Abstract
Introduction
Neuregulin-1 (NRG1) is one of susceptibility genes for schizophrenia and plays critical roles in glutamatergic, dopaminergic and GABAergic signaling. Using mutant mice heterozygous for Nrg1 (Nrg1+/−) we studied the effects of Nrg1 signaling on behavioral and electrophysiological measures relevant to schizophrenia.
Experimental Procedure
Behavior of Nrg1+/− mice and their wild type littermates was evaluated using pre-pulse inhibition, contextual fear conditioning, novel object recognition, locomotor, and social choice paradigms. Event-related potentials (ERPs) were recorded to assess auditory gating and novel stimulus detection.
Results
Gating of ERPs was unaffected in Nrg1+/− mice, but mismatch negativity in response to novel stimuli was attenuated. The Nrg1+/− mice exhibited behavioral deficits in contextual fear conditioning and social interactions, while locomotor activity, pre-pulse inhibition and novel object recognition were not impaired.
Summary
Nrg1+/− mice had impairments in a subset of behavioral and electrophysiological tasks relevant to the negative/cognitive symptom domains of schizophrenia that are thought to be influenced by glutamatergic and dopaminergic neurotransmission. These mice are a valuable tool for studying endophenotypes of schizophrenia, but highlight that single genes can not account for the complex pathophysiology of the disorder.
Keywords: Neuregulin, schizophrenia, mice, animal models, event related potentials, social, behavior
1. Introduction
Schizophrenia is a devastating psychiatric illness that affects about 1% of the world's population (Lewis and Levitt, 2002). Recent molecular genetics studies have identified multiple candidate genes for susceptibility for schizophrenia, such as Neuregulin-1 (NRG1), DISC1, Dysbindin, COMT, and GAD67 (Guidotti et al., 2000; Harrison and Weinberger, 2005; O'Tuathaigh et al., 2007a).
NRG1 is of particular interest because much of its physiological and molecular activity has been characterized previously and is relevant to the proposed pathophysiology of schizophrenia (Stefansson et al., 2002) (Wen et al., 1992; Yang et al., 2003) (Corfas et al., 2004; Dong et al., 1995; Marchionni et al., 1993; Plowman et al., 1993). NRG1 functions as a growth and differentiation factor and binds the erbB family of receptor tyrosine kinases, specifically erbB2, 3 and 4. Many isoforms of NRG1 are created by alternative splicing, but types 1, 2 and 3 constitute the major forms (Hashimoto et al., 2004). Several of these isoforms have been linked to aspects of neuronal development that are thought to contribute to the onset of schizophrenia. For instance, NRG1 type 1, also known as neu differentiation factor, modulates growth and differentiation of neural crest cells (Corfas et al., 1995; Goodearl et al., 1995). NRG1 type 2 promotes oligodendrocyte production and myelination in the CNS, while type 3 is involved in the development of Schwann cells (Edwards and Bottenstein, 2006; Marchionni et al., 1999; Meyer et al., 1997). NRG1 type 3 is thought to be the most abundant type of NRG1 found in the cortical regions of the brain. It has been shown to be important for normal sensory motor gating and memory functions as well as nicotine mediated hippocampal activity (Chen et al., 2008; Zhong et al., 2008). Of note, one line of Nrg1 transgenic mice were found to have decreased N-methyl-D-aspartic acid (NMDA) receptor binding when compared to their wild type littermates (Stefansson et al., 2002). Additionally, NRG1 binds preferentially to the ErbB4 receptor, which can modulate the function of glutamatergic receptors, further supporting its possible role in the pathophysiology of schizophrenia (Hahn et al., 2006; Huang et al., 2000; Kwon et al., 2005; Li et al., 2007).
Changes in NRG1 expression may profoundly effect the development and function of the brain through all of the previously discussed mechanisms and, therefore, may contribute to impairments seen in schizophrenia. Individuals with schizophrenia perform poorly across a range of cognitive, behavioral, and physiological measures. Problems with working memory and social interaction can contribute to an inability to function in society (Addington and Addington, 2000; Bowen et al., 1994; Cohen et al., 2006; Corrigan et al., 1994). Additionally, deficits in gating, mismatch negativity (MMN), and pre-pulse inhibition (PPI) of startle are well described endophenotypes of schizophrenia that may underlie some of the aforementioned problems (Boutros et al., 2004; Freedman et al., 1996; Grillon et al., 1992; Javitt et al., 1998; Light et al., 2000; Parwani et al., 2000; Umbricht and Krljes, 2005). Performing similar measures in mice has added a valuable tool for researchers to model schizophrenia. Fear conditioning and novel object recognition paradigms are used to determine if mice display deficits in memory (Crawley, 1999; Hashimoto et al., 2007; Powell et al., 2007). Gating, mismatch negativity, and PPI are evaluated in animals using tasks that are very similar to those used in the human population. Studies from our group as well as others have demonstrated such schizophrenia endophenotypes in rodent models using these tasks (Ehrlichman et al., 2008; Maxwell et al., 2006; Simosky et al., 2003; Swerdlow et al., 1998; Umbricht et al., 2005).
Several groups have reported increases in NRG1 in postmortem brain studies. This may suggest that mice that over-express Nrg1, rather than Nrg1+/−, may be a better model for schizophrenia. It is unknown, however, whether the modest increases in NRG1 previously observed are associated with enhanced NRG1 –erbB4 signaling (Chong et al., 2008; Hashimoto et al., 2004; Law et al., 2006). Our group has reported a significant enhancement in erbB4 signaling, when postmortem brain tissues were stimulated with NRG1 (Hahn et al., 2006). In our Western analysis, we failed to detect significant differences in NRG1 expression between the schizophrenia and control groups (Hahn et al., 2006). Interestingly, Nrg1 induced activation of erbB4 in mice was found to have a trend for an increase in Nrg1+/− examined in this study (unpublished observation).
The purpose of this study is to examine the impact of Nrg1 on electrophysiological and social behaviors that have been implicated for schizophrenia. NRG1 has many isoforms and it is not entirely clear as to which isoforms are dysregulated in brains of patients with schizophrenia. Therefore, we chose to examine mice mutated for the activity of all isoforms among various mutant lines available. To accomplish this goal, we chose the mutants developed by Meyer and Birchmeier that contain mutations in EGF domain of all isoforms, which is critical for binding of Nrg1 to the receptors. In various lines of Nrg1+/− mice, recent studies showed deficits in physiology and behaviors (Chen et al., 2008; Duffy et al., 2008; O'Tuathaigh et al., 2008; Stefansson et al., 2002). We anticipated a reduction in gating of P20 and N40 event related potentials (ERPs) and an attenuation of MMN in response to novel stimuli. In addition to those electrophysiological measures, we expected the Nrg1+/− mice in this study to display impairments in PPI, memory tasks including contextual fear conditioning and novel object recognition, social interaction and increased locomotor activity.
2. Results
ERPs
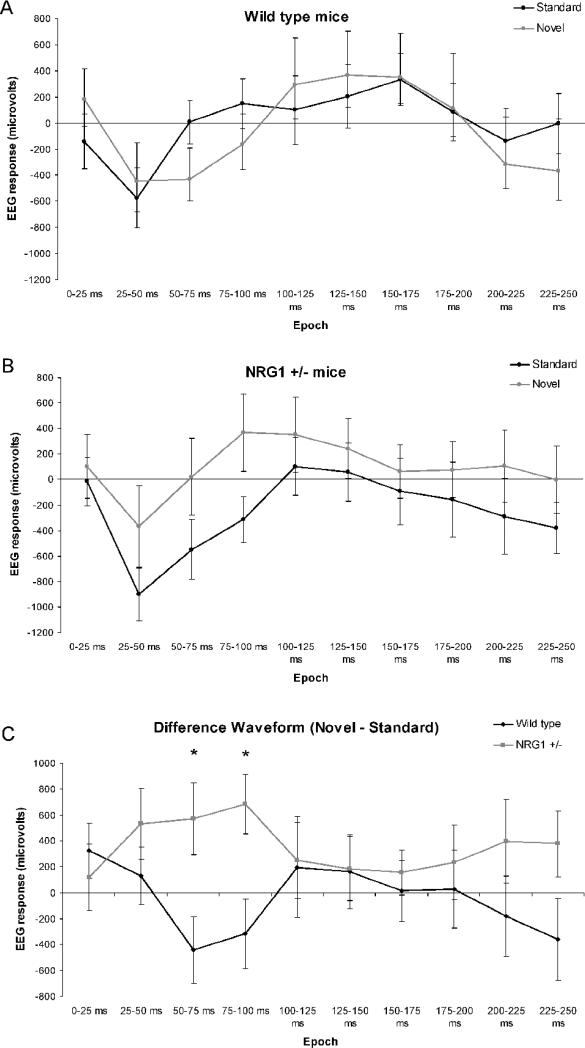
A reduction in the amplitude of the second response (S2) referenced to the first (S1) was found across genotypes for both P20 and N40 ERPs (P20: F(1,26) = 38.729, p<0.001, N40: F(1,26) = 26.34, p < 0.001). There was no main effect of genotype on either P20 or N40 response (p > 0.05 for both). There was no stimulus by gene interaction in P20 or N40 (p > 0.05 for both). These data do not support the hypothesis that Nrg1 signaling modulates gating of ERPs. Wild type mice displayed a MMN from 50-75 ms (p<0.05). Nrg1+/− mice did not display the normal pattern of MMN. Rather, mutant mice had a positive difference wave (novel – standard) from 50-100ms (50-75 ms p<0.05, 75-100 ms p<0.05). There was a significant difference between genotypes for MMN using an independent t-test at 50-100 ms (50-75 ms t = 2.36, df = 26, p < 0.05; 75-100 ms t = 2.49, df = 26, p < 0.05) (Figure 1).
Figure 1. Mismatch Negativity.
The area under the curve in response to the standard (black line) and novel (gray line) stimuli for (A) wild type and (B) Nrg1+/− mice are shown. (C) The difference in area (standard – novel) for both the wild type (black line) and Nrg1+/− (gray line) are shown. A significant difference between wild type and Nrg1+/− waveforms exists at 50-100 ms and 75-100 ms. * p < 0.05
Startle and Pre-pulse inhibition
An rmANOVA showed no main effects of genotype on startle (F(1, 26) = 0.002, p > 0.05) or PPI (F(1, 26) = 0.67, p > 0.05). There was a main effect of startle intensity (F(7, 182) = 12.8 , p < 0.001) but no gene by startle interaction (F(7, 182) = 0.25, p > 0.05) indicating that both wild type and Nrg1+/− mice respond similarly to startle stimuli. There was a significant effect of pre-pulse intensity (F(2, 52) = 4.64, p < 0.05), but no interaction between pre-pulse intensity and genotype (F(2, 52) = 0.16, p > 0.05) (Table 1).
Table 1.
Startle and Pre-Pulse Inhibition
| Startle Intensity |
WT (V ± SEM) |
Nrg1+/− (V ± SEM) |
|---|---|---|
| 0 dB |
9.49 ± 0.67 |
9.08 ± 0.63 |
| 90 dB |
10.46 ± 0.82 |
10.56 ± 0.77 |
| 95 dB |
12.23 ± 2.12 |
15.55 ± 1.98 |
| 100 dB |
14.2 ± 2.32 |
15.23 ± 2.16 |
| 105 dB |
17.55 ± 2.58 |
19.2 ± 2.40 |
| 110 dB |
24.94 ± 5.17 |
24.33 ± 4.81 |
| 115 dB |
28.05 ± 6.79 |
25.92 ± 6.32 |
| 120 dB |
33.78 ± 5.97 |
29.75 ± 5.55 |
| PPI Intensity |
WT (% inhibition ± SEM) |
Nrg1+/− (% inhibition ± SEM) |
|---|---|---|
| + 4 dB |
−2.54 ± 10.63 |
10.94 ± 9.89 |
| + 8 dB |
13.17 ± 11.27 |
20.18 ± 10.49 |
| + 16 dB |
15.6 ± 10.35 |
27.19 ± 9.63 |
The mean voltages ± SEM are shown for wild type and Nrg1+/− mice for all startle intensities. The mean percent inhibition ± SEM is shown for wild type and Nrg1+/− mice for each pre-pulse intensity level (+4, +8 and +16 dB over background).
In a separate cohort of mice treated with saline and amphetamine there was a significant main effect of drug (F(1, 14) = 6.29, p < 0.05) and startle intensity (F(7, 98) = 8.13, p < 0.001). There was also a significant interaction of drug and startle intensity (F(7, 98) = 3.45, p < 0.005) indicating that treatment with amphetamine reduced the level of startle in wild type and Nrg1+/− mice compared to treatment with saline. However, there was no three way interaction of gene, startle intensity, and drug (F(7, 98)=1.05, p > 0.05) showing that amphetamine affected both wild type and Nrg1+/− mice similarly in the startle experiment. When examining the pre-pulse inhibition experiment there were no significant main effects of gene (F(1, 14) = 2.08, p > 0.05), pre-pulse intensity (F(2, 28) = 1.76, p > 0.05), or drug (F(1, 14)= 0.79, p > 0.05). Additionally, there was no significant two-way interactions between drug and pre-pulse intensity (F(2, 28) = 2.72, p > 0.05) showing that amphetamine blocked the ability of wild type and Nrg1+/− mice to gate using the pre-pulses. Nor was there a three-way interaction between drug, pre-pulse intensity, and gene (F(2, 28) = 0.94, p > 0.05) indicating that wild type and Nrg1+/− mice were not differentially affected by amphetamine (Table 2).
Table 2.
Startle and Pre-Pulse Inhibition (Saline vs. Amphetamine)
| Startle Intensity |
WT (Saline) |
WT (Amph) |
Nrg1+/− (Saline) |
Nrg1+/− (Amph) |
|---|---|---|---|---|
| 0 dB |
6.1 ± 1.47 |
8.63 ± 1.66 |
9.25 ± 1.47 |
7.83 ± 1.66 |
| 90 dB |
8.73 ± 2.10 |
9.73 ± 1.87 |
7.9 ± 2.10 |
8.10 ± 1.87 |
| 95 dB |
18.28 ± 3.72 |
15.85 ± 3.37 |
11.9 ± 3.72 |
14.45 ± 3.37 |
| 100 dB |
25.98 ± 7.00 |
16.35 ± 3.61 |
18.88 ± 7.00 |
16.08 ± 3.61 |
| 105 dB |
41.75 ± 14.38 |
19.30 ± 3.79 |
25.93 ± 14.38 |
18.13 ± 3.79 |
| 110 dB |
58.05 ± 17.46 |
27.98 ± 9.75 |
29.3 ± 17.46 |
24.85 ± 9.75 |
| 115 dB |
59.48 ± 18.97 |
30.68 ± 10.26 |
45.32 ± 18.97 |
27.53 ± 10.26 |
| 120 dB |
85.95 ± 27.31 |
34.05 ± 7.88 |
42.83 ± 27.31 |
28.60 ± 7.88 |
| PPI Intensity |
WT (Saline) |
WT (Amph) |
Nrg1+/− (Saline) |
Nrg1+/− (Amph) |
|---|---|---|---|---|
| + 4 dB |
6.54 ± 16.15 |
−10.81 ± 15.83 |
−30.41 ± 21.01 |
−6.54 ± 25.93 |
| + 8 dB |
7.87 ± 13.25 |
−9.57 ± 13.95 |
−25.68 ± 18.30 |
−44.51 ± 23.35 |
| + 16 dB |
29.28 ± 11.68 |
−8.62 ± 17.13 |
10.98 ± 14.07 |
−31.59 ± 24.95 |
The mean voltages ± SEM are shown for wild type and Nrg1+/− mice for all startle intensities following treatment with saline or amphetamine. The mean percent inhibition ± SEM is shown for wild type and Nrg1+/− mice for each pre-pulse intensity level (+4, +8 and +16 dB over background) following treatment with saline or amphetamine.
Locomotor activity
An rmANOVA showed that there was no main effect of genotype on activity (mean ± SEM; wild type: 1595.2 ± 129.9; Nrg1+/−: 1554.3 ± 114.6) (F(1, 22)=.06, p > 0.05). There was no time by genotype interaction (F(5,110) = 0.62, p > 0.05) across the testing interval.
Contextual Fear Conditioning
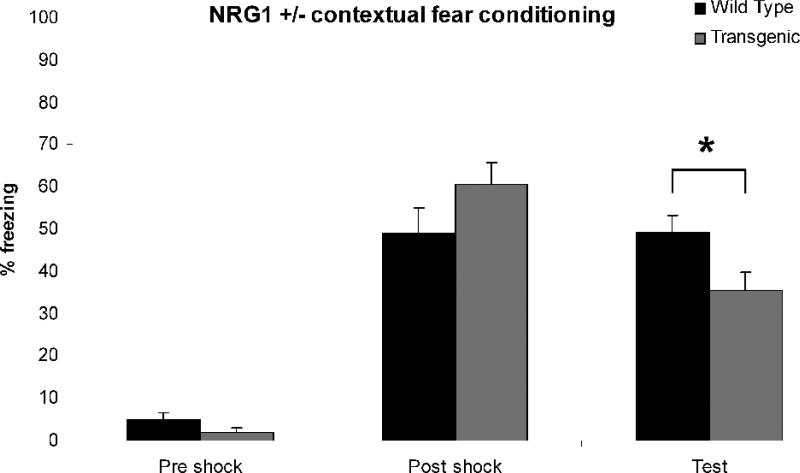
There was no main effect of genotype (p > 0.05). There was a main effect of session (p < 0.01) and a session by genotype interaction (F(2,36) = 4.56, p < 0.05). A Fisher LSD post hoc test indicated no difference between genotypes for either the pre- or immediate post- shock freezing (p > 0.05). There was a significant reduction in freezing by the Nrg1+/− mice compared to wild type littermates during the session (p < 0.05) (Figure 2).
Figure 2. Contextual Fear Conditioning.
Comparison of wild type (black) and Nrg1+/− (gray) mice revealed no significant difference between genotypes for the degree of pre- or immediate post-shock freezing. There was a significant decrease in freezing of Nrg1+/− mice during the test phase. * p < 0.05
Novel Object Recognition
No difference in novel object exploration was found between wild type (mean ± SEM: 66.63% ± 1.62) and Nrg1+/− mice (mean ± SEM: 60.61% ± 3.87) (t = 1.36, df = 13, p > 0.05).
Social Interaction
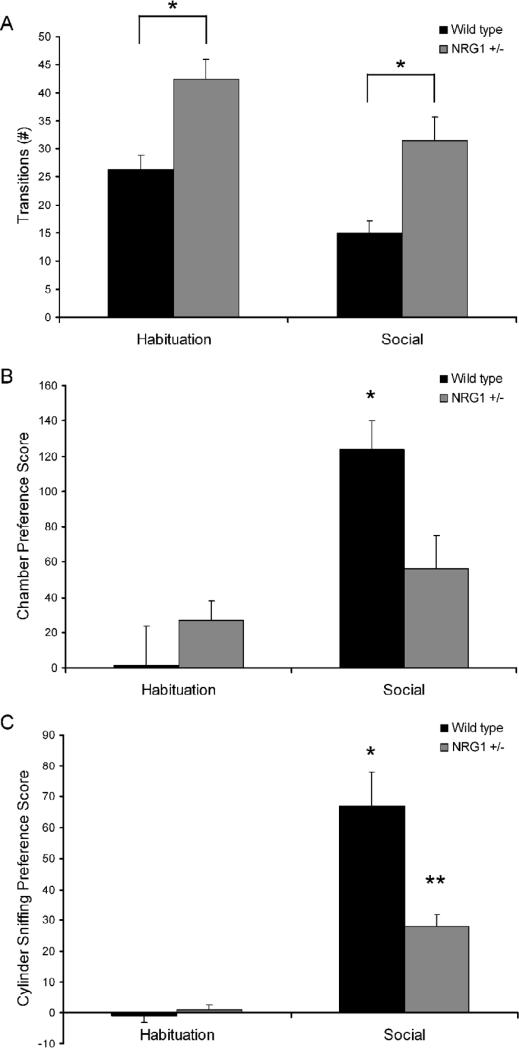
The Nrg1+/− mice showed increased transitions relative to wild type mice during the social choice test. A rmANOVA showed a significant main effect of time (F(1,17) = 35.274, p < 0.01) and a significant main effect of genotype (F(1,18) = 8.1, p < 0.05; paired t test p < 0.05 between wild type and Nrg1+/− mice during habituation and social phases) on number of transitions between chambers during social testing (Figure 3A). There was no significant interaction between time by genotype (F(1,17)=0.019, p > 0.05), indicating that both wild type and Nrg1+/− mice habituated to the 3-chambered apparatus in a similar manner.
Figure 3. Social Choice Task.
Habituation scores are the mean of the two 5-minute habituation periods (mean of Phase 1A and 1B), during which the cylinders were empty. Social scores are from the 5-minute Social Phase (Phase 2), during which the social cylinder contained a stimulus mouse, and the nonsocial cylinder contained a novel object. (A) Nrg1+/− mice showed increased transitions relative to wild type mice during the social choice test, suggesting that decreased social interactions were not related to a decrease in entries into the social chamber. (B) Nrg1+/− mice showed significantly lower levels of approach toward stimulus mice relative to wild type mice. (C) Nrg1+/− mice also showed significantly lower levels of direct sniffing of the stimulus mice, relative to wild type mice.
During the social phase (stimulus mouse in the “social cylinder”), the Nrg1+/− mice showed significantly lower levels of approach toward stimulus mice relative to wild type mice (significant main effect of time (F(1,17)=21.850; p < 0.001); significant interaction between time by genotype (F(1,17)=10.329; p = 0.005) (Figure 3B). In addition, Nrg1+/− mice also showed significantly lower levels of direct sniffing of the stimulus mice, relative to wild type mice (significant main effect of genotype on (F(1,17) = 11.7, p = 0.003); significant main effect of time (F(1,17) = 92.643, p < 0.001); significant interaction between time by genotype (F(1,17) = 17.6, p < 0.001) (Figure 3C)). During Phase 3 (free interaction phase) of the social choice test, none of the Nrg1+/− or wild type mice attacked (bit) a stimulus mouse, and thus there was no significant difference between mutant and wild type mice in aggressive attack behaviors.
3. Discussion
Expectations, summary of results
Multiple studies have identified NRG1 as a candidate susceptibility gene for schizophrenia (Harrison and Weinberger, 2005; Stefansson et al., 2003; Weinberger, 2005). We expected Nrg1+/− mice to exhibit behavior consistent with mouse models that have been shown to exhibit predictive or construct validity for positive and negative symptoms as well as cognitive and electrophysiological deficits in schizophrenia (Swerdlow and Geyer, 1998). Anticipated results included reduced amplitude and gating of the P20 and N40 ERPs, disrupted mismatch negativity, reduced pre-pulse inhibition, impaired memory function in the Contextual Fear Conditioning and Novel Object Recognition tasks, decreased social interaction and increased locomotor activity (Duffy et al., 2008; Karl et al., 2007; Moghaddam and Adams, 1998; Siegel et al., in press). We anticipated such alterations in locomotor activity in Nrg1+/− mice because of possible interactions between NRG1 and NMDA function (Stefansson et al., 2002). It is important to note that NMDA antagonists can cause increased, decreased or no change in locomotor activity depending on dose. Therefore, the interaction between the specific Nrg1 mutation and NMDA receptor function may result in differing locomotor profiles in the various Nrg1 hypomorphic lines.
Nrg1+/− mice exhibited some, but not all, of these endophenotypes. Nrg1+/− mice had reduced freezing in the contextual fear paradigm, disrupted MMN, and reduced sociability compared to wild type controls. No differences were found in locomotor activity, P20 or N40 gating and amplitude, prepulse inhibition, or novel object recognition.
Significance as mouse model of schizophrenia
Our results differ from previous locomotor analysis of Nrg1-Tm+/− mice, suggesting that alterations in the expression of the various Nrg1 isoforms may have differential effects on task specific behaviors (Karl et al., 2007). The observation of increased transitions among Nrg1+/− mice during the social interaction task also suggests that subtle differences in the metric of activity can influence the outcome. As noted below, increased transitions can not explain the reduction in social behavior. Nrg1+/− mice exhibited impairments in experience related memory (contextual fear) but not in object related memory. Studies show that multiple brain regions are required in the creation of a conditioned response (CR), including thalamus, hippocampus and sensory cortex. LeDoux and colleagues showed that hippocampus and amygdala are important for memory consolidation and that conditioned-unconditioned stimulus convergence happens primarily in the amygdala (Davis and Shi, 2000; LeDoux, 1995; Maren, 2001). Similarly, hippocampus and amygdala both play a role in the retrieval and expression of fear memories. Novel object recognition has been shown to primarily involve the perirhinal cortex and hippocampus (Awipi and Davachi, 2008; Bachevalier and Nemanic, 2008; Buffalo et al., 2006; Cornejo et al., 2008). Thus, the hippocampus is critical for both contextual fear and novel object recognition. Alternatively, amygdalar activation is more related to contextual fear memories than novel object recognition and the perirhinal cortex is more critical for novel object recognition (Awipi and Davachi, 2008; Bachevalier and Nemanic, 2008; Buffalo et al., 2006; Cornejo et al., 2008; LeDoux, 1995; Maren, 2001). Impaired performance of Nrg1+/− mice in contextual fear testing but not novel object recognition suggests that the amygdala may be more affected by constitutive Nrg1 deficiencies than other regions of interest. The lack of significant differences in novel object recognition between Nrg1+/− mice and control mice suggest that Nrg1 deficiency does not alter the hippocampus or perirhinal cortex sufficiently to cause quantifiable changes in the novel object recognition task.
Our finding of reduced sociability in Nrg1+/− mice is somewhat different from previous reports in Nrg1-TM +/− mice. A previous study of the latter mice found no difference in sociability relative to wild type mice (no difference in social approach toward the first stimulus mouse that is introduced), but a decrease in “preference for social novelty” (decrease in approach to a stimulus mouse that is introduced second, and is relatively unfamiliar) (O'Tuathaigh et al., 2007b). Also, while our study found no difference in aggressive attack behaviors between Nrg1+/− and wild type mice, previous studies have found an increase in aggressive behaviors in Nrg1-TM +/− mice relative to wild type mice, both in a resident-intruder test and a novel environment (O'Tuathaigh et al., 2007b; O'Tuathaigh et al., 2008). The differences between our findings and those of previous studies may be due to the difference in the mutation being studied (Nrg1+/− vs. Nrg1-TM+/−). Moreover, previous studies used time in the chamber as the only dependent variable for analysis in the social choice task (O'Tuathaigh et al., 2007b), whereas we analyzed both time in chamber and direct sniffing time (sniffing of cylinder containing the stimlus mouse vs. sniffing of cylinder containing the novel object). Also, in our social choice task, we used gonadectomized A/J stimulus mice in an effort to minimize sexual and aggressive motivations of test mice. Because the previous study used intact C57BL/6J stimulus mice, it is possible that the higher level of social approach of Nrg1-TM+/− mice may have been influenced by aggressive motivations (O'Tuathaigh et al., 2007b). Future studies of Nrg1+/− mice in the social choice test using non-gonadectomized stimulus mice would be useful in order to determine the degree to which the reduced sociability phenotype that we observed is generalizable to various types of stimulus mice, and to better understand the degree to which the sociability phenotype in Nrg1+/− mice can be influenced by aggressive or sexual motivations of the test mice. Although the present study was mainly interested in the effects of genotype on the various phenotypes, future studies will include larger samples of males and females, to test the effect of sex on the phenotypes. The mouse model used to examine social interactions in this study has both face and construct validity for decreased social interactions in schizophrenia. The predictive value of these social deficits with regard to treatment of social withdrawal in schizophrenia is worthy of further study, but this is a challenge because there are not yet medications that effectively treat negative symptoms of schizophrenia (Siegel et al., in press).
Not all of the behaviors exhibited by Nrg1+/− mice are consistent with schizophrenia. Nrg1+/− mice showed no change in either P20 or N40 amplitude or gating, suggesting that Nrg1-ErbB signaling deficits are not linked to P50 or N100 deficits seen in schizophrenia. This lack of change in P20 and N40 amplitude is consistent with a lack of change in PPI in Nrg1+/− mice in the current study. However, others have reported that Nrg1-Tm+/− mice have impaired PPI (Stefansson et al., 2002). Differences in results among these studies may be due to the specific nature of the Nrg1 mutations in each line of mice or differences in compensatory mechanisms for deficiencies in various Nrg1 types. Alternatively, the lack of PPI impairment in Nrg1+/− mice used in this study could be related to their hybrid background strain as evidenced by the relatively low maximal PPI levels and high SEM seen in Nrg1+/− and wild type littermates. The mice used in this study had maximal PPI levels ranging from 15-30%, whereas in a comparable study with a positive PPI finding the mice had maximal levels ranging from 50 to 70% (Stefansson et al., 2002). Many tasks, including ERPs (Connolly et al., 2003; Maxwell et al., 2006; Metzger et al., 2007; Siegel et al., 2003), PPI (Bullock et al., 1997; Ralph et al., 2001; Willott et al., 2003), sociability (Brodkin, 2007; Fairless et al., 2008; Moy et al., 2004; Moy et al., 2007; Yang et al., 2007) as well as novel object recognition and fear conditioning (Orsini et al., 2004; Sik et al., 2003; Voikar et al., 2005) vary across different inbred strains. Of note, the NRG1 mutant mice are created on a 129/B6 background that would incorporate the inherent capabilities and weaknesses of these two strains.
Although Nrg1+/− mice did not show deficits in P20 amplitude or gating, they did demonstrate disrupted mismatch negativity. This MMN disruption is found in humans with schizophrenia and is correlated with functional outcome in patients (Light and Braff, 2005). Furthermore, deficits in MMN have been correlated with an impaired ability to detect and parse novel stimuli in humans, suggesting that Nrg1+/− mice may have impaired ability to differentiate novel from familiar auditory stimuli (Javitt et al., 2000). To our knowledge, other variants of Nrg1+/− mice have not been tested with auditory ERPs for comparison. Future studies analyzing ERP data from the various Nrg1+/− mouse lines may help identify which Nrg1 types are most closely associated with the electrophysiological abnormalities commonly found in schizophrenia.
Limitations
One limitation of the present study is that there may be compensatory changes in other proteins and systems in response to a reduction in Nrg1. Thus, we are unable to determine if the changes in endophenotypes are caused directly by reduced Nrg1 expression or by changes in other proteins in response to reduced Nrg1. This is consistent with how genetic alterations would exist in humans since such allelic heterogeneity is present throughout development and maturation. Additionally, people with schizophrenia display a range of symptoms and endophenotypic deficits. Each endophenotype may present with different severities and one should not expect a single genetic mutation to recreate all possible deficits found in schizophrenia. Indeed, multiple genes and environmental factors, such as prenatal infection, urban environment, maternal stress and season of birth, have all been linked to small changes in relative risk for the disorder (Castrogiovanni et al., 1998; Izumoto et al., 1999; King et al., 2005; Pedersen and Mortensen, 2006). Additional limitations include mixed gender cohorts and a limited number of animals in several experiments.
Future Studies
As noted above, the predictive validity of reduced sociability in mice for human treatment development is difficult to establish because there are currently no medications that effectively reduce negative symptoms. Further studies aimed at pharmacologically rescuing the sociability phenotype in mouse models (e.g. using oxytocin or drugs that modulate glutamatergic signaling) may facilitate development of novel medications to treat this domain. If progress in both human and mouse studies is able to establish predictive validity of this model, studies involving all Nrg1 hypomorphic variants may foster novel drug development to target selective types of Nrg1 found to be most closely related to specific functional deficits.
4. Experimental Procedure
Animals
Nrg1+/− mice were obtained from C. Birchmeier (Meyer and Birchmeier, 1995) and bred on a C57BL/6/129 hybrid background at the University of Pennsylvania. Briefly, exon 6 of the neuregulin gene is fused to beta-galactosidase, which results in partial deletion of the EGF like domains of all three major types of Nrg1. All protocols were performed in accordance with University Laboratory Animal Resources guidelines and were approved by the Institutional Animal Care and Use Committee. Mice were group housed, three to four per cage, in a light- and temperature controlled Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility. All efforts were made to minimize animal pain and discomfort. Water and standard rodent chow were available ad lib. Experiments were conducted during the light phase between 10 am and 3 pm. Each experiment utilized a separate cohort of mice and all testing times were balanced for genotype (i.e. animals from both genotypes were either tested simultaneously (ERP, PPI, locomotion) or alternating fashion (sociability).
Genotyping
DNAs were isolated from mouse tails using RED Extract-N-Amp Tissue PCR kit (Sigma). Tissue extracts were mixed with primers 1, 2 and 3 and with Extract-N-Amp PCR reaction mix. PCR was conducted by 35 cycles of amplifications with the annealing temperature of 60°C. Primer 1 (NDF-): 5'-TGC TGC TTT CTT CGC TCT TCA GAA GC -3' Primer 2 (NDF+): 5'- GAG ATG GTC ATG TCC TTG TCA CTA AC -3' Primer 3 (NDFneo): 5'- CGA ATT CGC CAA TGA CAA GAC GCT G -3'.
Electrode Implantation
Animals underwent stereotaxic implantation of electrode assemblies (PlasticsOne, Roanoke, VA) for nonanesthetized recording of auditory ERPs. Animals were anesthetized with isoflurane and unipolar recording electrodes were placed in the CA3 hippocampal region (1.4 mm posterior, 2.65 mm lateral, and 2.75 mm deep relative to the bregma) and referenced to the ipsilateral frontal sinus (Connolly et al., 2003; Connolly et al., 2004; Maxwell et al., 2004). The electroencephalogram (EEG) recorded from this configuration will strongly reflect hippocampal and frontal cortical activity due to placement of the positive and negative electrodes, respectively. Other generators will influence the EEG as an inverse function of their distance from the recording sites. ERPs recorded from this electrode configuration are characteristically similar in appearance to human recordings from the Cz scalp location as illustrated in the third figure from a prior publication by our group (Siegel et al., 2003). The electrode pedestal was secured to the skull using a methyl methacrylate-polymer compound (Ortho Jet; Lang Dental, Wheeling, IL) and ethyl cyanoacrylate (Loctite; Henkel KGaA, Düsseldorf, Germany). All animals were housed singly post electrode placement and received a minimum of 1 week recovery before ERP recordings.
ERP Recording
The recording session consisted of an acclimation run immediately followed by the testing run. Each run included stimuli for a P20 and N40 amplitude and gating task immediately followed by a novelty task. Stimuli were generated and recorded by Micro1401 hardware and Spike 5 software (Cambridge Electronic Design, Cambridge, England) and were delivered through speakers attached to the cage top. Mice were tested in their home cages, which were fitted with special tops to accommodate speakers and electrode cables and placed inside a Faraday cage for a 15 min acclimation period prior to the recording session. All tones were played at 85 dB SPL compared with 70 dB background white noise. The sound pressure was calibrated inside the cage from the approximate height of the animals head with a sound level meter (Radio Shack, Cat. No. 33-2055). Nrg1+/− mice were sex and age matched to wild type controls (male n = 8, female n = 6 for each genotype). A ninth male hypomorphic mouse lost his electrode during recording; data from this subject as well as the matched wild type control was therefore discarded. An independent t-test (Statistica, Statsoft, Tulsa, OK) confirms no significant difference in age between genotypes (p=0.77). The mean age of Nrg1+/− mice was 17.82 weeks and the average age of wild type controls was 16.85 weeks at ERP testing. All ERP data were analyzed with a repeated measure ANOVA (rmANOVA) and Fisher LSD post hoc (Statistica, Statsoft, Tulsa, OK). Mice used in ERP testing were not used in other experiments.
For the P20 and N40 gating and amplitude task, eighty white-noise clicks (10 ms duration) were presented in pairs 500 ms apart with a 9-sec interpair interval at 85 dB. Waveforms were filtered between 1 and 500 Hz, baseline corrected at the average value 50 ms prior to stimulus onset, and individual sweeps were rejected for movement artifact according to a criterion of two times the root mean squared amplitude per mouse. Average waves were created from 50 ms pre-stimulus to 200 ms post-stimulus. The novelty task consisted of 24 standard tones (7 kHz) followed by a deviant tone that ranged from 5 kHz to 9 kHz in 100 Hz increments. All tones were sinusoidal, 50 ms in duration, and separated by a 500 ms inter-stimulus interval. The order of deviant tones was randomly selected so that half were higher and half lower frequency than the standard tone. Consequently, the mean frequencies of the standard and deviant tones were equal. Each novel tone was used only once for a total of 40 sets of standard/deviant trials at 85 dB. Only the 24th standard tone was used to balance the number of trials when comparing the standard and novel responses. Waveforms were filtered between 1 and 500 Hz, baseline corrected at the average value 50 ms prior to stimulus onset and individual sweeps were rejected for movement artifact based on a criteria of two times the root mean squared amplitude per mouse. No more than 5 trials were rejected from any mouse that was included in the study. Average waves were created from 25 ms prior to stimulus onset until 250 ms post stimulus. The areas under the curve of the standard and novel waveforms were calculated in 25 ms epochs for the Nrg1+/− and wild type mice. The difference in area (novel – standard) was used to evaluate the MMN (Ehrlichman et al., 2008).
Pre-pulse Inhibition
Startle responses and inhibition of startle response after presentation of a non-startling pre-pulse were registered by an accelerometer in response to acoustic stimuli delivered by a white noise generator (4–19 kHz) in a four-chamber system (San Diego Instruments, San Diego, CA). All mice were between 14 and 22 weeks of age at time of testing. After the mouse (15 Nrg1+/−, 5 male, 10 female; 13 wild type, 5 male, 8 female) was placed in the test chamber, the sessions began with a 5-min acclimation interval to a background white noise of 60 dB. This was followed by a block of five 120-dB startle pulses in an effort to make the subsequent startle responses less variable. During the next 10-min block, startle responses were measured to 40-ms pulses of 0 (control), 90, 95, 100, 105, 110, 115, and 120-dB sound pressure. Each pulse was presented five times in random order with an interstimulus interval randomized from 10 to 20 s with a mean of 15 s. The startle portion of the session concluded with an additional block of five 120-dB pulses to assess potential effects of habituation. Startle trials were followed by a 10-min block of PPI trials. Each prepulse trial consisted of a 20-ms prepulse 4, 8, or 16-dB above background noise (60 dB) followed by a 40-ms pulse of 120 dB 100 ms later. Five trials of each prepulse, along with 10 startle-only trials, were presented in random order. Startle responses were collected as 60, 1-ms readings, which were averaged over the collection interval to obtain an average measure for each trial using San Diego Instruments Startle Reflex Software. For the dopamine agonist challenge mice (8 Nrg1+/−, 2 male, 6 female; 8 wild type, 5 male, 3 female) were given an i.p. injection of either saline or 5 mg/kg d-amphetamine 10 minutes prior to the first tone presentation. PPI was calculated as the percent difference in startle units following the pre-pulse/startle pair as compared to the startle tone alone. Nrg1+/− and wild type mice were compared using an rmANOVA ( Statistica, Statsoft, Tulsa, OK).
Contextual Fear Conditioning
The contextual fear conditioning experiments were performed in a rectangular chamber (16”L×6”W×8 3/8” H) as previously described by Graves and colleagues (Graves et al., 2003). Mice (Nrg1+/−: male n = 4, female n = 7; wild type: male n = 7, female n = 2) were handled for three consecutive days before training, for 1 min each day. All mice were between 12 and 18 weeks of age at time of testing. For the training session, two 2-sec 1.5-mA scrambled footshocks were delivered at 2 min and 2.5 min after placing the mice into the conditioning chamber. Mice were removed from the chamber and returned to their home cages after 3 min. Freezing was assessed prior to and after the shock (pre- and post- shock sessions). 24 hours after training, mice were placed back into the same chamber (conditioned context) in the absence of shock for 5 min and their freezing behavior was assessed during this period (testing period). All training and testing sessions were conducted and analyzed by an individual blind to the genotypes of the animals examined. Nrg1+/− and wild type mice were compared using an rmANOVA and a Fisher LSD post hoc (Statistica, Statsoft, Tulsa, OK).
Novel Object Recognition
The novel object recognition experiment was performed as previously described by Wood and colleagues (Wood et al., 2006). All mice (Nrg1+/−: male n = 4, female n = 4; wild type: male n = 5, female n = 2) were handled for 1 min a day for 2 days and then habituated to the experimental apparatus (a rectangular open field) with 5 min of exploration in the absence of objects before training. All mice were between 16 and 22 weeks of age at time of testing. During the training session, mice were placed back in the experimental apparatus for 15 min. Two identical objects were placed at specific locations in the open field for mice to freely explore. 24 hrs later, mice were placed back into the rectangular environment for 15 min for the testing session. Two objects were again present at the same location, but one of the familiar objects was replaced by a novel one. All testing and training sessions were videotaped and analyzed by an individual blind to the genotype of the animals. Arenas were drawn using the MED Associates software (MED Associates, VT) such that each object was segmented from the background to properly allow the software to detect mouse-object interactions. Software settings were as follows: animal size (10-6000), animal color (Dark in a light background), max animal movement per frame (20 pixels), animal orientation direction by tail shape (minimum elongation of 1.5). Each mouse was scored on the percentage of time it spent on exploring each object during the training and testing session. Time spent on exploring was defined as any time the mouse approached or spent sniffing the object while oriented towards it. Scores were compared using an independent t-test (Statistica, Statsoft, Tulsa, OK).
Locomotor Testing
Locomotor testing was similar to previously described procedures (Halene and Siegel, 2008; Sadalge et al., 2003). Mice (Nrg1+/−: male n = 6, female n = 6, wild type: male n = 6, female n = 6) were allowed to acclimate to the testing room for at least 15 min prior to recoding. All mice were between 14 and 22 weeks of age at time of testing. After acclimation, the mice were placed in a home cage environment inside an automated locomotor activity photobeam frame (30 ×24 ×8 cm) with sensors arranged in an 8-beam array strip with 1.25” spacing (MED Associates, VT). For 30 min, the distance traveled was recorded by a computer using MED PC software that was set to record the number of times the horizontal light beams were broken in 5 minute bins. All testing was conducted during the light phase and results were analyzed using an rmANOVA (Statistica, Statsoft, Tulsa, OK).
Sociability
The sociability of a separate cohort of 11 Nrg1+/− mice (7 females and 4 males) was compared to that of 8 wild type mice (2 females and 6 males). The degree of social approach for a test mouse (Nrg1+/− or wild type) towards a novel, unfamiliar stimulus mouse was measured in a social choice test in a three-chambered apparatus as previously described (Brodkin et al., 2004; Brodkin, 2007; Fairless et al., 2008; Sankoorikal et al., 2006). The three-chambered apparatus had no top or bottom and consisted of a center chamber and two end chambers, with dimensions that have been reported previously (Sankoorikal et al., 2006). Behavioral testing was videotaped with a Sony digital videocamera with NightShot (infrared) feature for recording in low light. To minimize the general stress level of the mice, the testing room was very dimly lit; the lighting within all chambers measured at 1 – 2 lux during testing. Prior to the start of the test, one end chamber was designated the “social chamber,” into which a stimulus mouse would be introduced, and the other end chamber was designated the “nonsocial chamber.” The end chamber designated as the social chamber was varied in a counterbalanced sequence among tests. Before each test, the apparatus was placed on a clean mat and clean mouse bedding. Two identical, clear, Plexiglas cylinders (each 7 cm in diameter, 12.2 cm tall) with removable, black, Plexiglas lids were placed in the apparatus, one in each end chamber. The stimulus mouse could move around easily within the cylinder. The cylinders had multiple holes (1 cm in diameter) to allow for air exchange between the inside and outside of the cylinder. Auditory, visual, and olfactory investigation between a mouse inside and a mouse outside the cylinder was thus possible. Between tests, the apparatus, cylinders, and paperweight were all washed with copious amounts of water and dried before testing the next mouse.
The following modifications of the previously described procedure were made. Because we were primarily interested in measuring nonaggressive and nonsexual affiliative social interactions of test mice, we tried to minimize aggressive and sexual motivations of the test mouse towards the stimulus mouse by pairing castrated male A/J stimulus mice with male test mice, and ovariectomized female A/J stimulus mice with female test mice. The habituation phase, (Phase 1) during which time the test mouse was allowed to initially explore the apparatus, lasted 10 minutes, which was split into two 5 min intervals for data analysis (Phase 1A and Phase 1B) and then averaged. At the beginning of the Social Phase (Phase 2), which lasted 5 minutes, a gonadectomized A/J stimulus mouse was placed in the “social cylinder” in the social side of the apparatus, and simultaneously, an inanimate object (black plastic block) was inserted into the “nonsocial cylinder”. During the free interaction phase (Phase 3), which lasted 5 minutes, the cylinders were removed and the test mouse and stimulus mouse were allowed to interact freely, in order to determine whether the social approach behavior may have been influenced by aggressive motivations. Free interaction was terminated if there was more than 3 seconds of aggressive, attack behavior (biting, vigorous lunging).
The mean +/− standard error was calculated for the following variables for each group of mice defined by genotype: time in social and nonsocial chambers, time sniffing the social and the nonsocial cylinders, and numbers of transitions between chambers. Time sniffing the social and nonsocial cylinders was operationally defined as the time during which the test mouse made direct nose-to-cylinder contact with the social and nonsocial cylinders, respectively. These values were calculated for the Habituation Phase (Phase 1A,stimulus mouse absent, 0-5 minutes and Phase 1B, stimulus mouse absent, 5-10 minutes) as well as for the Social Phase (Phase 2 ;stimulus mouse present, 10-15 minutes). A chamber preference score was calculated for each mouse by subtracting the time spent in the nonsocial side from the time spent in the social side of the apparatus for each phase. In addition, a cylinder sniffing preference score was calculated by subtracting the time spent sniffing the nonsocial cylinder from the time spent sniffing the social cylinder for each phase. A positive score signified a predominance of approach or sniffing towards the social side, whereas a negative score implied a predominance of approach or sniffing towards the nonsocial side. For statistical analysis, the mean of the chamber preference scores for each mouse in habituation Phase 1A and Phase 1B was calculated, as was the mean of the cylinder sniffing preference score for each mouse in Habituation Phase 1A and 1B. Nrg1+/− vs. wild type chamber preference scores and cylinder sniffing preference scores during habituation (mean of Phase 1A and Phase 1B) vs. social (Phase 2) periods were compared with repeated measures ANOVAs. The number of transitions of Nrg1+/− vs. wild type mice was compared during the habituation vs. social phases, using a rmANOVA.
Acknowledgments
The authors would like to thank Dr. Carmen Birchmeier for providing the mice used in this study and for her helpful input throughout the process. Funding provided by NIMH P50 MH064045 (SJ Siegel Project PI, RE Gur Center PI), RO1MH075916 (C Hahn), R01MH080718 (ES Brodkin).
Abbreviations
- Nrg1
neuregulin-1
- NMDA
N-methyl-D-aspartic acid
- PPI
pre-pulse inhibition
- MMN
mismatch negativity
- ERP
event-related potentials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Addington D. Neurocognitive and social functioning in schizophrenia: a 2.5 year follow-up study. Schizophr Res. 2000;44:47–56. doi: 10.1016/s0920-9964(99)00160-7. [DOI] [PubMed] [Google Scholar]
- Awipi T, Davachi L. Content-specific source encoding in the human medial temporal lobe. J Exp Psychol Learn Mem Cogn. 2008;34:769–79. doi: 10.1037/0278-7393.34.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res. 2004;126:203–15. doi: 10.1016/j.psychres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bowen L, Wallace CJ, Glynn SM, Nuechterlein KH, Lutzker JR, Kuehnel TG. Schizophrenic individuals’ cognitive functioning and performance in interpersonal interactions and skills training procedures. J Psychiatr Res. 1994;28:289–301. doi: 10.1016/0022-3956(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–7. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learn Mem. 2006;13:638–43. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock AE, Slobe BS, Vazquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav Neurosci. 1997;111:1353–60. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- Castrogiovanni P, Iapichino S, Pacchierotti C, Pieraccini F. Season of birth in psychiatry. A review. Neuropsychobiology. 1998;37:175–81. doi: 10.1159/000026499. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, Rosoklija G, Liu RC, Gingrich JA, Small S, Moore H, Dwork AJ, Talmage DA, Role LW. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–83. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res. 2008;100:270–80. doi: 10.1016/j.schres.2007.12.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Forbes CB, Mann MC, Blanchard JJ. Specific cognitive deficits and differential domains of social functioning impairment in schizophrenia. Schizophr Res. 2006;81:227–38. doi: 10.1016/j.schres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell CR, Kanes SJ, Abel T, Liang Y, Tokarczyk J, Bilker WB, Turetsky BI, Gur RE, Siegel SJ. Inhibition of auditory evoked potentials and prepulse inhibition of startle in DBA/2J and DBA/2Hsd inbred mouse substrains. Brain Res. 2003;992:85–95. doi: 10.1016/j.brainres.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–88. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–15. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–80. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Cornejo BJ, Mesches MH, Benke TA. A single early-life seizure impairs short-term memory but does not alter spatial learning, recognition memory, or anxiety. Epilepsy Behav. 2008 doi: 10.1016/j.yebeh.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan PW, Green MF, Toomey R. Cognitive correlates to social cue perception in schizophrenia. Psychiatry Res. 1994;53:141–51. doi: 10.1016/0165-1781(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The amygdala. Curr Biol. 2000;10:R131. doi: 10.1016/s0960-9822(00)00345-6. [DOI] [PubMed] [Google Scholar]
- Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–96. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Duffy L, Cappas E, Scimone A, Schofield PR, Karl T. Behavioral profile of a heterozygous mutant mouse model for EGF-like domain neuregulin 1. Behav Neurosci. 2008;122:748–59. doi: 10.1037/0735-7044.122.4.748. [DOI] [PubMed] [Google Scholar]
- Edwards JM, Bottenstein JE. Neuregulin 1 growth factors regulate proliferation but not apoptosis of a CNS neuronal progenitor cell line. Brain Res. 2006;1108:63–75. doi: 10.1016/j.brainres.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J Cogn Neurosci. 2008;20:1403–14. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–7. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects. Human recordings, computer simulation, and an animal model. Arch Gen Psychiatry. 1996;53:1114–21. doi: 10.1001/archpsyc.1996.01830120052009. [DOI] [PubMed] [Google Scholar]
- Goodearl AD, Yee AG, Sandrock AW, Jr., Corfas G, Fischbach GD. ARIA is concentrated in the synaptic basal lamina of the developing chick neuromuscular junction. J Cell Biol. 1995;130:1423–34. doi: 10.1083/jcb.130.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–76. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Charney DS, Krystal J, Braff D. Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biol Psychiatry. 1992;32:939–43. doi: 10.1016/0006-3223(92)90183-z. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–9. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–8. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Halene TB, Siegel SJ. Antipsychotic-like properties of phosphodiesterase 4 inhibitors: evaluation of 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (RO-20-1724) with auditory event-related potentials and prepulse inhibition of startle. J Pharmacol Exp Ther. 2008;326:230–9. doi: 10.1124/jpet.108.138586. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2007;32:514–21. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–55. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Izumoto Y, Inoue S, Yasuda N. Schizophrenia and the influenza epidemics of 1957 in Japan. Biol Psychiatry. 1999;46:119–24. doi: 10.1016/s0006-3223(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalogr Clin Neurophysiol. 1998;108:143–53. doi: 10.1016/s0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–7. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: implications for understanding schizophrenia. Genes Brain Behav. 2007;6:677–87. doi: 10.1111/j.1601-183X.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- King S, Laplante D, Joober R. Understanding putative risk factors for schizophrenia: retrospective and prospective studies. J Psychiatry Neurosci. 2005;30:342–8. [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–83. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–52. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–35. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–97. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry. 2000;157:767–71. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–36. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl AD, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–8. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Cannella B, Hoban C, Gao YL, Garcia-Arenas R, Lawson D, Happel E, Noel F, Tofilon P, Gwynne D, Raine CS. Neuregulin in neuron/glial interactions in the central nervous system. GGF2 diminishes autoimmune demyelination, promotes oligodendrocyte progenitor expansion, and enhances remyelination. Adv Exp Med Biol. 1999;468:283–95. [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, Turetsky BI, Bilker WB, Lenox RH, Siegel SJ. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004;29:739–46. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316:315–24. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Metzger KL, Maxwell CR, Liang Y, Siegel SJ. Effects of nicotine vary across two auditory evoked potentials in the mouse. Biol Psychiatry. 2007;61:23–30. doi: 10.1016/j.biopsych.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–90. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–86. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–52. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Babovic D, O'Meara G, Clifford JJ, Croke DT, Waddington JL. Susceptibility genes for schizophrenia: characterisation of mutant mouse models at the level of phenotypic behaviour. Neurosci Biobehav Rev. 2007a;31:60–78. doi: 10.1016/j.neubiorev.2006.04.002. [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Babovic D, O'Sullivan GJ, Clifford JJ, Tighe O, Croke DT, Harvey R, Waddington JL. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007b;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CM, O'Connor AM, O'Sullivan GJ, Lai D, Harvey R, Croke DT, Waddington JL. Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous ‘knockout’ of the schizophrenia risk gene neuregulin-1. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:462–6. doi: 10.1016/j.pnpbp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Conversi D, Cabib S. Selective improvement of strain-dependent performances of cognitive tasks by food restriction. Neurobiol Learn Mem. 2004;81:96–9. doi: 10.1016/s1074-7427(03)00075-3. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–9. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Mortensen PB. Urbanization and traffic related exposures as risk factors for schizophrenia. BMC Psychiatry. 2006;6:2. doi: 10.1186/1471-244X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman GD, Green JM, Culouscou JM, Carlton GW, Rothwell VM, Buckley S. Heregulin induces tyrosine phosphorylation of HER4/p180erbB4. Nature. 1993;366:473–5. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Hori SE, Leslie R, Andrieux A, Schellinck H, Thorne M, Robertson GS. Cognitive impairments in the STOP null mouse model of schizophrenia. Behav Neurosci. 2007;121:826–35. doi: 10.1037/0735-7044.121.5.826. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Geyer MA. Strain-specific effects of amphetamine on prepulse inhibition and patterns of locomotor behavior in mice. J Pharmacol Exp Ther. 2001;298:148–55. [PubMed] [Google Scholar]
- Sadalge A, Coughlin L, Fu H, Wang B, Valladares O, Valentino R, Blendy JA. alpha 1d Adrenoceptor signaling is required for stimulus induced locomotor activity. Mol Psychiatry. 2003;8:664–72. doi: 10.1038/sj.mp.4001351. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–23. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, Kanes SJ, Turetsky BI. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–82. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Dankert M, Phillips JM. Psychosis and Schizophrenia (chapter 55). In: Waldman S, Terzic A, editors. Pharmacology and Therapeutics: Principles to Practice. Elsevier; Philadelphia: in press. [Google Scholar]
- Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147:49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl) 2003;165:386–96. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–92. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, Ingason A, Gulcher JR, Stefansson K, St Clair D. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–7. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Bakshi V, Waikar M, Taaid N, Geyer MA. Seroquel, clozapine and chlorpromazine restore sensorimotor gating in ketamine-treated rats. Psychopharmacology (Berl) 1998;140:75–80. doi: 10.1007/s002130050741. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotki D, Latanov A, Nitsch R, Lipp HP. Deviance-related electrophysiological activity in mice: is there mismatch negativity in mice? Clin Neurophysiol. 2005;116:353–63. doi: 10.1016/j.clinph.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–52. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Genetic mechanisms of psychosis: in vivo and postmortem genomics. Clin Ther. 2005;27(Suppl A):S8–15. doi: 10.1016/j.clinthera.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, Trail G, Hu S, Silbiger SM, Levy RB, et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–72. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- Willott JF, Tanner L, O'Steen J, Johnson KR, Bogue MA, Gagnon L. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav Neurosci. 2003;117:716–27. doi: 10.1037/0735-7044.117.4.716. [DOI] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem. 2006;13:609–17. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JZ, Si TM, Ruan Y, Ling YS, Han YH, Wang XL, Zhou M, Zhang HY, Kong QM, Liu C, Zhang DR, Yu YQ, Liu SZ, Ju GZ, Shu L, Ma DL, Zhang D. Association study of neuregulin 1 gene with schizophrenia. Mol Psychiatry. 2003;8:706–9. doi: 10.1038/sj.mp.4001377. [DOI] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–21. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Du C, Hancock M, Mertz M, Talmage DA, Role LW. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. J Neurosci. 2008;28:9111–6. doi: 10.1523/JNEUROSCI.0381-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]