Abstract
Purposes
The aim of this study was to investigate the relation between plasma level of glutamate and extent of radiographic bone erosion of the TMJ in patients with early RA in relation to inflammatory disease activity as well as estradiol and testosterone.
Patients and Methods
47 patients (29 women and 18 men) of whom 24 were seropositive were included shortly after being diagnosed with RA. Radiographic signs of bone tissue resorption (erosions) in the TMJ was recorded by cone-beam CT images and an erosion score (0 – 24) was calculated for each patient. Venous blood was analyzed for rheumatoid factor, C-reactive protein, erythrocyte sedimentation rate, leukocyte particle count, glutamate, estradiol and testosterone. Nonparametric and parametric statistical methods were used in the analysis when applicable.
Results
Resorptive changes of the TMJ were found in a major part of the patients. There was a significant positive correlation between plasma level of glutamate and extension of radiographic erosions that was strongest in the patients with low levels of C-reactive protein, estradiol or testosterone. On the other hand erosions were correlated with C-reactive protein in patients with high levels of estradiol. The highest levels of glutamate were found in patients with low levels of C-reactive protein and estradiol.
Conclusion
This study shows that a majority of patients with early RA presents radiographic signs of bone tissue resorption of the TMJ and that circulating glutamate is associated with the extent of these changes. The relationship between glutamate and bone resorption seems to be influenced by systemic inflammatory activity as well as estradiol and testosterone levels.
Key Indexing Terms: Bone resorption, Glutamate, Radiographic tomography, Rheumatoid arthritis, Sex steroid hormones, Temporomandibular joint
Introduction
Bone tissue destruction is a major consequence of the inflammatory disorder rheumatoid arthritis (RA). Involvement of the temporomandibular joint (TMJ) in RA often leads to changes in the jaw relation like anterior open bite with impaired chewing ability as a result (1, 2, 3) and early diagnosis and prediction of such changes are therefore very important. However, the mechanisms behind the development of local tissue destruction are not well known.
The radiographic sign of erosion is generally considered to be an indicator of active bone resorption (4). Radiographic involvement of the TMJ has been reported to occur in 45 –71% of patients in the late phase of RA (5, 6). However, very little is known about how early such changes appear and to what extent. In addition, the relation between systemic inflammatory activity and bone resorption in the early phase of RA is not well known regarding the TMJ.
The excitatory amino acid glutamate contributes to the inflammatory process in patients with RA via peripheral N-methyl D-aspartate (NMDA) or non-NMDA receptors (7, 8). Presence of glutamate-containing nerve fibers has been reported in bone tissue (9, 10). Glutamate, which is also produced by osteoblasts, is involved in bone formation and resorption through NMDA receptors on osteoblasts and osteoclasts (11). Indeed, the NMDA antagonist MK-801 inhibits bone resorption in vitro by an action on mature osteoclasts (12). There are thus reasons to suspect that glutamate and the NMDA receptor are involved in the articular bone destruction occurring in RA.
Elevated plasma levels of the neurotransmitter glutamate have been reported in RA (13), possibly partly due to activation of thrombocytes (14).
Thrombocytes release glutamate ex vivo when challenged with aggregating stimuli such as the rheumatoid factor (14). Continuous thrombocyte stimulation with ensuing release of glutamate into the blood might also be accomplished by proinflammatory cytokines such as tumor necrosis factor (TNF) and interleukin-1β (IL-1β) since thrombocytes have been shown to express cytokine receptors (15) that reduces thrombocyte reuptake of glutamate (14). However, it is not known whether this is an early event.
An additional factor to consider in articular tissue destruction is the possible influence of the sex steroid hormones. Both estradiol and testosterone have immunomodulating effects that may explain some of the sex differences in the course of RA (16). Estradiol has proinflammatory or anti-inflammatory effects in RA depending on the immune stimulus, the cell types involved, the specific microenvironment, the timing in relation to the disease course and reproductive status, the concentration of estrogens, the variability in expression of estrogen receptors and the intracellular metabolism of estrogens (17, 18, 19). However, the menopausal state with its lower steroid hormone levels is responsible for the major part of the differences in prevalence between men and women (19). In male RA patients, estradiol levels were found to be higher than in healthy men and the levels were positively correlated to indices of inflammation (16). Reduced testosterone levels are considered to decrease bone production (20) and testosterone may therefore be a background factor to TMJ bone tissue resorption. If, and to what extent, glutamate influences TMJ bone tissue resorption in an estradiol- or testosterone-dependent manner is however unknown.
Our hypothesis is that glutamate influence TMJ bone tissue resorption in a sex steroid dependent manner in patients with systemic inflammation.
The aim of this study was to investigate the relation between plasma level of glutamate and radiographic bone resorption of the TMJ in patients with early RA in relation to systemic inflammatory activity and level of the sex-steroid hormones estradiol and testosterone.
Materials and methods
Patients
Forty-seven patients, 29 females and 18 males with RA were included in the study. Of these, 28 (60%) were seropositive (Table 1). There was no statistically significant gender difference regarding demographic or other background variables investigated. The patients were consecutively asked to participate in the study after being diagnosed at the Department of Rheumatology, Karolinska University Hospital in Huddinge, Sweden. The inclusion criteria were diagnosis of RA according to the ACR criteria (19), age above 18 and verbal consent. Exclusion criteria were current malignancies, TMJ surgery or trauma within 2 years, recent intra-articular glucocorticoid injection in the TMJ (within 1 month) and diseases other than RA as a cause of craniofacial pain. The patients gave their informed consent according to the Declaration of Helsinki.
Table 1.
Demographic and background data for 47 patients with early rheumatoid arthritis.
| Total | |||||||
|---|---|---|---|---|---|---|---|
| Median | 25th | 75th | % abn | n | |||
| Gender | (M/F) | 18/29 | |||||
| Age | years | 62 | 54 | 67 | 47 | ||
| Duration of general joint symptoms | months | 7 | 4 | 10 | 47 | ||
| Duration from RA diagnosis | days | 23 | 12 | 36 | 47 | ||
| anti-cyclic citrullinated peptide | IU/mL | 30 | 0 | 420 | 51 | 37 | |
| Rheumatoid factor | IU/mL | 23 | 0 | 165 | 51 | 47 | |
| Erythrocyte sedimentation rate | mm/h | 22 | 17 | 32 | 36 | 47 | |
| Leukocyte particle count | 10 9 /L | 7.5 | 5.6 | 8.7 | 54 | 39 | |
| C-reactive protein | mg/L | 0 | 0 | 17.5 | 43 | 47 | |
| Disease actvity score 28 | 0 – 10 | 6 | 17 | 32 | 44 | ||
| Number of involved joint regions | 0 – 9 | 4 | 3 | 6 | 47 | ||
| Estradiol | males | pmol/L | 65 | 62 | 83 | 17 | |
| females | pmol/L | 41 | 26 | 66 | 25 | ||
| Testosterone | males | nmol/L | 12 | 7.9 | 14 | 17 | |
| females | nmol/L | 0 | 0 | 0.9 | 25 | ||
% abn = percentage of observations with abnormal values, n = number of observations, M = males, F = females, IU = international units. The following values was considered abnormal: rheumatoid factor ≥ 20 IU, erythrocyte sedimentation rate > 24 mm/h leukocyte particle count < 8.8 109/L and C-reactive protein ≥ 3 mg/L. The disease activity score for 28 joints was assessed at the time for diagnosis.
The design of the project was approved by the regional ethical committee at Karolinska Institutet, Stockholm, Sweden (03–204) and by the local radiation committee at Karolinska University Hospital in Huddinge, Sweden (19/03).
Medication
When they entered the study eighty percent of the patients were on methotrexate treatment, which was initiated at the time of RA diagnosis (Table 1). Another 56% had NSAID medication, 25% received glucocorticoid (either by oral or intra-articular administration) and 7% salazopyrin. Two female patients received estradiol supplementation.
Clinical examination
The patients were examined, blood samples were collected and radiographs taken on the same day, a median (25th percentile – 75th percentile) of 23 (12–36) after their entrance into the study (Table 1).
The disease activity score comprising 28 joints (DAS28) was assessed at the Department of Rheumatology at the time of diagnosis.
Radiographic examinations
Radiographic examination of the TMJ region was performed with a cone-beam computer tomograph (NewTom QR DVT mod 9000, Verona, Italy). The NewTom allowed three-dimensional digital reconstruction of the specific region of interest in the craniofacial area, i.e. the TMJ region, including sections of the examined region in any desired plane. The examinations were performed with a standardized head position with the TMJ located in the centre of the field as the region of interest. The maximum size of the region of interest was 130×130×130 mm and scanning time 70 seconds. Three-dimensional reconstructions were processed and analysed by the NewTom software (NewTom 3G).
Primary reconstructions (0.3 mm thickness) were created separately for the temporal component and the condylar component. The primary reconstruction of the temporal component was parallel to the hard palate and the primary reconstruction of the condylar components was obtained perpendicular to the mandibular ramus line. Transaxial reconstructions (0.5 mm thickness) were then produced for the temporal and condylar components with the condyle as a reference for the localization of the sections. The left and right side were reconstructed separately.
The computer tomograhy images were viewed from the frontal aspect of the condyle and from the sagital aspect of the temporal component. Three transaxial regions (lateral, central and medial) of the temporal component were analyzed in the anterior and central parts of each region. The transaxial sections of the condyle, one central (the section with the largest condylar width) and one anterior were analyzed in the lateral, central and medial parts. These regions were evaluated for presence or absence of erosions. Erosion was defined as a local area with decreased density of the cortical joint surface, sometimes including the adjacent subcortical bone, and considered as a radiographic sign of bone tissue resorption. The extension of erosive changes was expressed as a score ranging from 0 to 12 for each TMJ (Fig. 1–4). The sum of the score of the right and left side for each individual was used in the analysis. The reading of the erosions was made by a skilled radiologist and when erosions were observed their presence was verified from the frontal, sagital and axial views.
Figure 1.
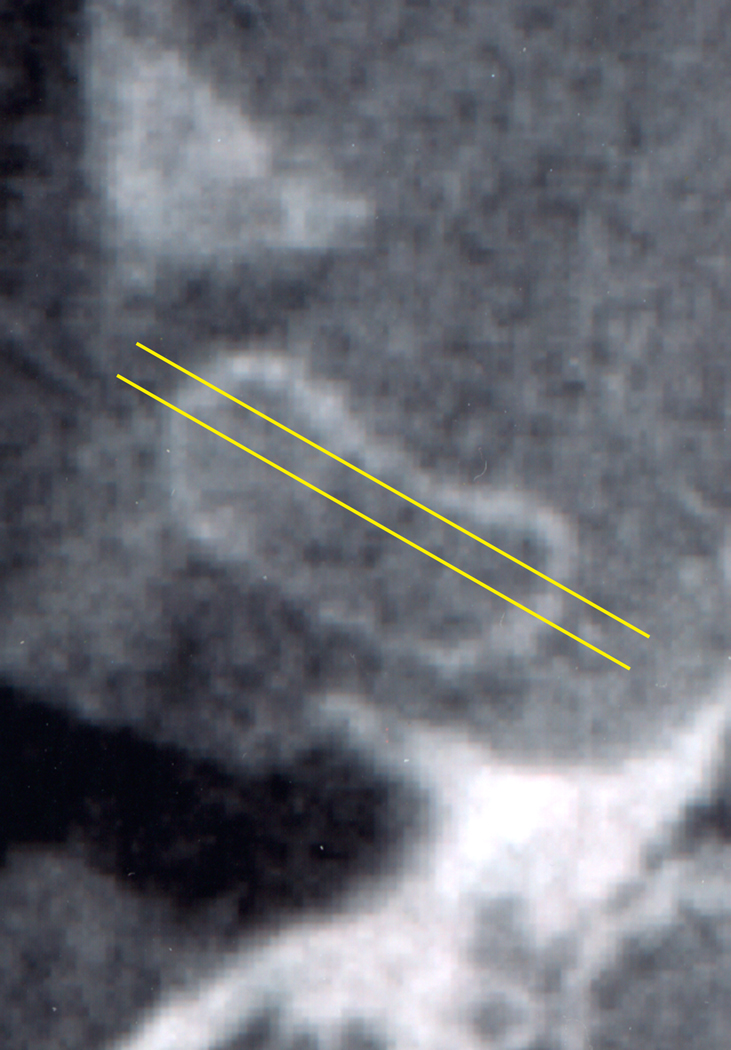
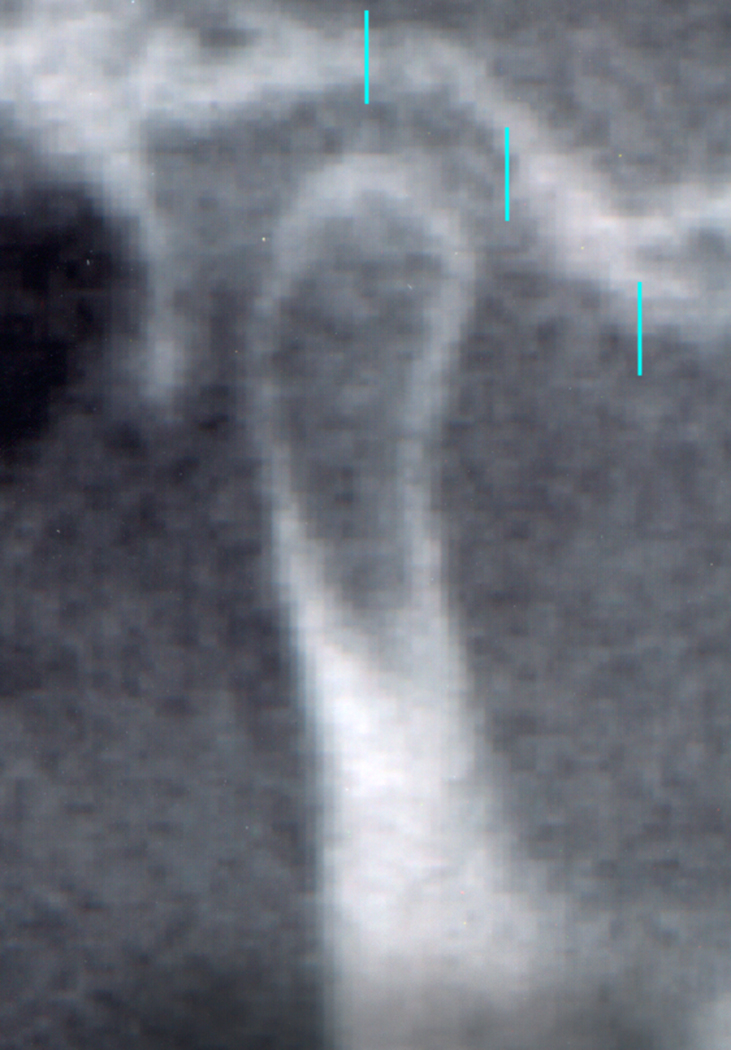
Axial view of the left temporomandibular joint showing the position of the two transectional sections of the condylar component (A = anterior section, C = central section).
Figure 4.
Lateral view of the temporal component of the temporomandibular joint showing the medial section divided into an anterior (A) and central (C) part.
Intra-observer consistency between repeated readings of erosions was tested by a randomized selection of twenty joints from 10 individuals. The scoring system was tested in a blinded fashion by the same radiologist 2–3 years apart. On a patient level the repeated readings showed a difference of one score unit between the two occasions in three patients, whereas the readings of the other seven patients showed no differences.
Blood samples and laboratory procedures
Venous blood samples were collected the same day as the clinical and radiographic examinations. For assessment of the systemic inflammatory activity the inflammatory markers erythrocyte sedimentation rate (normal range: ≤ 24 mm/hour), C-reactive protein (normal range: < 3 mg/L), leukocyte particle count (normal range: 3.5–8.8 ×10(9)/L), rheumatoid factor (normal range: < 20 IU) and anti-cyclic citrullinated peptide (anti-CCP; normal range: < 25 IU /mL) as well as the estradiol and testosterone levels were analyzed in serum. These analyses were performed according to standard procedures at the Department of Laboratory Medicine (Clinical Chemistry and Immunology), Karolinska University Hospital, Huddinge, Sweden. The normal reference ranges according to the laboratory for estradiol levels in fertile women was 100 – 1500 pmol/L, in postmenopausal women <50 pmol/L and in men 50 – 150 pmol/L. The corresponding reference ranges for testosterone are in females 20 – 49 years of age: 0.3 – 2.6 and females >50 years: 0.3 – 2.4 and in males 20 – 49 years of age: 10 – 30 nmol/L and males >50 years: 10 – 30 nmol/L.
Venous blood was also collected in an EDTA tube that was immediately cooled and centrifuged (1500g for 10 min at +4°C) and then frozen (−70 °C) and later examined for glutamate. Glutamate concentration in plasma was analysed by the Amplex® Red Glutamic acid/glutamate oxidase assay (A-12221, Molecular Probes Inc, Eugene, OR, USA). The glutamate assay had a detection level < 0.01 µmol/L according to the manufacturer.
Statistics
Median as well as the 25th and 75th percentiles was used for descriptive statistics. The significance of the differences between groups was calculated with the Mann-Whitney U-test and the significance of univariate correlations was calculated with Spearman rank correlation coefficient (rs). The patients were allocated into two groups with estradiol levels below or above the arbitrary limit 65 pmol/L for statistical analysis of the specific relation between erosions and systemic inflammatory activity in relation to the estradiol level. A probability level of less than 0.05 was considered significant.
Results
Glutamate
The plasma level of glutamate was median (25th-75th percentile) 4 (1 – 7) µmol/L. There was no significant gender difference regarding glutamate or any significant correlation between glutamate and age. Table 2
Table 2.
Glutamate concentration in plasma (mmol/L) in 47 patients with early rheumatoid arthritis
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Percentile | Percentile | |||||||
| Duration of disease | Median | 25 | 75 | n | Median | 25 | 75 | n |
| Less than 7 months | 0,5 | 0,5 | 5,6 | 7 | 4,4 | 2,1 | 8,0 | 14 |
| Seven months or more | 7,1 | 2,9 | 9,8 | 11 | 2,8 | 0,8 | 4,8 | 15 |
The difference between males and females was significant in the group of patients with duration of disease shorter than 7 months (p = 0.044) as well as in the group of patients with longer duration of disease (p = 0.017). The difference in glutamate level between the groups with shorter and longer duration of disease was also significant in the males (p = 0.016).
Radiographic erosions
A total of 72% of the patients with RA had erosive changes in the TMJ with a median score (25th – 75th percentile) of 2 (0 – 4). The maximum erosion score was 9, which was found in one patient. There was no significant correlation between erosions and age or any difference between genders.
Glutamate and radiographic erosions
The plasma level of glutamate was positively correlated to extension of erosions (rs = 0.36, n = 47, p = 0.014).
Influence of disease duration on the association between glutamate and radiographic erosions
The correlation between glutamate and erosions was stronger in the subgroup with disease duration < 7 months (rs = 0.47, n = 21, p = 0.032) than in the group with longer duration (rs = 0.24, n = 26, p = 0.240).
Influence of systemic inflammatory activity on the association between glutamate and radiographic erosions
There were stronger positive correlations between glutamate and erosions in the subgroup of patients with C-reactive protein levels of < 3 mg/L (rs = 0.55, n = 27, p = 0.002), erythrocyte sedimentation rate ≤ 24 mm (rs = 0.42, n = 30, p = 0.020) or leukocyte particle count < 7.5 (rs = 0.56, n = 18, p = 0.016). The association between glutamate and erosion was not significantly influenced by anti-CCP.
Systemic inflammatory activity versus estradiol and testosterone levels
Estradiol was significantly correlated to C-reactive protein, rheumatoid factor and leukocyte particle count (rs = 0.33, n = 42, p = 0.036, rs = 0.33, n = 42, p = 0.034 and rs = 0.48, n = 39, p < 0.001, respectively). Testosterone was not significantly correlated to any of the inflammatory markers.
Influence of estradiol and testosterone on the association between glutamate and radiographic erosions
There was a stronger positive correlation between glutamate and erosions in the patients with estradiol levels < 50 pmol/L (rs = 0.69, n = 16, p = 0.004) compared to all patients. There was also a stronger correlation between glutamate and erosions in patients with testosterone levels ≤ 1.2 nmol/L (rs = 0.56, n = 21, p = 0.008).
Influence of systemic inflammatory activity and estradiol on the association between glutamate and radiographic erosions
There was a stronger positive correlation between glutamate and erosions in the patients with C-reactive protein < 3 mg/L and estradiol levels < 50 pmol/L (rs = 0.89, n = 13, p < 0.001) compared to all patients as well as in the patients with erythrocyte sedimentation rate ≤ 24 mm and estradiol levels < 50 pmol/L (rs = 0.82, n = 12, p < 0.001).
The relation found between erosions and C-reactive protein depended on the level of estradiol, i.e. there was a positive correlation between erosions and C-reactive protein in the patients with estradiol levels ≥ 65 pmol/L (rs = 0.68, n = 16, p = 0.004). Accordingly, the erosion score was higher in the patients with higher levels of C-reactive protein and estradiol than in the patients with lower levels of C-reactive protein and higher level of estradiol (p= 0.025, n= 16; Table 3).
Table 3.
TMJ erosion score (median and 25th/75th percentiles) in relation to estradiol and C-reactive protein in blood samples from 42 patients with early rheumatoid arthritis.
| Estradiol | ||||||||
|---|---|---|---|---|---|---|---|---|
| < 65 pmol/L | ≥ 65 pmol/L | |||||||
| Percentile | Percentile | |||||||
| Median | 25th | 75th | n | Median | 25th | 75th | n | |
| C-reactive protein | ||||||||
| < 3 mg/L | 2.0 | 1.0 | 4.2 | 18 | 0 | 0 | 2.0 | 6 |
| ≥ 3 mg/L | 1.5 | 0 | 2.8 | 8 | 3.5 | 1.5 | 5.3 | 10 |
The relation between glutamate and estradiol was significant after allowing for the level of C-reactive protein, i.e. the glutamate level was higher in the group with low C-reactive protein and lower estradiol than in the group with low C-reactive protein and higher estradiol (p= 0.014, n= 24; Table 4).
Table 4.
Plasma glutamate concentration (µmol/L; median and 25th/75th percentiles) in relation to estradiol and C-reactive protein in blood samples from 42 patients with early rheumatoid arthritis.
| Estradiol | ||||||||
|---|---|---|---|---|---|---|---|---|
| < 65 pmol/L | ≥ 65 pmol/L | |||||||
| Percentile | Percentile | |||||||
| Median | 25th | 75th | n | Median | 25th | 75th | n | |
| C-reactive protein | ||||||||
| < 3 mg/L | 7.2 | 2.4 | 9.2 | 18 | 1.8 | 0.4 | 3.9 | 6 |
| ≥ 3 mg/L | 2.9 | 1.0 | 6.7 | 8 | 4.5 | 2.4 | 7.3 | 10 |
Influence of systemic inflammatory activity and testosterone on the association between glutamate and radiographic erosions
There was a stronger positive correlation between glutamate and erosions in the patients with C-reactive protein < 3 mg/L and testosterone levels ≤ 1.2 pmol/L (rs = 0.84, n = 14, p < 0.001) compared to all patients as well as in the patients with erythrocyte sedimentation rate ≤ 24 mm and testosterone levels ≤ 1.2 pmol/L (rs = 0.74, n = 13, p = 0.004).
Discussion
This study shows that a large part of newly diagnosed RA patients have radiographic signs of bone resorption in the TMJ despite very short duration of disease. Systemic glutamate is associated with this TMJ bone tissue resorption, particularly in patients with low systemic inflammatory activity and estradiol levels <50 pmol/L or testosterone levels ≤ 1.2 nmol/L. Systemic inflammatory activity, as expressed by C-reactive protein, erythrocyte sedimentation rate and leukocyte particle count, seems to be a factor that in combination with estradiol and testosterone influences the glutamate effect on TMJ bone tissue resorption. Glutamate may thus be a modulator of connective tissue cell function regarding bone tissue resorption in joints with RA especially in individuals with low systemic inflammatory activity.
The results of this study agree with the hypothesis that glutamate is associated with bone tissue resorption of the TMJ with RA as assessed by radiographic erosions. The causal relation between glutamate and bone resorption is, however, not fully understood. It seems that glutamate is released in the bone microenvironment by osteoblasts, activated osteoclasts and peripheral nerves as part of normal bone remodeling (22). In turn, activation of NMDA receptors stimulates osteoblast precursor proliferation, NFκB-mediated osteoclast differentiation and inhibition of osteoclast apoptosis and the resulting net signaling effect modulates changes in bone mass (23). The mature resorbing osteoclast is a target for glutamate by the NMDA receptor (24), which when activated results in bone tissue resorption in the healthy organism (10, 11). Fibroblast-like synoviocytes from patients with active RA express glutamate receptor mRNA for the glutamate NMDA, kainate and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors, where activated kainate/AMPA receptors increase the release of proinflammatory cytokine IL-6 and subsequently matrix metalloproteinase 2, an enzyme highly involved in matrix degradation (25). Our findings indicate that glutamate modulates bone tissue resorption, which is an intriguing pathophysiologic mechanism in RA as previously suggested by Flood and coworkers (25). However, the influence of systemic inflammatory activity and the sex steroid hormones estradiol and testosterone is a new finding. Flood and coworkers also found that glutamate receptor activation may contribute to the synovial hyperplasia and thus pannus tissue development by stimulation of synoviocyte proliferation. Whether glutamate regulates release of cytokines from synoviocytes or other synovial cells remains to be investigated.
Neither erosions nor glutamate levels in plasma were associated with gender in this study, except for the higher levels of glutamate in males after 7 months disease duration. Nevertheless, our results suggest an influence on the relationship between plasma glutamate and TMJ resorption by both estradiol and testosterone since this relation was considerably stronger in the patients with estradiol levels < 50 pmol/L and testosterone levels ≤ 1.2 nmol/L. This relation was also stronger in patients with low systemic inflammatory activity, suggesting a particular role for circulating glutamate in local bone tissue resorption without or with low systemic inflammatory influence. However, circulating glutamate may activate locally expressed receptors on e.g. osteoclasts or fibroblast like synoviocytes and modulate bone tissue resorption in patients with low systemic inflammation in combination with low estradiol or testosterone and thereby represent a bone resorbing mechanism independent on systemic inflammation. Potential sources of circulating glutamate are excess release from cells in inflamed tissues such as mast cells, platelets, lymphocytes, macrophages, neutrophils, fibroblasts and Schwann cells as well as afferent and sympathetic nerve terminals (7).
Estradiol has been found to increase the expression of osteoprotegrin by a mouse bone marrow stromal cell line from healthy mice, ST-2, via the estrogen receptor α (26). Osteoprotegrin binds to and inhibits the essential osteoclast differentiation factor “receptor activator for NFκB ligand” (RANKL) and therefore reduces osteoclastic bone resorption. Estradiol can also decrease proinflammatory cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages (27). These results are consistent with our findings in the RA patients with estradiol levels ≥ 65 pmol/L combined with low systemic inflammatory activity, in whom no erosions were found. Yoneda and coworkers found that estrogen deficiency also plays a crucial role in acceleration of bone lesions in autoimmune arthritis associated with RANKL-mediated osteoclastogenesis in a murine model for rheumatoid arthritis (28). This finding may explain the bone erosions in the patients with estradiol levels <65 pmol/L in combination with high systemic inflammatory activity. RANKL mRNA and protein have been identified in cultured synovial fibroblasts from patients with RA (29). Glutamate may modulate the RANK/RANKL system via estradiol and mediators of systemic inflammation (cytokines) and thereby bone metabolism in RA, but the effects of cytokine release is still unknown. Functional estrogen receptors have been found in fibroblast like synoviocytes, where estradiol exerts a stimulatory effect on the expression of matrix metalloproteinases and the tissue inhibitor of matrix metalloproteinase as well as the enzymatic activity of matrix metalloproteinases produced by these cells (30). Estrogen receptors have also been found in synovial lining cells, sublining fibroblasts, stromal cells of the articular disc and chondrocytes of the TMJ in male Wistar rats (31).
A remarkably large proportion of the patients, 72%, all with very short disease duration, showed signs of bone resorption in the TMJ. However, the extension of the resorption was in general small with a median score of 2 on a 0–24 scale. This finding, nevertheless, indicates that the TMJ is commonly and very early involved in RA, which has not been known previously. In an earlier study, 45% of RA patients with disease duration of 7–10 years had TMJ radiographic signs of bone tissue resorption (32). The considerably higher frequency reported in our study can be explained by the use of a different and more sensitive technique with computer tomography.
TMJ bone resorption in the present study was not directly associated with systemic inflammatory activity, as expressed by erythrocyte sedimentation rate, C-reactive protein, leukocyte particle count, rheumatoid factor or anti-CCP. However, the erosion score correlated to C-reactive protein in the patients with estradiol level ≥ 65 pmol/L, i.e. a combination of high levels of C-reactive protein and estradiol resulted in a high score of erosions. In previous studies of progression of radiographic changes in RA, time-averaged C-reactive protein levels were correlated to increase in Larsen score (33) and regarding the TMJ, progression during a period of 25–46 months was related to C-reactive protein level (34). The influence of estradiol was not investigated in those studies. The short disease duration as well as the cross-sectional character of the present study may also influence the relation between systemic inflammatory activity and erosions.
Since bone resorption was not significantly associated with age or gender in this study there is no support for a direct influence of these factors on the development of early bone tissue resorption in the TMJ. This is in agreement with a previous prospective study where the Larsen score of erosions did not show any difference between genders at study entry or after 2 years follow-up (35).
Estradiol levels have been found to correlate with several indices of inflammation, except rheumatoid factor (16). In agreement with our results no direct correlation was found between estradiol level and erosions.
Sixty percent of the female patients in this study had estrogen levels below 50 pmol/L. These patients were all above 50 years of age and may thus be considered as postmenopausal according to the reference values provided by the laboratory at the Karolinska University Hospital.
No attempt was made to adjust for multiple testing since this study partly had an exploratory aim and the intention was to minimize the risk of type II errors.
In conclusion, this study shows that a majority of patients with early RA presents radiographic signs of bone tissue resorption of the TMJ and that circulating glutamate is associated with the extent of these changes. The relationship between glutamate and bone resorption seems to be modulated by systemic inflammatory activity, estradiol and testosterone levels.
Figure 2.
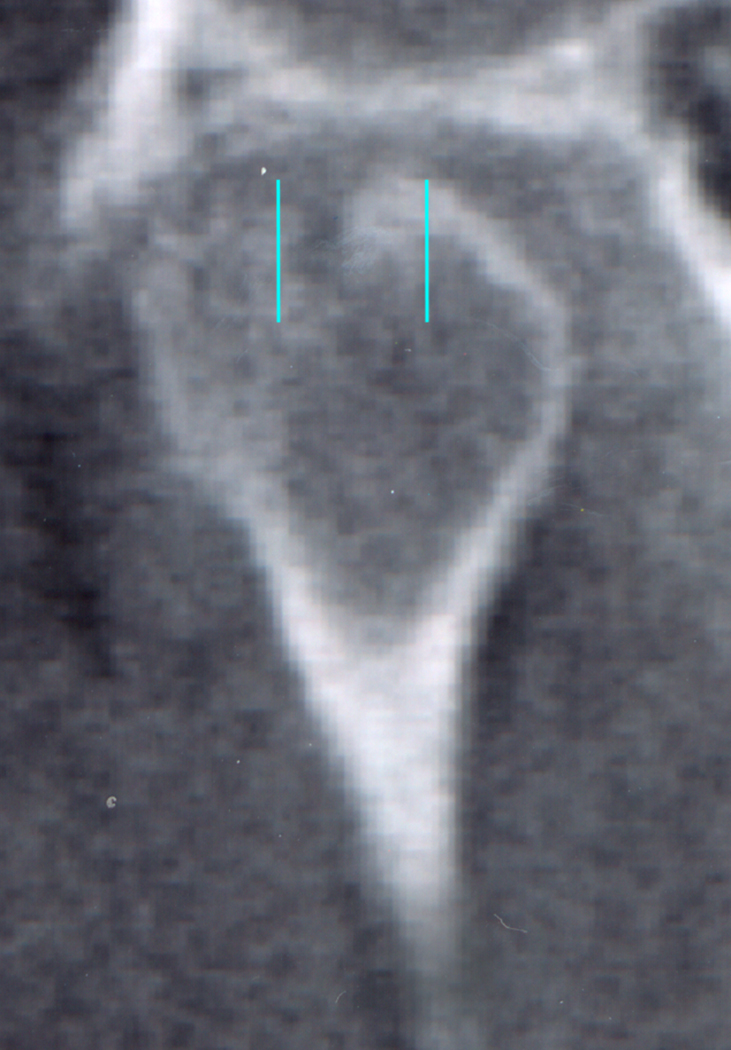
Frontal view of the left condyle of the temporomandibular joint showing the central section divided into a medial (M), central (C) and lateral (L) part.
Figure 3.
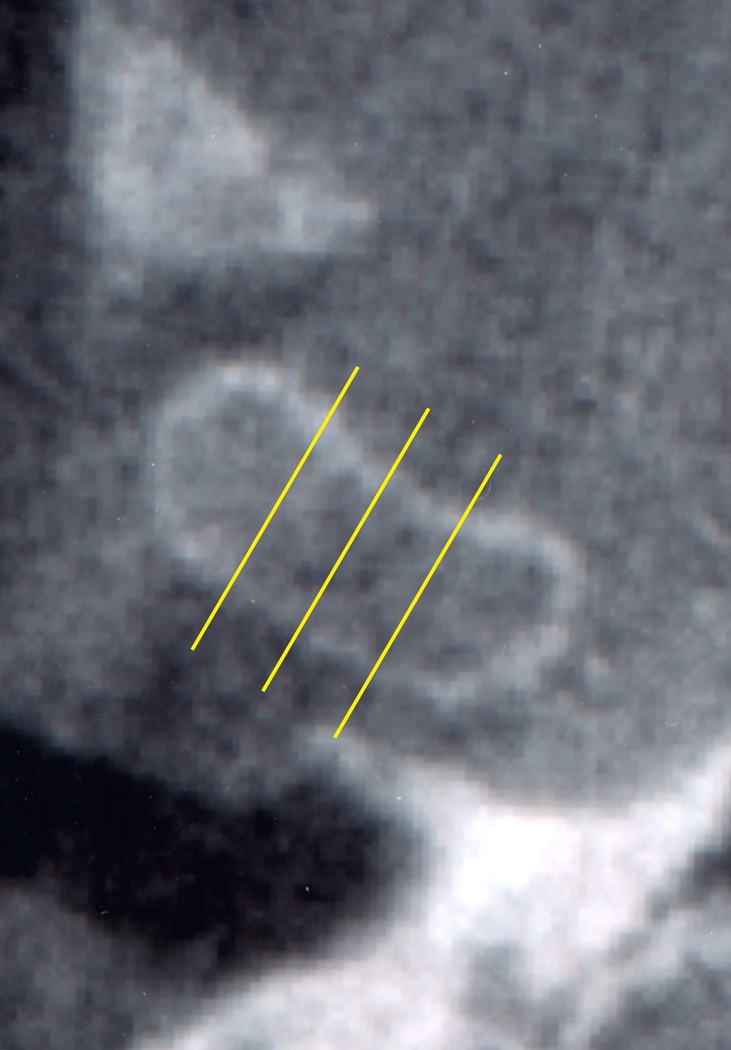
Axial view of the left temporomandibular joint showing the position of the transectional sections of the temporal component (M = medial section, C = central section, L= lateral section).
Acknowledgements
We thank Karin Trollsas for her skilful laboratory work and Marie-Louise Bjerdahl for her assistance at the clinic of the Department of Clinical Physiology. We also thank the personnel at the Departments of Rheumatology and Oral Radiology for their assistance with patients.
Sources of support
The National Institute of Dental and Craniofacial Research, The Institute of Odontology at Karolinska Institutet and The Swedish Dental society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anna-Kari Hajati, Email: Anna-Kari.Hajati@ki.se.
Per Alstergren, Email: Per.Alstergren@ki.se.
Karin Näsström, Email: Karin.Nasstrom@ki.se.
Johan Bratt, Email: Johan.Bratt@karolinska.se.
Sigvard Kopp, Email: Sigvard.Kopp@ki.se.
References
- 1.Helenius LM, Hallikainen D, Helenius I, Meurman JH, Kononen M, Leirisalo-Repo M, et al. A case-control study. Clinical and radiographic findings of the temporomandibular joint in patients with various rheumatic diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005 Apr;99(4):455–463. doi: 10.1016/j.tripleo.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 2.Tegelberg A, Kopp S. Subjective symptoms from the stomatognathic system in individuals with rheumatoid arthritis and osteoarthrosis. Swed Dent J. 1987;11(1–2):11–22. [PubMed] [Google Scholar]
- 3.Tegelberg A, Kopp S. Clinical findings in the stomatognathic system for individuals with rheumatoid arthritis and osteoarthrosis. Acta Odontol Scand. 1987 Apr;45(2):65–75. doi: 10.3109/00016358709098359. [DOI] [PubMed] [Google Scholar]
- 4.Voog U, Alstergren P, Eliasson S, Leibur E, Kallikorm R, Kopp S. Inflammatory mediators and radiographic changes in temporomandibular joints of patients with rheumatoid arthritis. Acta Odontol Scand. 2003 Feb;61(1):57–64. doi: 10.1080/ode.61.1.57.64. [DOI] [PubMed] [Google Scholar]
- 5.Gynther GW, Tronje G, Holmlund AB. Radiographic changes in the temporomandibular joint in patients with generalized osteoarthritis and rheumatoid arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996 May;81(5):613–618. doi: 10.1016/s1079-2104(96)80058-8. [DOI] [PubMed] [Google Scholar]
- 6.Nordahl S, Alstergren P, Eliasson S, Kopp S A longitudinal study. Radiographic signs of bone destruction in the arthritic temporomandibular joint with special reference to markers of disease activity. Rheumatology (Oxford) 2001 Jun;40(6):691–694. doi: 10.1093/rheumatology/40.6.691. [DOI] [PubMed] [Google Scholar]
- 7.Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint: key role in nociception and inflammation. Pain. 2000 May;86(1–2):69–74. doi: 10.1016/s0304-3959(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 8.McNearney T, Baethge BA, Cao S, Alam R, Lisse JR, Westlund KN. Excitatory amino acids, TNF-alpha, and chemokine levels in synovial fluids of patients with active arthropathies. Clin Exp Immunol. 2004 Sep;137(3):621–627. doi: 10.1111/j.1365-2249.2004.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chenu C. Glutamatergic regulation of bone remodeling. J Musculoskelet Neuronal Interact. 2002 Mar;2(3):282–284. [PubMed] [Google Scholar]
- 10.Chenu C. Glutamatergic regulation of bone resorption. J Musculoskelet Neuronal Interact. 2002 Sep;2(5):423–431. [PubMed] [Google Scholar]
- 11.Spencer GJ, McGrath CJ, Genever PG. Current perspectives on NMDA-type glutamate signalling in bone. Int J Biochem Cell Biol. 2007;39(6):1089–1104. doi: 10.1016/j.biocel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Chenu C, Serre CM, Raynal C, Burt-Pichat B, Delmas PD. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998 Apr;22(4):295–299. doi: 10.1016/s8756-3282(97)00295-0. [DOI] [PubMed] [Google Scholar]
- 13.Trang LE, Furst P, Odeback AC, Lovgren O. Plasma amino acids in rheumatoid arthritis. Scand J Rheumatol. 1985;14(4):393–402. doi: 10.3109/03009748509102044. [DOI] [PubMed] [Google Scholar]
- 14.Aliprandi A, Longoni M, Stanzani L, Tremolizzo L, Vaccaro M, Begni B, et al. Increased plasma glutamate in stroke patients might be linked to altered platelet release and uptake. J Cereb Blood Flow Metab. 2005 Apr;25(4):513–519. doi: 10.1038/sj.jcbfm.9600039. [DOI] [PubMed] [Google Scholar]
- 15.Loppnow H, Bil R, Hirt S, Schonbeck U, Herzberg M, Werdan K, et al. Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood. 1998 Jan 1;91(1):134–141. [PubMed] [Google Scholar]
- 16.Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Pizzorni C, et al. Anti-TNF and sex hormones. Ann N Y Acad Sci. 2006 Jun;1069:391–400. doi: 10.1196/annals.1351.037. [DOI] [PubMed] [Google Scholar]
- 17.Booji A, Biewenga-Booji CM, Huber-Bruning O, Cornelis C, Jacobs JW, Bijlsma JW. Androgens as adjuvant treatment in postmenopausal female patients with rheumatoid arthritis. Ann Rheum Dis. 1996 Nov;55(11):811–815. doi: 10.1136/ard.55.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007 Aug;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 19.Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002 Jun;23(3):279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002 Nov;61 Suppl 2:84–86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006 Apr 1;119(7):1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 23.Genever PG, Skerry TM. Regulation of spontaneous glutamate release activity in osteoblastic cells and its role in differentiation and survival: evidence for intrinsic glutamatergic signaling in bone. FASEB J. 2001 Jul;15(9):1586–1588. doi: 10.1096/fj.00-0594fje. [DOI] [PubMed] [Google Scholar]
- 24.Flood S, Parri R, Williams A, Duance V, Mason D. Modulation of interleukin-6 and matrix metalloproteinase 2 expression in human fibroblast-like synoviocytes by functional ionotropic glutamate receptors. Arthritis Rheum. 2007 Aug;56(8):2523–2534. doi: 10.1002/art.22829. [DOI] [PubMed] [Google Scholar]
- 25.Saika M, Inoue D, Kido S, Matsumoto T. 17beta-estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-alpha. Endocrinology. 2001 Jun;142(6):2205–2212. doi: 10.1210/endo.142.6.8220. [DOI] [PubMed] [Google Scholar]
- 26.Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004 Jun;50(6):1967–1975. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 27.Yoneda T, Ishimaru N, Arakaki R, Kobayashi M, Izawa T, Moriyama K, et al. Estrogen deficiency accelerates murine autoimmune arthritis associated with receptor activator of nuclear factor-kappa B ligand-mediated osteoclastogenesis. Endocrinology. 2004 May;145(5):2384–2391. doi: 10.1210/en.2003-1536. [DOI] [PubMed] [Google Scholar]
- 28.Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001 May;44(5):1003–1012. doi: 10.1002/1529-0131(200105)44:5<1003::AID-ANR179>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Khalkhali-Ellis Z, Seftor EA, Nieva DR, Handa RJ, Price RH, Jr, Kirschmann DA, et al. Estrogen and progesterone regulation of human fibroblast-like synoviocyte function in vitro: implications in rheumatoid arthritis. J Rheumatol. 2000 Jul;27(7):1622–1631. [PubMed] [Google Scholar]
- 30.Yamada K, Nozawa-Inoue K, Kawano Y, Kohno S, Amizuka N, Iwanaga T, et al. Expression of estrogen receptor alpha (ER alpha) in the rat temporomandibular joint. Anat Rec A Discov Mol Cell Evol Biol. 2003 Oct;274(2):934–941. doi: 10.1002/ar.a.10107. [DOI] [PubMed] [Google Scholar]
- 31.Celiker R, Gokce-Kutsal Y, Eryilmaz M. Temporomandibular joint involvement in rheumatoid arthritis. Relationship with disease activity. Scand J Rheumatol. 1995;24(1):22–25. doi: 10.3109/03009749509095149. [DOI] [PubMed] [Google Scholar]
- 32.Plant MJ, Williams AL, O'Sullivan MM, Lewis PA, Coles EC, Jessop JD. Relationship between time-integrated C-reactive protein levels and radiologic progression in patients with rheumatoid arthritis. Arthritis Rheum. 2000 Jul;43(7):1473–1477. doi: 10.1002/1529-0131(200007)43:7<1473::AID-ANR9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Voog U, Alstergren P, Eliasson S, Leibur E, Kallikorm R, Kopp S. Progression of radiographic changes in the temporomandibular joints of patients with rheumatoid arthritis in relation to inflammatory markers and mediators in the blood. Acta Odontol Scand. 2004 Feb;62(1):7–13. doi: 10.1080/00016350310007860. [DOI] [PubMed] [Google Scholar]
- 34.Tengstrand B, Ahlmen M, Hafstrom I. The influence of sex on rheumatoid arthritis: a prospective study of onset and outcome after 2 years. J Rheumatol. 2004 Feb;31(2):214–222. [PubMed] [Google Scholar]
- 35.Tengstrand B, Carlstrom K, Fellander-Tsai L, Hafstrom I. Abnormal levels of serum dehydroepiandrosterone, estrone, and estradiol in men with rheumatoid arthritis: high correlation between serum estradiol and current degree of inflammation. J Rheumatol. 2003 Nov;30(11):2338–2343. [PubMed] [Google Scholar]