent manner (Fig. 3B). Trolox (200 µM) almost completely prevented cell death induced by the addition of H2O2 in BSO-pretreated cells. Other antioxidants such as butylated hydroxyanisole and butylated hydroxytoluene also prevented the increase in the ROS levels and the death induced after the addition of H2O2 (data not shown).
Fig. 3.
Effect of Trolox on intracellular ROS levels and viability. The culture medium of H9c2 cells pretreated with BSO (0.1 mM) was replaced by one containing BSO and Trolox (0, 10, 50 or 200 µM) 1 h before the addition of H2O2 at a final concentration of 250 µM. (A) Levels of intracellular ROS were determined using DCFH2-DA at 15 min after the addition of H2O2. (B) Cell viability 6 h after the addition of H2O2. Data represent the means ± SD of three independent experiments. * p<0.05 compared with cells treated with H2O2 in the absence of Trolox.
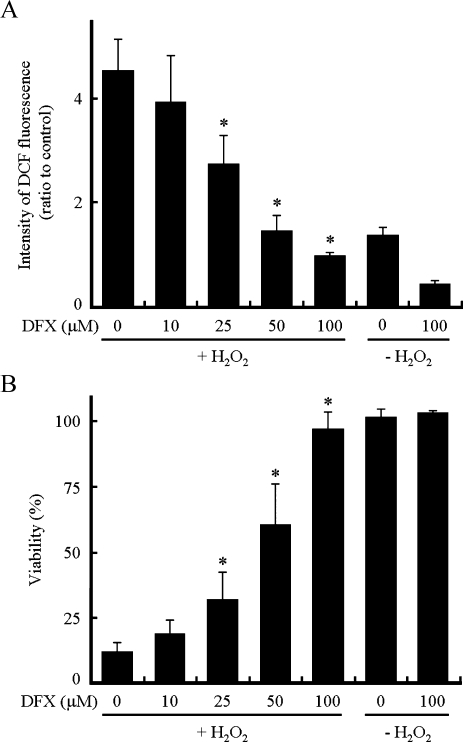
We examined the effect of DFX, an iron chelator, on intracellular ROS levels and cell viability, because H2O2 is converted to HO·, the most highly reactive species of ROS, under biological conditions through the Fenton reaction between H2O2 and ferrous ion [2]. As shown in Fig. 4A, DFX inhibited the increase in DCF fluorescence intensity in a dose-dependent manner, and at 100 µM DFX the increase was no longer observed. DFX also prevented cell death induced by H2O2 in a dose-dependent manner in cells pretreated with BSO, and completely abolished cell death at 100 µM DFX (Fig. 4B). These results indicate that the increase in ROS levels is associated with the cell death in BSO-pretreated H9c2 cells after the addition of H2O2.
Fig. 4.
Effect of DFX on intracellular ROS levels and viability. The culture medium of H9c2 cells pretreated with BSO (0.1 mM) was replaced by one containing BSO and DFX (0, 10, 25, 50 or 100 µM) 1 h before the addition of H2O2 at a final concentration of 250 µM. (A) Levels of intracellular ROS were determined using DCFH2-DA at 15 min after the addition of H2O2. (B) Cell viability 6 h after the addition of H2O2. Data represent the means ± SD of three independent experiments. * p<0.05 compared with cells treated with H2O2 in the absence of DFX.
Relationship between hypertrophy and ROS levels
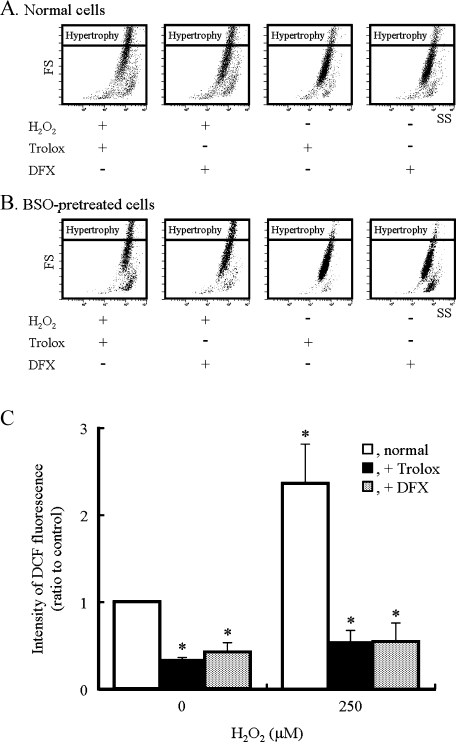
To determine whether ROS is associated with the cell hypertrophy induced by the addition of H2O2, we examined the effects of Trolox and DFX on hypertrophy in normal cells. In the presence of Trolox, the percentage of the population of normal cells belonging to a region of hypertrophy was approximately 42% at 72 h after the addition of H2O2, but that of cells without H2O2 treatment was about 11% (Fig. 5A). When the cells were incubated with DFX alone for 72 h, cell death was observed (data not shown). To remove the toxic effect of DFX, the medium containing DFX was exchanged for fresh medium 3 h after the addition of H2O2. After incubation with DFX, the percentage of the normal cell population belonging to a region of hypertrophy was approximately 28% at 72 h after the addition of H2O2, although the fraction of hypertrophic cells without exposure to H2O2 was approximately 12% (Fig. 5B). After incubation with Trolox or DFX, the fraction of BSO-pretreated cells belonging to a region of hypertrophy was approximately 35% or 33%, respectively, 72 h after the addition of H2O2; that of cells without H2O2 was about 10% in both cases (Fig. 5B). In cells pretreated with (Figs. 3A and 4A) or without (Fig. 5C) BSO, the intensity of DCF fluorescence observed after the addition of H2O2 in the presence of Trolox or DFX was lower than the basal intensity in normal cells incubated without H2O2. These results indicated that cell hypertrophy is induced even when intracellular ROS levels do not increase after the addition of H2O2.
Fig. 5.
Effects of Trolox and DFX on hypertrophy. The culture medium of H9c2 cells was replaced by one containing Trolox (200 µM) or DFX (100 µM) with (B) or without (A) BSO 1 h before the addition of H2O2 at a final concentration of 250 µM. The size of cells was analyzed 72 h after the addition of H2O2 using a flow cytometer. FS and SS were recorded for each cell to determine the relative size and density, respectively. The culture medium containing DFX was replaced with the fresh medium 3 h after the addition of H2O2. Similar results were obtained in two additional independent experiments using different cell preparations. (C) ROS levels obtained 15 min after the addition of H2O2 in normal cells in the presence or absence of Trolox or DFX. Data represent the means ± SD of three independent experiments. * p<0.05 compared with normal cells untreated with H2O2.
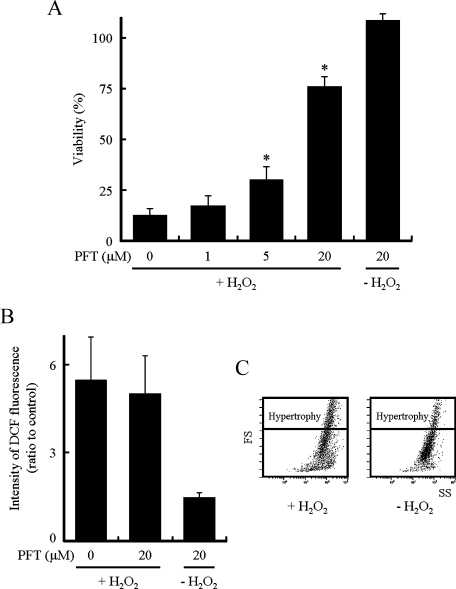
Because several studies have demonstrated that the addition of H2O2 induces activation of p53 protein and then cell death [17–19], we examined the effect of PFT, an inhibitor of p53 [20, 21], on the death and ROS levels in cells incubated with H2O2. PFT prevented cell death induced by H2O2 in BSO-pretreated H9c2 cells and cell viability was approximately 76% in the presence of PFT (Fig. 6A). PFT did not significantly affect the increase in DCF fluorescence intensity after the addition of H2O2 (Fig. 6B). These data indicate that PFT inhibits cell death without significantly influencing the ROS levels after the addition of H2O2 in BSO-pretreated H9c2 cells. We examined whether hypertrophy is induced in the presence of PFT where ROS levels are remarkably increased after the addition of H2O2. As shown in Fig. 6C, the fraction of the population of cells belonging to a region of hypertrophy increased (approximately 38%) in the presence of PFT at 72 h after the addition of H2O2. PFT itself had no effect on the cell size and viability of normal (data not shown) and BSO-pretreated cells in the absence of H2O2 (Fig. 6C). We found that the development of hypertrophy might not be connected with the levels of intracellular ROS after the addition of H2O2 in H9c2 cells.
Fig. 6.
Effects of PFT on ROS levels and hypertrophy. The culture medium of H9c2 cells pretreated with BSO was replaced by one containing BSO and PFT (0, 1, 5 and 20 µM) at 1 h before the addition of H2O2 at a final concentration of 250 µM. (A) Cell viability 6 h after the addition of H2O2. (B) Levels of intracellular ROS were determined using DCFH2-DA at 15 min after the addition of H2O2. Data represent the means ± SD of three independent experiments. (C) The size of cells was analyzed 72 h after the addition of H2O2 using a flow cytometer. Similar results were obtained in two additional independent experiments using different cell preparations. * p<0.05 compared with cells treated with H2O2 in the absence of PFT.
Discussion
We demonstrated that the addition of H2O2 induces cell hypertrophy in normal H9c2 cells and cell death in BSO-pretreated H9c2 cells. BSO would deteriorate the ability of H9c2 cells to scavenge ROS, because BSO inhibits de novo synthesis of GSH, a key compound playing roles in the function to prevent accumulation of ROS, and then results in lower amounts of intracellular GSH [6, 22]. A remarkable increase in intracellular ROS generation was observed after the addition of H2O2 in BSO-pretreated cells as compared with normal cells (Fig. 2). Trolox and DFX significantly prevented the increase in intracellular ROS generation and cell death by the addition of H2O2 in BSO-pretreated H9c2 cells (Figs. 3 and 4).
We showed that normal H9c2 cells exhibited a slight increase in intracellular ROS levels followed by cell hypertrophy upon the addition of H2O2 (Figs. 1 and 2). Kwon et al. [23] and Chen et al. [15] proposed that H2O2-treatement at a low concentration induces cell hypertrophy. Pimentel et al. [14] demonstrated that mechanical stretching of neonatal rat ventricular myocytes, which generates O2·−, induces hypertrophy and/or cell death, depending on the stretch levels. These results indicate the possibility that low levels of ROS induce hypertrophy and high levels of ROS induce cell death. In the present study, we also observed that cell death induced by the addition of H2O2 was prevented by an inhibitor of p53, PFT, in BSO-pretreated H9c2 cells. L’Ecuyer et al. [21] demonstrated that H2O2-treatment induces DNA injury in H9c2 cells. DNA damage activates p53, which mediates cell death in many types of cells containing cardiomyocytes. From these findings, we speculate that PFT inhibited the cell death as a consequence of inhibiting p53 function, although the detailed mechanism of H2O2 cytotoxicity is not clearly understood. Prevention of H2O2-induced cell death by PFT led to hypertrophy in BSO-pretreated H9c2 cells though levels of intracellular ROS dramatically increased (Fig. 6). In contrast, the addition of H2O2 induced cell hypertrophy even when Trolox and DFX lowered the ROS levels below basal levels in both H9c2 cells pretreated with or without BSO (Figs. 3–5). From these findings, we conclude that the development of hypertrophy might not be connected with the levels of intracellular ROS after the addition of H2O2 to H9c2 cells.
In the presence of Trolox or DFX, the intensity of DCF fluorescence was not increased in H9c2 cells after the addition of H2O2, implying a possibility that the added H2O2 itself did not induce or hardly induced the increase in DCF-fluorescence as compared with other oxygen species such as O2·− and HO·. Trolox is a cell-permeable, water-soluble derivative of vitamin E and can scavenge free radicals such as O·− and HO· [24]. In line with other studies that the oxidation of DCFH2 by H2O2 requires mediation by a catalyst such as peroxidase in cell free system [25, 26], we have also confirmed that H2O2 itself doesn’t directly oxidize DCFH2. DCF fluorescence may reflect generated HO· in the present experiment system, because DFX, that is able to lower the levels of intracellular free iron and inhibit the Fenton reaction [2, 27], suppressed the increase in DCF fluorescence intensity in H9c2 cells after the addition of H2O2. We have also confirmed that hypertrophy developed in H2O2-dose dependent manner in H9c2 cells in the presence of Trolox and that catalase inhibited the hypertrophy. From these findings, we hypothesize that H2O2 itself participates in the induction of hypertrophy in H9c2 cells. The present study would provide a new insight into the hypertrophy in H9c2 cells, a cardiomyocyte cell line, from a standpoint of sensitivity to ROS generated after the addition of H2O2. To clarify our hypothesis, further studies are needed using primary cultured cardiomyocytes, which should prove the useful information to understand the role of H2O2 in the development of cardiomyocyte hypertrophy.
Pretreatment with BSO decreased the content of intracellular GSH to 30% as compared with that of normal H9c2 cells [28]. GSH not only plays key roles in the function to prevent accumulation of ROS, but also regulates redox signaling [29, 30]. Tanaka et al. [28] observed that GSH-depleted H9c2 cells underwent cell hypertrophy after the addition of H2O2 in the presence of exogenous thiol compounds and also proposed a requirement of thiol for the induction of cell hypertrophy. In the present study, BSO-pretreated H9c2 cells underwent death by the addition of H2O2, although the treatment of BSO may result in the increase in intracellular cysteine content due to inhibition of γ-glutamylcysteine synthetase. In addition, non-thiol compounds Trolox and DFX inhibited the cell death in BSO-pretreated H9c2 cells after the addition of H2O2 and these cells underwent hypertrophy. From these findings, we think that GSH, but not cysteine, plays a role of antioxidants and that GSH itself could not be essential for the development of hypertrophy in H9c2 cells exposed to H2O2.
In the present study, we conclude that H9c2 cardiomyocytes exhibit the differential sensitivity to intracellular ROS generation with regard to hypertrophic versus death responses to exogenously added H2O2, and propose the possibility that cell hypertrophy is developed independently of intracellular ROS, but cell death is dependent on, in H9c2 cells. The fact that prevention of H2O2-induced cell death led to the hypertrophy in H9c2 cells suggests a possibility that the prevention of cardiomyocyte death induced by oxidative stress involves a risk of hypertrophy.
Abbreviations
- Ang II
angiotensin II
- BSO
L-buthionine-sulfoximine
- DCFH2-DA
2',7'-dichlorofluorescin diacetate
- DFX
deferoxamine
- DMEM
Dulbecco’s modified Eagle’s medium
- GPx
glutathione peroxidase
- HO·
hydroxyl radical
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
phosphate-buffered saline
- PFT
pifithrin-α
- ROS
reactive oxygen species
- O2·−
superoxide anion radical
References
- 1.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Valko M., Rhodes C.J., Monocol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Christen Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 4.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 5.Keith M., Geranmayegan A., Sole M.J., Kurian R., Robinson A., Omran A.S., Jeejeebhoy K.N. Increased oxidative stress in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 6.Marklund S.L., Westman N.G., Lundgren E., Roos G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982;42:1955–1961. [PubMed] [Google Scholar]
- 7.Zweier J.L., Flaherty J.T., Weisfeldt M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelos M.G., Kutala V.K., Torres C.A., He G., Stoner J.D., Mohammad M., Kuppusamy P. Hypoxic reperfusion of the ischemic heart and oxygen radical generation. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H341–H347. doi: 10.1152/ajpheart.00223.2005. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S., Pulinilkunnil T., Yuen G., Kewalramani G., An D., Qi D., Abrahani A., Rodrigues B. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H768–H776. doi: 10.1152/ajpheart.00038.2005. [DOI] [PubMed] [Google Scholar]
- 10.Takemoto M., Node K., Nakagami H., Liao Y., Grimm M., Takemoto Y., Kitakaze M., Liao J.K. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J. Clin. Invest. 2001;108:1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grishko V., Pastukh V., Solodushko V., Gillespie M., Azuma J., Schaffer S. Apoptotic cascade initiated by angiotensin II in neonatal cardiomyocytes: role of DNA damage. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2364–H2372. doi: 10.1152/ajpheart.00408.2003. [DOI] [PubMed] [Google Scholar]
- 12.Remondino A., Kwon S.H., Communal C., Pimentel D.R., Sawyer D.B., Singh K., Colucci W.S. β-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ. Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 13.Gupta M.K., Neelakantan T.V., Sanghamitra M., Tyagi R.K., Dinda A., Maulik S., Mukhopadhyay C.K., Goswami S.K. An assessment of the role of reactive oxygen species and redox signaling in norepinephrine-induced apoptosis and hypertrophy of H9c2 cardiac myoblasts. Antioxid. Redox Signal. 2006;8:1081–1093. doi: 10.1089/ars.2006.8.1081. [DOI] [PubMed] [Google Scholar]
- 14.Pimentel D.R., Amin J.K., Xiao L., Miller T., Viereck J., Oliver-Krasinski J., Baliga R., Wang J., Siwik D.A., Singh K., Pagano P., Colucci W.S., Sawyer D.B. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ. Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q.M., Tu V.C., Wu Y., Bahl J.J. Hydrogen peroxide dose dependent induction of cell death or hypertrophy in cardiomyocytes. Arch. Biochem. Biophys. 2000;373:242–248. doi: 10.1006/abbi.1999.1558. [DOI] [PubMed] [Google Scholar]
- 16.Timolati F., Ott D., Pentassuglia L., Giraud M.N., Perriard J.C., Suter T.M., Zuppinger C. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J. Mol. Cell Cardiol. 2006;41:845–854. doi: 10.1016/j.yjmcc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 17.von Harsdorf R., Li P.F., Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 18.Mao Y., Song G., Cai Q., Liu M., Luo H., Shi M., Ouyang G., Bao S.D. Hydrogen peroxide-induced apoptosis in human gastric carcinoma MGC803 cells. Cell Biol. Int. 2006;30:332–337. doi: 10.1016/j.cellbi.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Yan W., Chen X. GPX2, a direct target of p63, inhibits oxidative stress-induced apoptosis in p53-dependent manner. J. Biol. Chem. 2006;281:7856–7862. doi: 10.1074/jbc.M512655200. [DOI] [PubMed] [Google Scholar]
- 20.Komarov P.G., Komarova E.A., Kondratov R.V., Christov-Tselkov K., Coon J.S., Chernov M.V., Gudkov A.V. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 21.L’Ecuyer T., Sanjeev S., Thomas R., Novak R., Das L., Campbell W., Heide R.V. DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1273–H1280. doi: 10.1152/ajpheart.00738.2005. [DOI] [PubMed] [Google Scholar]
- 22.Li S., Li X., Rozanski G.J. Regulation of glutathione in cardiac myocytes. J. Mol. Cell Cardiol. 2003;35:1145–1152. doi: 10.1016/s0022-2828(03)00230-x. [DOI] [PubMed] [Google Scholar]
- 23.Kwon S.H., Pimentel D.R., Remondino A., Sawyer D.B., Colucci W.S. H2O2 regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J. Mol. Cell Cardiol. 2003;35:615–621. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 24.Kuwabara M., Asanuma T., Niwa K., Inanami O. Regulation of cell survival and death signals induced by oxidative stress. J. Clin. Biochem. Nutr. 2008;43:51–57. doi: 10.3164/jcbn.2008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myhre O., Andersen J.M., Aarnes H., Fonnum F. Evaluation of the probes 2',7'-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 26.Setsukinai K., Urano Y., Kakinuma K., Majima H.J., Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- 27.Krausse-Opatz B., Wittkop U., Gutzki F.M., Schmidt C., Jürgens-Saathoff B., Meier S., Beckmann B., Takikawa O., Morgan M.A., Tsikas D., Stichtenoth D.O., Wagner A.D., Zeidler H., Köhler L. Free iron ions decrease indoleamine 2,3-dioxygenase expression and reduce IFNγ-induced inhibition of Chlamydia trachomatis infection. Microb. Pathog. 2009;46:289–297. doi: 10.1016/j.micpath.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H., Sakurai K., Takahashi K., Fujimoto Y. Requirement of intracellular free thiols for hydrogen peroxide-induced hypertrophy in cardiomyocytes. J. Cell Biochem. 2003;89:644–655. doi: 10.1002/jcb.10568. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel D.R., Adachi T., Ido Y., Heibeck T., Jiang B., Lee Y., Melendez J.A., Cohen R.A., Colucci W.S. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. J. Mol. Cell Cardiol. 2006;41:613–622. doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Dalle-Donne I., Rossi R., Giustarini D., Colombo R., Milzani A. S-glutathionylation in protein redox regulation. Free Radic. Biol. Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]