INTRODUCTION
Since 2002 there has been a rapid expansion in access to antiretroviral therapy (ART) in low- and middle-income countries. By the end of 2007, UNAIDS estimated that over 2 million individuals were receiving ART in Sub-Saharan Africa, an increase of 54% in one year in this region alone (World Health Organization et al. 2008). Despite this remarkable achievement, and growing evidence that ART services in sub-Saharan Africa have had a major impact on morbidity and mortality (Boulle et al. 2008; Jahn et al. 2008) there are notable gaps in our knowledge about the operations and effectiveness of large-scale ART programmes.
Of particular concern is the absence of gender-specific data on ART programmes. While there have been concerns that women may be disadvantaged in their ability to access to ART (Box et al. 2003; Muula et al. 2007; Braitstein et al. 2008), evidence suggests that disproportionately more women than men have initiated ART in developing countries (Braitstein et al. 2008; Keiser 2008). A systematic review found that after accounting for HIV prevalence, proportionally more women than men were on treatment (Muula et al. 2007). How gender may influence ART outcomes remains unclear, and the results of previous research is mixed. Collazos found that women had better clinical and viro-immunological responses to ART than men and concluded that gender played a small but significant role in outcomes (Collazos et al. 2007). Studies in Malawi provided evidence that men were at increased risk of mortality on treatment (Ferradini et al. 2006; Chen et al. 2008). In contrast, a number of studies (Hogg et al. 2001; Weidle et al. 2002; Seyler et al. 2003; Hacker et al. 2004; Nicastri et al. 2005) and two reviews on gender and ART (Braitstein et al. 2006; Nicastri et al. 2007) found little evidence that gender influenced responses to ART.
Socio-economic factors are likely to impact on ART outcomes. The ART-LINC collaboration found that the free provision of ART was associated with reduced mortality (Braitstein et al. 2006). This had major implications for ART programmes in developing countries where patients are living in conditions of low socio-economic status (SES) and are unable to pay user fees for access to ART. For example, a recent study of children accessing ART in Cape Town found that half of the households had a monthly income lower than the national subsistence line, while 75% had an income of <$200/month (Marais et al. 2008)(Marais, Esser et al. 2008). These patients received free treatment, but spent $1–$6 on transport to the clinic (Marais et al. 2008). It is plausible that socio-economic factors including monthly income play a role in survival on ART and retention within ART programmes, but data on these issues from Africa are few.
As ART programmes in developing countries mature, their ability to retain patients on treatment becomes increasingly important. In fact, Boulle (Boulle et al. 2008) suggests that patient retention may be a good overall indicator of programme effectiveness. A recent systematic review found that at 2 years, African ART programmes had retained about 60% of their patients (Rosen et al. 2007) largely due to high loss to follow-up (LTFU). The underlying determinants of this are not well understood though.
Given the importance of understanding the impact of gender and income on the survival and retention of patients on treatment, we investigated these issues in a large public sector ART service in Cape Town, South Africa.
METHODS
Study setting & population
The Gugulethu clinic provides ART to residents of Nyanga, Cape Town, and has been described previously (Bekker et al. 2003; Lawn et al. 2005; Bekker et al. 2006). Nyanga is a predominantly African township on the outskirts of Cape Town with a population estimated at 300,000. Estimated antenatal HIV seroprevalence in 2006 was 29% (Western Cape Provincial Department of Health. 2007).
Patients are eligible for ART on the basis of the 2002 World Health Organisation (WHO) guidelines (WHO stage 4 disease or CD4 cell count below 200 cells/µL). Patients undergo clinical and psycho-social assessment and three group treatment-readiness sessions to ensure that they are ready to initiate and remain on long-term therapy. Patients are then assigned to a community-based therapeutic counsellor for on-site support and follow-up with home visits. This therapeutic support contributes to high rates of adherence and viral suppression and good ascertainment of outcomes (Orrell et al. 2003; Lawn et al. 2006).
Demographic and clinical data were collected via standard data collection forms. Income was measured in South African Rand (ZAR) per month. Income was unevenly distributed, with half of the patients (54%) reporting no personal monthly income. Among those who earned any income, the median was R740/month* (IQR, 620–1040). Monthly income was thus included as a binary variable (some monthly income vs none). WHO staging was done according to 2002 WHO clinical guidelines. CD4 cell count was done using flow cytometry (FACSCount™, Becton Dickinson, New Jersey, USA). HIV RNA viral load was tested with branch DNA hybridisation technique (VersantTM HIV-1 RNA 3.0 branched chain DNA assay (Bayer Healthcare, Leverkusen, Germany).
Analysis
This cohort study included all adults (>=15 years old) in the database who were initiating ART for the first time. For the purposes of this paper, the date of ART initiation was regarded as the baseline visit. Cleaning, coding and analysis of data were done in Intercooled STATA 10.0 for Windows (STATA Corporation, College Station, TX). Follow-up time was calculated from the date of ART initiation and was divided into ‘early’ and ‘late’ ART. The early ART period was analysed as the first 4 months on ART; the late ART period was from 4 to 12 months on ART. Patients were regarded as LTFU if they had started ART and were absent from the clinic for longer than 3 months. Permanent deferrals were those patients who were referred but did not start ART for any reason besides mortality (including LTFU before ART initiation and refusal of ART).
Differences between groups were tested using the Wilcoxon sum rank test for medians and the chi-square test for proportions. Separate proportional hazards regression was used to estimate the crude and adjusted impact of covariates on the hazard of death on ART and the hazard of LTFU. The proportional hazards assumption was confirmed using Schoenfeld and scaled Schoenfeld residuals. Throughout the analysis, 2-sided statistical tests were used at alpha=0.05.
RESULTS
Enrolment and follow-up
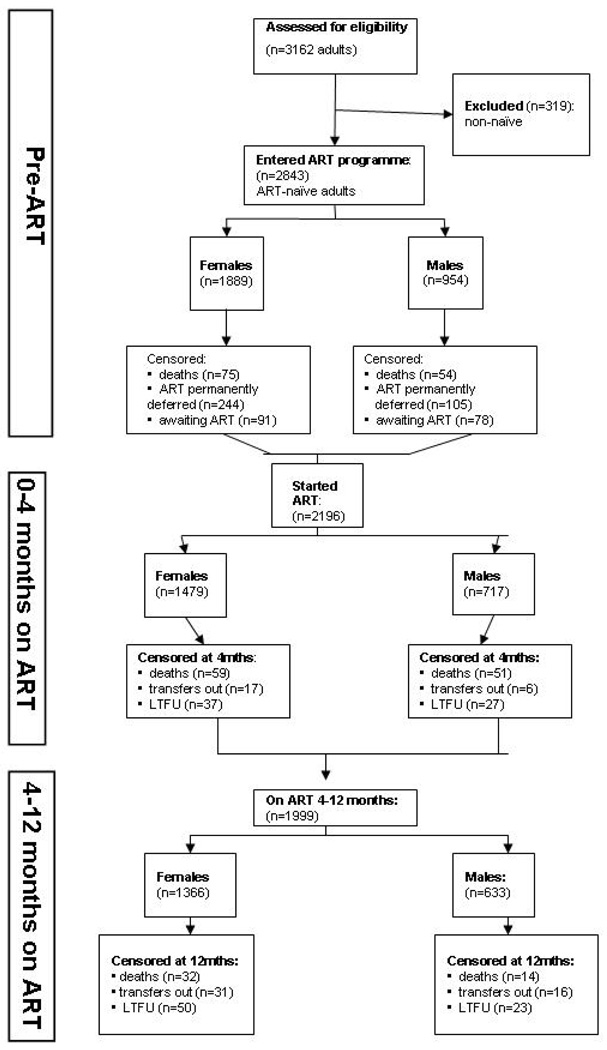
A total of 3,162 adults were referred for treatment between September 2002 and April 2007 (Figure 1). All patients who had started treatment elsewhere (n=319) were excluded as being non-naïve. During the period prior to ART initiation, 647 (23%) adults were censored due to death (n=129) or permanent deferral (n=349) or were still awaiting treatment (n=169). A total of 2,196 patients started ART and were included in this analysis, 67% of whom (n=1,479) were female. The total duration of follow-up was 1,650.8 person-years (females, 1,146.1 person-years and males, 504.7 person-years, p=0.005). The median duration of follow-up was 365 person-days (IQR, 183–365). Median follow-up among women was 365 person-days (IQR, 202–365) and among men 343 person-days (IQR, 145–365). During early ART, 9% of patients (n=197) were censored due to death (n=110), LTFU (n=64) or transfer (n=23). During late ART, 8% (n=166) of patients were censored. LTFU was the main cause (n=73), while 47 were transferred out and 46 died.
Figure 1.
Description of an adult ART programme cohort, Gugulethu, South Africa: September 2002 – April 2007
Baseline characteristics
As shown in Table 1, there were significant gender differences in all key baseline characteristics except income. Of the total study population, 67% (n=1,479) were female. Women were younger than men (31 years vs 36 years, p<0.001) and healthier, with higher median CD4 cell counts (107 vs 89 cells/µL; p<0.001) and lower median log viral loads (4.80 vs 4.95; p<0.001). Overall, 46% of patients (n=864) had some monthly income, and there was no gender difference in the distribution of income (p=0.505).
Table 1.
Demographic and clinical characteristics of 2,196 adults at ART initiation in Gugulethu, Cape Town, South Africa
| Characteristic | Total cohort (n=2,196) |
Women (n=1,479, 67%) |
Men (n=717, 33%) |
p-value |
|---|---|---|---|---|
| Age, median (y) (IQR) | 33 (28–39) |
31 (27–36) |
36 (31–42) |
p<0.001 |
| CD4 cell count (cells/µL), median (IQR) | 102 (48–158) |
107 (56–160) |
89 (39–149) |
p<0.001 |
| CD4 cell count (cells/µL), n (%) | p<0.001 | |||
| <50 | 527 (26%) |
315 (23%) |
209 (32%) |
|
| 51–100 | 468 (23%) |
313 (23%) |
155 (24%) |
|
| 101–150 | 452 (22%) |
320 (23%) |
132 (20%) |
|
| > 150 | 576 (29%) |
415 (30%) |
161 (25%) |
|
| WHO stage at entry, n (%) | p<0.001 | |||
| I & II | 495 (23%) |
382 (26%) |
113 (16%) |
|
| III | 1,197 (55%) |
802 (54%) |
395 (55%) |
|
| IV | 502 (23%) |
294 (20%) |
208 (29%) |
|
| HIV RNA level (log10 copies/ml), median (IQR) |
4.84 (4.41–5.27) |
4.80 (4.34–5.22) |
4.95 (4.54–5.40) |
p<0.001 |
| HIV RNA level (log10 copies/ml), n (%) | p<0.001 | |||
| <= 5 log | 1,181 (60%) |
841 (63%) |
340 (53%) |
|
| > 5 log | 803 (40%) |
504 (37%) |
299 (47%) |
|
| Monthly income, n(%) | p=0.505 | |||
| No income | 1,007 (54%) |
694 (54%) |
313 (53%) |
|
| Some income | 864 (46%) |
583 (46%) |
281 (47%) |
‘Early’ ART period
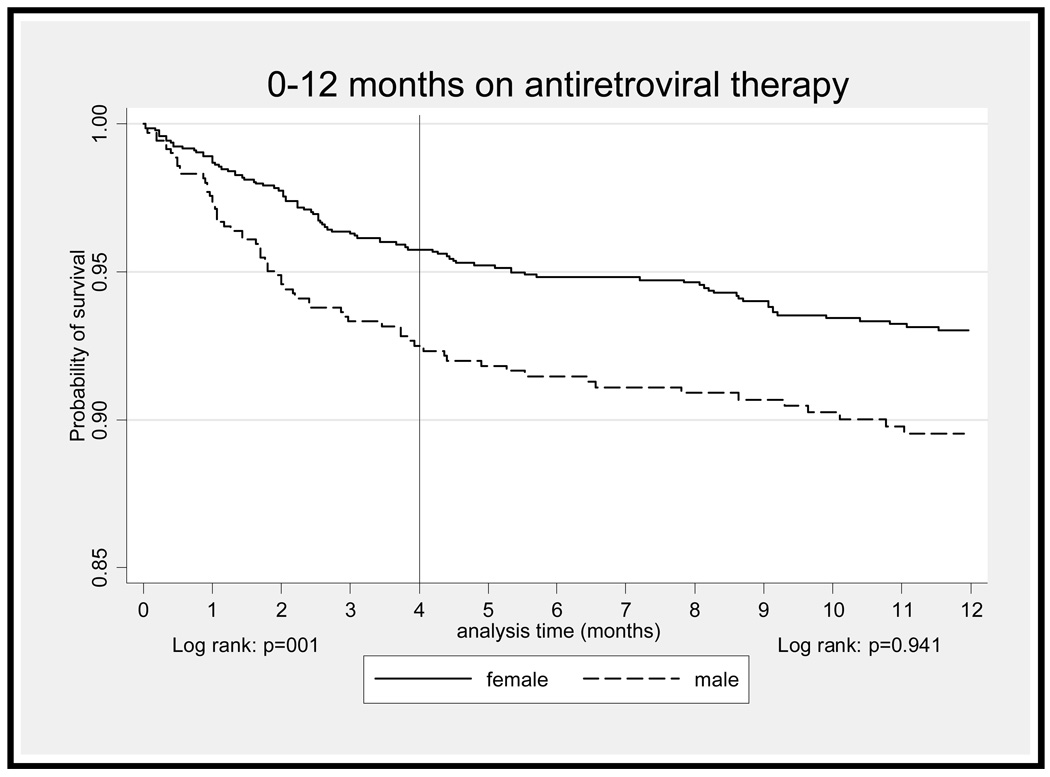
Early mortality on ART was very high. In the first year on ART, 156 deaths occurred. Of these, 71% (n=110) occurred in the first four months on treatment. In this period, 4% (n=59) of female and 7% (n=51) of male patients died (p=0.004). The overall death rate was 15.78/100 person-years and was far higher among men than women (22.8 vs 12.5/100 person-years; p=0.002). In the first four months on ART (Table 2), men had nearly twice the crude risk of mortality compared with women (HR 1.83, 95% CI, 1.26–2.66; p=0.002) (Figure 2). Other risk factors strongly associated with death in bivariate analysis were age, CD4 count, WHO stage and viral load. After adjustment for baseline characteristics (Table 2), there was no association with male gender (adjusted HR 1.46, 95% CI, 0.96–2.22; p=0.076). In multivariate analysis, only CD4 count and WHO stage at baseline significantly impacted on mortality. Having some monthly income was not significantly associated with survival during early ART in crude (HR 0.72, 95% CI 0.48–1.07; p=0.106) or multivariate analysis (adjusted HR 0.78, 95% CI, 0.52–1.18; p=0.246).
Table 2.
Cox proportional hazard models of crude and adjusted associations between patient characteristics and survival:0–4 months and 4–12 months on antiretroviral therapy
| 0–4 months ART | 4–12 months ART | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Deaths, n(%) | univariate model | multivariate model | Deaths, n(%) | univariate model | multivariate model | ||||
| HR (95% CI) | p-value | HR (95 CI%) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Gender | ||||||||||
| Female | 59 (53.6) | 1.0 | 1.0 | 32 (69.6) | 1.0 | 1.0 | ||||
| Male | 51 (46.3) | 1.83 (1.26–2.66) | 0.002 | 1.46 (0.96–2.22) | 0.076 | 14 (30.4) | 0.98 (0.52–1.84) | 0.956 | 0.70 (0.37–1.34) | 0.280 |
| Age (years) | 1.02 (1.00–1.04) | 0.027 | 1.01 (0.99–1.04) | 0.270 | 1.05 (1.02–1.09) | 0.001 | 1.05 (1.02–1.09) | <0.001 | ||
| CD4 (cells/µL) | ||||||||||
| <50 | 55 (52.9) | 1.0 | 1.0 | 17(37) | 1.00 | 1.0 | ||||
| 51–100 | 18 (17.3) | 0.35 (0.20–0.59) | <0.001 | 0.42 (0.24–0.73) | 0.002 | 15 (32.6) | 0.86 (0.43–1.73) | 0.682 | 0.86 (0.43–1.75) | 0.683 |
| 101–150 | 14 (13.5) | 0.28 (0.15–0.50) | <0.001 | 0.37 (0.19–0.71) | 0.003 | 7 (15.2) | 0.41 (0.17–0.98) | 0.046 | 0.42 (0.17–1.05) | 0.064 |
| >150 | 17 (16.4) | 0.27 (0.16–0.46) | <0.001 | 0.35 (0.19–0.63) | <0.001 | 7 (15.2) | 0.33 (0.14–0.81) | 0.015 | 0.35 (0.14–0.87) | 0.023 |
| WHO stage | ||||||||||
| I & II | 7 (6.4) | 1.0 | 1.0 | 5 (10.9) | 1.0 | 1.0 | ||||
| III | 55 (50) | 3.29 (1.50–7.23) | 0.003 | 3.41 (1.21–9.58) | 0.020 | 26 (56.5) | 2.10 (0.81–5.47) | 0.128 | 1.63 (0.61–4.32) | 0.326 |
| IV | 48 (44) | 7.06 (3.19–15.60) | <0.001 | 6.10 (2.14–17.37) | 0.001 | 15 (32.6) | 3.08 (1.12–8.46) | 0.030 | 2.18 (0.77–6.18) | 0.144 |
| HIV RNA level, log10 copies/ml, | ||||||||||
| <=5 Log | 45 (45.9) | 1.0 | 1.0 | 25 (54.4) | 1.0 | 1.0 | ||||
| >5 Log | 53 (54.1) | 1.77 (1.19–2.63) | 0.005 | 1.21 (0.80–1.83) | 0.369 | 21 (45.6) | 1.31 (0.73–2,33) | 0.366 | 1.01 (0.56–1.83) | 0.973 |
| Monthly income | ||||||||||
| No income | 61 (61) | 1.0 | 31 (67.4) | 1.0 | 1.0 | |||||
| Some income | 39 (39) | 0.72 (0.48–1.07) | 0.106 | 0.78 (0.52–1.18) | 0.246 | 15 (32.6) | 0.52 (0.28–0.96) | 0.038 | 0.47 (0.25–0.88) | 0.018 |
Figure 2.
Kaplan-Meier survival estimates of survival by patient gender: 0–4 months and 4–12 months on antiretroviral therapy
Late ART period
The death rate dropped substantially during late ART. One third of all deaths at a year (n=46) occurred during this period. In this period, 3% (n=32) of female and 3% (n=14) of male patients died (p=0.969). The overall mortality rate was 3.7/100 person-years and there was no gender difference in mortality (3.5 vs 3.8/100 person-years; p=0.817) (Figure 2). In this period (Table 2), there was no association between male gender and survival in crude (HR 0.98, 95% CI, 0.52–1.84; p=0.946) or multivariate analyses (adjusted HR 0.70, 95% CI, 0.37–1.34; p=0.280). Factors strongly associated with survival during late ART in crude analysis were age (p=0.001), CD4 count 101–150 and CD4>150 vs <50 cells/µL (respectively p=0.046, p=0.015), WHO stage IV vs stages I & II (p=0.030) and having some monthly income vs none (p=0.038). After adjustment, age (p<0.001), CD4 count >150 cells/µL (p=0.023) and monthly income (p=0.018) remained significantly associated with survival (Table 2). There was a 55% reduction in the risk of mortality among those patients earning some monthly income compared with none (adjusted HR 0.47, 95% CI, 0.25–0.88; p=0.018).
Total time on ART
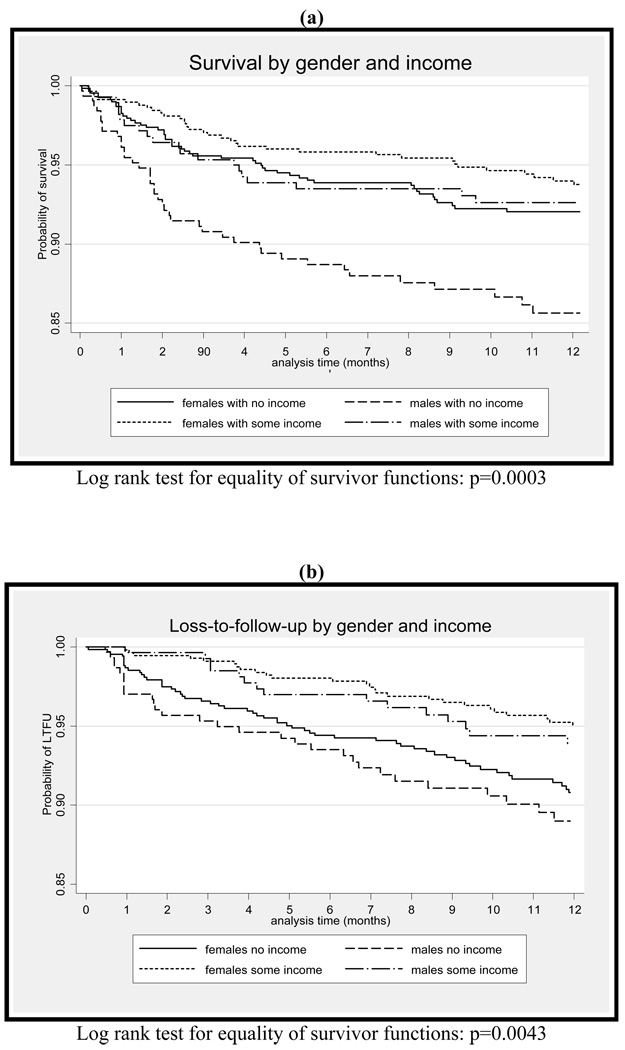
To investigate the potential interaction of gender and income in influencing outcomes on ART, we analysed survival on ART by gender and income strata. Compared to women with no income as the reference group, men with no monthly income had nearly twice the crude hazard of death (HR 1.89, 95% CI, 1.25–2.86; p=0.002) (Figure 3a). However there was no difference in the crude hazard of death for men (HR 0.94, 95% CI, 0.56–1.58; p=0.821) and women (HR 0.75, 95% CI, 0.49–1.16; p=0.201) with some monthly income compared with women with none. In a multivariate model including age, CD4 count, WHO stage, viral load and a separate term for the interaction of gender and income, there was no evidence of any interaction on a multiplicative scale between gender and income (p=0.228).
Figure 3.
Kaplan-Meier estimates: the impact of gender and income on (a) survival and (b) loss-to-follow-up during the first 12 months on antiretroviral therapy
Loss to follow-up
Of 2,196 patients who initiated ART, 137 (6%) were LTFU at one year on therapy. During the first 12 months on ART, 6% (n=87) of female and 7% (n=50) of male patients were LTFU (p=0.055). There was no significant difference in gender distribution between those LTFU and those who were alive and on treatment at one year (p=0.240). Neither median CD4 cell count (p=0.881) nor median log viral load (p=0.490) differed between those LTFU and those who survived. However, income was strongly protective against LTFU (Table 3). The risk of being LTFU was halved among those earning any income compared with no income in both univariate (HR 0.53, 95% CI, 0.37–0.77; p=0.001) and multivariate analyses (HR 0.56, 95% CI, 0.39–0.82; p=0.002) (Table 3). To investigate the potential interaction of gender and income on ART outcomes, we also analysed LTFU on ART by gender and income strata. Compared with women with no income as the reference group, women with some income had a 48% reduction in the crude hazard of LTFU (HR 0.52, 95% CI, 0.33–0.83; p=0.005) (Figure 3b). This finding persisted in a multivariate model with all baseline characteristics and a separate term for the interaction of gender and income (adjusted HR 0.56, 95% CI, 0.36–0.90; p=0.015). There was no significant difference in the crude hazard of LTFU among males with no income (HR 1.2, 95% CI 0.78–1.91; p=0.377), and males with some income (HR 0.66, 95% CI, 0.38–1.16; p=0.149), compared with women with no income.
Table 3.
Cox proportional hazard models: crude and adjusted associations between patient characteristics at baseline and loss-to-follow-up at one year on antiretroviral therapy
| Patients LTFU, n (%) |
Univariate model | Multivariate model | |||
|---|---|---|---|---|---|
| HR (95% CI) | p- value |
HR (95% CI) | p-value | ||
| Gender Female |
87 (63.5) | 1.0 | 1.0 | ||
| Male | 50 (36.5) | 1.30 (0.92–1.84) | 0.141 | 1.38 (0.94–2.03) | 0.100 |
| Age (years) | 0.98 (0.96–1.00) | 0.047 | 0.98 (0.96–1.00) | 0.102 | |
| CD4 (cells/µL) <50 |
41 (30.2) | 1.0 | 1.0 | ||
| 51–100 | 24 (17.7) | 0.59 (0.36–0.98) | 0.041 | 0.62 (0.37–1.05) | 0.077 |
| 101–150 | 27 (17.7) | 0.61 (0.37–1.02) | 0.057 | 0.57 (0.33–1.00) | 0.049 |
| >150 | 47 (34.6) | 0.96 (0.63–1.45) | 0.836 | 1.01 (0.64–1.59) | 0.971 |
| WHO stage I & II |
25 (25.6) | 1.0 | 1.0 | ||
| III | 72 (52.6) | 0.82 (0.55–1.23) | 0.341 | 0.78 (0.50–1.21) | 0.274 |
| IV | 30 (21.9) | 0.85 (0.52–1.38) | 0.508 | 0.75 (0.44–1.28) | 0.294 |
| HIV RNA level, log10 copies/ml, <=5 Log |
81 (60) | 1.0 | 1.0 | ||
| >5 Log | 54 ((40) | 1.04 (0.73–1.46) | 0.843 | 1.13 (0.78–1.64) | 0.520 |
| Monthly income No income |
86 (66.7) | 1.0 | 1.0 | ||
| Some income | 43 (33.3) | 0.53 (0.37–0.77) | 0.001 | 0.56 (0.39–0.82) | 0.002 |
DISCUSSION
In this cohort there were significant gender differences in key demographic and HIV disease characteristics at entry into the ART programme. Men were older and had more advanced disease, with lower CD4 cell counts and higher viral loads. These gender-associated disparities explained the higher mortality rate among men than women during early ART. During late ART, some baseline characteristics (age, CD4 count and monthly income) impacted on mortality in multivariate analysis, while others (including gender) did not. Having some monthly income significantly reduced the risk of mortality during later ART as well as the risk of being LTFU, independent of demographic and HIV disease characteristics.
This study took place in a large, well-maintained cohort. The counsellors ensured near-complete ascertainment of outcomes which strengthened findings related to death and LTFU. The division of study time into early and late ART allowed exploration of the changing impact of baseline disparities on survival. Although the characteristics of this programme, and the patients within it, are broadly representative of public sector ART services in sub-Saharan Africa, the generalisability of these results may be limited and the links between gender and ART programme outcomes requires investigation in other settings.
Mortality during early ART was extremely high, as individuals - particularly men - entered the programme with advanced disease. This is similar to reports from collaborative and single site studies (Braitstein et al. 2006; Stringer et al. 2006; Boulle et al. 2008; Braitstein et al. 2008; Chen et al. 2008; Keiser 2008). In multivariate analysis, there was no evidence of gender-based differences in survival on treatment. This concurs with findings from numerous studies (Hogg et al. 2001; Weidle et al. 2002; Seyler et al. 2003; Hacker et al. 2004. Nicastri et al. 2005; Stringer et al. 2006) and from two reviews, one comparing low and high income countries (Braitstein et al. 2006) and the other a systematic review of published studies on gender and ART (Nicastri et al. 2007). In contrast, two studies in Malawi reported an increased risk for men which persisted after adjustment (Ferradini et al. 2006; Chen et al. 2008). This may have been due to incomplete or missing CD4 measurements in these two studies; without complete data on this important covariate it would be difficult to adjust adequately for the effect of CD4 on mortality. In contrast, baseline CD4 cell counts were available for 92% of the Gugulethu cohort.
More women than men entered this ART programme, as in other developing countries (Braitstein et al. 2006; Muula et al. 2007; Chen et al. 2008). In addition, men started treatment with more advanced disease than women, as has been reported in other African settings (Boulle et al. 2008; Braitstein et al. 2008; Chen et al. 2008). These data demonstrate that late presentation of men resulted in increased mortality in the first four months on treatment. Thus, contrary to concerns that gender differentials may limit women’s access to ART, our study provided additional evidence that men may be particularly disadvantaged in access to treatment in resource-constrained settings (Braitstein et al. 2008; Keiser 2008). Previous studies have suggested a range of complex psycho-social reasons (Braitstein et al. 2008; Chen et al. 2008; Keiser 2008). In addition, and perhaps more importantly, structural obstacles may prevent men from accessing health care services including ART (Braitstein et al. 2008; Keiser 2008). In South Africa, ART is offered through the primary health care (PHC) service, which offers preventive services aimed primarily at the needs of women, not men. PHC services need urgent attention to increase men’s exposure to health services, which is likely to increase their chances of earlier diagnosis and treatment.
Age played an important role in early and late survival in this ART programme. This has important implications as men generally enter the ART programme at later ages than women, and are often infected at an older age than women (UNFPA. 2003). This provides additional evidence to support earlier diagnosis and treatment for men (Lawn & Wood. 2006; Braitstein et al. 2008).
The issue of patient retention in ART programmes is of major import in developing countries which are rapidly expanding access to HIV treatment. A recent systematic review found that African ART programmes retained about 60% of patients after two years on therapy (Rosen et al. 2007). In Malawi, 50% of patients reported to be LTFU had actually died, most of them shortly after missing their last clinic visit (Yu et al. 2007). Similarly, Braitstein (Braitstein et al. 2006) suggests that with different assumptions of outcomes for those LTFU, true mortality in their study might have been as high as 15% and not 6.4%. The low rate of LTFU in Gugulethu, due to the active role of the Sizophila adherence counsellors, increases confidence in our findings. While it is vital to continue to enrol new patients on ART, programmes need to find ways to keep these patients on treatment.
Our finding that individuals with no monthly income experience poorer outcomes on ART has important programmatic implications for public ART programmes in Africa. The ART-LINC collaborative study (Braitstein et al. 2006) found that free HIV treatment in low-income countries was highly protective against mortality (adjusted HR 0.23; 95% CI 0.08–0.78). In this cohort, as in all South African government ART roll-out sites, treatment was free. Despite this, monthly income was strongly associated with survival overall (Table 2) and stratified by gender (Figure 3a). Income was also strongly associated with LTFU (Table 3). These findings suggest that even when treatment is free, having some income still impacts on outcomes on therapy. While the underlying mechanisms require further investigation, these associations are highly plausible: in a situation of limited resources, patients may have to choose, for example, between food for their family and transport to reach an ART clinic.
Socio-economic status is difficult to quantify and measure in the South African context where race-based denial of economic opportunities, and very high levels of inequality, further complicate commonly used definitions of socio-economic status such as income, education or employment (Myer et al. 2004). Nonetheless, our binary measure of income demonstrated persistent associations with programme outcomes. The South African Government uses a household monthly income of ≤$100/month to indicate indigent familiesf (Marais et al. 2008). Using this definition, an estimated 57% of all South Africans were living below the poverty income line by 2001 (Schwabe 2004). In this cohort, over half of the patients had no monthly income. For many of those patients who reported some monthly income, this was in the form of a government disability grant. Although there was no gender difference in the distribution of income, there was some interaction between gender and income (Figures 3a & 3b). Compared with women with no income, men with no monthly income had nearly twice the crude hazard of death (HR 1.89, 95% CI, 1.25–2.86; p=0.002). In addition, among women with some income compared with women with none, there was a 48% reduction in the crude hazard of LTFU (HR 0.52, 95% CI, 0.33–0.83; p=0.005). This finding persisted in multivariate analysis (adjusted HR 0.56, 95% CI, 0.36–0.90; p=0.015). Gender differences in the associations between income and outcomes on ART require further investigation, and highlight the importance of understanding social determinants of health for individuals in HIV care and treatment services. Socio-economic interventions (such as a basic income grant, as has been considered in South Africa) - either for HIV-infected individuals specifically or for all individuals below the poverty line - require further attention.
In summary, men entering this ART programme were older and had more advanced HIV disease than women. This late presentation resulted in increased early mortality on ART for men compared with women. Treatment programmes should prioritise earlier diagnosis and treatment of men. Further studies should explore the role of socio-economic factors in survival and patient retention in ART programmes in developing countries.
Acknowledgements
M.C., L.-G.B, L.M. and R.W. are all partially funded by the National Institutes of Health through a CIPRA Grant no. U19 AI53217. R.W. is also partially funded by an R01 grant A1058736-01A1. M.C. & R.W. are partially funded through the IeDEA-SA Grant no 5U01AI069924-02.
M.C. & L.M. conceived and designed the study. M.C. undertook statistical analysis and wrote the paper. L.M. assisted with data inerpretation and revision. R.K. assisted with data collection. L.-G.B. helped establish the study cohort and assisted in data interpretation and revision. R.W. assisted with data interpretation and revision.
"Support for this study was provided by the US National Institute of Allergy and Infectious Diseases (NIAID) through the Comprehensive International Program of Research on AIDS (CIPRA) network, Grant U19 AI53217 and the International epidemiological Database to Evaluate AIDS, Southern Africa (IeDEA-SA), Grant no 5U01AI069924-02."
Footnotes
ZAR1=US$7
$1 = ±R7
Conflicts of interest
The authors declare no conflict of interest.
REFERENCES
- Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96(4):315–320. [PubMed] [Google Scholar]
- Bekker LG, Orrell C, Reader L, Matoti K, Cohen K, Martell R, et al. Antiretroviral therapy in a community clinic--early lessons from a pilot project. S Afr Med J. 2003;93(6):458–462. [PubMed] [Google Scholar]
- Boulle A, Bock P, Osler M, et al. Antiretroviral therapy and early mortality in South Africa. Bulletin of the World Health Organisation. 2008;86(9):678–687. doi: 10.2471/BLT.07.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box TL, Olsen M, Oddone EZ, Keitz SA. Healthcare Access and Utilization by Patients Infected with Human Immunodeficienc Virus: Does Gender matter? Journal of Women's Health. 2003;12(4):391–397. doi: 10.1089/154099903765448907. [DOI] [PubMed] [Google Scholar]
- Braitstein P, Boulle A, Nash D, et al. Gender and the Use of Antiretroviral Treatment in Resource-Constrained Settings: Findings from a Multicenter Collaboration. J Womens Health (Larchmt) 2008;17(1):47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- Braitstein P, Brinkhof M, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- Chen SC-C, Yu JK-L, Harries AD, et al. Increased mortality of male adults with AIDS related to poor compliance to antiretroviral therapy in Malawi. Tropical Medicine & International Health. 2008;13(4):513–519. doi: 10.1111/j.1365-3156.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- Collazos J, Asensi V, Carton JA. Sex differences in the clinical, immunological and virological parameters of HIV-infected patients treated with HAART. Aids. 2007;21(7):835–843. doi: 10.1097/QAD.0b013e3280b0774a. [DOI] [PubMed] [Google Scholar]
- Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367(9519):1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- Hacker MA, Petersen ML, Enriquez M, Bastos FI. Highly active antiretroviral therapy in Brazil: the challenge of universal access in a context of social inequality. Rev Panam Salud Publica. 2004;16(2):78–83. doi: 10.1590/s1020-49892004000800002. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viiral load after initiating triple-drug therapy. Jama. 2001;286(20):2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- Jahn A, Floyd S, Crampin AC, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. The Lancet. 2008:371. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser O The ART-LINC Collaboration of the International Databases to Evaluate AIDS (IeDEA) Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13(7):1–10. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn S, Wood R. How can earlier entry of patients into antiretroviral programs in low-income countries be promoted? Clin Inf Dis. 2006;42(3):431–432. doi: 10.1086/499527. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa implications for program evaluation. Clin Infect Dis. 2006;43(6):770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa:implications for programme design. Aids. 2005;19(18):2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- Marais BJ, Esser M, Godwin S, Rabie H, Cotton MF. Poverty and human immunodeficiency virus in children: a view from the Western Cape, South Africa. Ann N Y Acad Sci. 2008;1136:21–27. doi: 10.1196/annals.1425.012. [DOI] [PubMed] [Google Scholar]
- Muula AS, Ngulube TJ, Siziya S, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer L, Ehrlich RI, Susser ES. Social epidemiology in South Africa. Epidemiol Rev. 2004;26:112–123. doi: 10.1093/epirev/mxh004. [DOI] [PubMed] [Google Scholar]
- Nicastri E, Angeletti C, Palmisano L, et al. Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. Aids. 2005;19(6):577–583. doi: 10.1097/01.aids.0000163934.22273.06. [DOI] [PubMed] [Google Scholar]
- Nicastri E, Leone S, Angeletti C, et al. Sex issues in HIV-1-infected persons during highly active antiretroviral therapy: a systematic review. J Antimicrob Chemother. 2007;60(4):724–732. doi: 10.1093/jac/dkm302. [DOI] [PubMed] [Google Scholar]
- Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. Aids. 2003;17(9):1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe C Human Sciences Research Council. Fact Sheet: Poverty in South Africa. 2004 [Google Scholar]
- Seyler C, Anglaret X, Dakoury-Dogbo N, et al. Medium-term survival, morbidity and immunovirological evolution in HIV-infected adults receiving antiretroviral therapy, Abidjan, Cote d'Ivoire. Antivir Ther. 2003;8(5):385–393. [PubMed] [Google Scholar]
- Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- UNFPA. State of World Population 2003: Making 1 billion count: Investing in Adolescents' Health and Rights. [Accessed 20 August 2008];UNFPA. 2003 Available from http://www.unfpa.org/swp/2003/english.html. [Google Scholar]
- Weidle PJ, Malamba S, Mwebaze R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet. 2002;360(9326):34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- Western Cape Provincial Department of Health. HIV Prevalence in the Western Cape:Results of the 2006 HIV Antenatal Provincial and Area Surveys. Cape Town. 2007 [Google Scholar]
- World Health Organization, UNAIDS, and UNICEF. Towards Universal access: Scaling up priority HIV/AIDS interventions in the health sector: Progress report: 2008. World Health Organization. 2008 [Google Scholar]
- Yu JK-L, Chen SC-C, Wang K-Y, et al. True outcomes for patients on antiretroviral therapy who are 'lost-to-follow-up' in Malawi. Bulletin of the World Health Organisation. 2007;85(7) doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]