Abstract
The intrinsic conformational preferences of the restricted phenylalanine analogue generated by including the α and β carbon atoms into a cyclohexane ring (1-amino-2-phenylcyclohexanecarboxylic acid, c6Phe) have been determined using quantum mechanical calculations. Specifically, the conformational profile of the N-acetyl-N’-methylamide derivative of the c6Phe stereoisomers exhibiting either a cis or a trans relative orientation between the amino and phenyl substituents has been analyzed in different environments (gas phase, chloroform and aqueous solutions). Calculations were performed using B3LYP, MP2 and HF methods combined with the 6-31+G(d,p) and 6-311++G(d,p) basis sets, and a self-consistent reaction-field (SCRF) method was applied to analyze the influence of the solvent. The amino acids investigated can be viewed as constrained phenylalanine analogues with a rigidly oriented aromatic side chain that may interact with the peptide backbone not only sterically but also electronically through the aromatic π orbitals. Their conformational propensities have been found to be strongly influenced by the specific orientation of the aromatic substituent in each stereoisomer and the conformation adopted by the cyclohexane ring, as well as by the environment.
Introduction
The incorporation of restricted amino acids into peptide chains is a powerful tool for the construction of peptide analogues with well-defined three-dimensional arrangements. α,α-Dialkylated residues are particularly useful because tetrasubstitution at the α carbon severely restricts the conformational space available to the peptide backbone and this leads to the stabilization of certain elements of secondary structure.1 Thus, the simplest α,α-dialkylated amino acid, (α-methyl)alanine (also known as α-aminoisobutyric acid, Aib) is found almost exclusively in the 310-/α-helical region of the Ramachandran map.1 In comparison, higher homologues of Aib with linear side chains prefer the fully-extended conformation, whereas their cyclic counterparts, 1-aminocycloalkanecarboxylic acids (Acnc), strictly parallel the behavior of Aib, with some distortion in the case of the highly strained cyclopropane derivative (n = 3).1
The repertoire of non-natural amino acids useful in peptide design may be expanded by attaching a substituent to the cycloalkane moiety of Acnc. This combines the presence of side-chain functionality, which may be essential for peptide-receptor recognition when dealing with a bioactive peptide, with the well-defined conformational properties of the Acnc residues. The new side-chain functionality incorporated may be selectively oriented by selecting a certain cycloalkane size and stereochemistry. Given that side chains are directly involved in molecular recognition processes, their three-dimensional arrangement is crucial for adequate peptide-receptor interaction. Moreover, side chains may play a role in directing the conformational preferences of the peptide backbone.2
In recent years, we have been working on the synthesis3 and structural study4 –both theoretically and experimentally– of the amino acids obtained by incorporating a phenyl substituent at one of the β carbons of Acnc for n = 3–6. The compounds thus generated are phenylalanine (Phe) analogues and we designate them as cnPhe, where n refers to the ring size, as in Acnc. The cyclic nature of the cnPhe residues precludes rotation about the Cα—Cβ bond and the orientation of the aromatic side chain is therefore dictated by the size (n value) and stereochemistry of the cycloalkane ring. It should be noted that the phenyl substituent incorporated at the β position may exhibit either a cis or a trans relative orientation with respect to the amino function, respectively giving rise to cis- or trans-cnPhe derivatives. The cnPhe residues with diverse ring size and stereochemistry constitute a set of phenylalanine analogues with distinct well-defined side-chain arrangements. They are excellent tools to investigate the ability of the side chain to influence the peptide backbone conformation4 since the tightly held aromatic substituent may interact with the backbone not only sterically but also electronically through the aromatic π orbitals. Indeed, the different spatial orientation attained by the aromatic substituent in the cnPhe residues has proven useful in several applications related to the stabilization of particular peptide backbone conformations.4,5
In particular, we have shown that the cyclohexane members (c6Phe) are able to stabilize different types of β-turns depending on the phenyl side-chain orientation,4b,e at variance with the behavior exhibited by the natural amino acid.4e,f These experimental results prove that the phenylalanine cyclohexane analogues (c6Phe) are able to modulate the peptide backbone conformation, and show therefore great promise as phenylalanine substitutes in the design of peptides with controlled fold in the backbone. In early studies, molecular mechanics (MM) simulations were used to explore the conformational propensities of c6Phe.4d The minimum energy conformations located were subsequently used as starting points for energy minimizations at the HF/6-31G(d) quantum mechanical level. However, such a study cannot be considered as a complete description of the conformational propensities of c6Phe due to the intrinsic limitations associated with MM methods. As a matter of fact, only 7 and 8 minima were characterized for the N-acetyl-N’-methylamide derivatives of cis-c6Phe and trans-c6Phe, respectively, in that MM study,4d whereas the number of minima expected for these compounds is significantly higher. The reason for this is that quantum mechanical calculations provided as many as 11 different minima for the analogous unsubstituted Ac6c derivative.6 Incorporation of a phenyl substituent into Ac6c to generate cis- or trans-c6Phe is expected to increase the number of minima because the symmetry of the molecule is broken [conformations characterized by (φ,ψ) and (–φ,–ψ) dihedral angles are equivalent for an achiral amino acid such as Ac6c but not for the non-symmetrical c6Phe residues], and therefore each minimum energy conformation characterized for Ac6c should translate, in principle, into two different c6Phe minima. Obviously, some of the expected minima may be annihilated, in the same way as new minima without precedent in Ac6c may appear in c6Phe induced by the presence of the aromatic substituent. In this context, a comparison with the cyclopentane series is instructive: quantum mechanical calculations provided 5 minimum energy conformations for Ac5c,7 while 8 and 10 minima, respectively, were located for cis-c5Phe and trans-c5Phe.4a
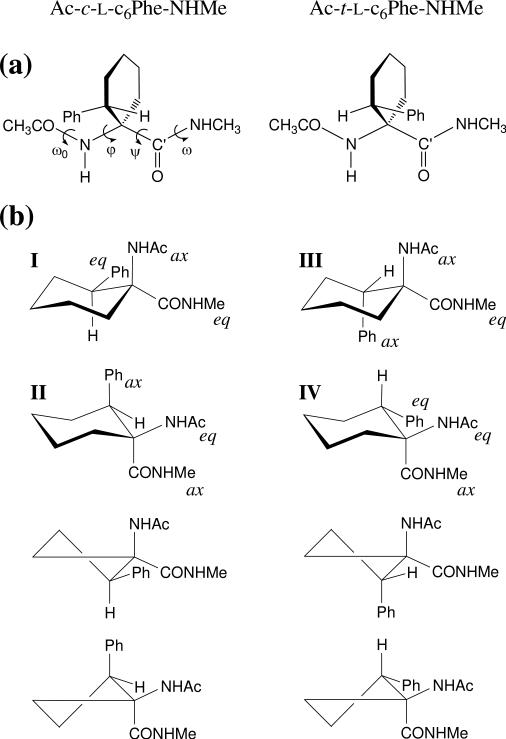
In this work, we present a complete conformational study of the N-acetyl-N’-methylamide derivatives of the cyclohexane analogues of L-phenylalanine exhibiting a cis or a trans relative orientation between the phenyl substituent and the amino function, hereafter denoted as Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe, respectively (Figure 1a). Density functional theory (DFT) calculations at the B3LYP/6-31+G(d,p) level have been used to locate and characterize the minimum energy conformations for these compounds. In order to obtain reliable estimates, the relative energies of all these structures have been re-evaluated using different theoretical levels. Moreover, the influence of the solvent on the conformational preferences of the compounds under study has been analyzed using a self-consistent reaction-field (SCRF) method. The intrinsic conformational preferences calculated in the present work for the c6Phe derivatives have been compared with those described recently for the unsubstituted Ac6c residue.6
Figure 1.
(a) Structure of the compounds investigated, Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe, that incorporate, respectively, the cis and trans cyclohexane analogues of l-phenylalanine. The backbone dihedral angles are indicated for the cis derivative. (b) Chair and half-chair conformations considered as starting geometries for the cyclohexane ring in the compounds under study. The substituents located in axial (ax) or equatorial (eq) positions are indicated for the chair conformations.
Methods
All the calculations were performed using the Gaussian 03 computer program.8 In order to characterize the minimum energy conformations of Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe, a systematic exploration of the conformational space was performed. Given that each flexible backbone dihedral angle (φ,ψ) is expected to exhibit three minima, 9 minima can be anticipated for the potential energy surface E=E(φ,ψ). Regarding the cyclohexane ring, the two possible chair conformations (Figure 1b) were initially considered for each compound. Additionally, two half-chairs (Figure 1b) were included as starting geometries. Although the latter arrangement is very high in energy and is not expected to be found in any energy minima, it is a good starting point to explore cyclohexane conformations different from chair, such as boat or twist. Accordingly, for each c6Phe derivative under study, 9 (φ,ψ minima) × 4 (2 chair + 2 half-chair cyclohexane conformations) = 36 structures were used as starting points for subsequent full geometry optimizations.
All geometry optimizations were performed using the B3LYP functional9,10 combined with the 6-31+G(d,p) basis set,11 i.e. B3LYP/6-31+G(d,p). Frequency analyses were carried out to verify the nature of the minimum state of all the stationary points obtained and to calculate the zero-point vibrational energies (ZPVE) and both thermal and entropic corrections. These statistical terms were then used to compute the conformational Gibbs free energies in the gas phase at 298K (ΔGgp). To analyze the influence of the size of the basis set and the theoretical method used on the conformational energies, single point calculations at the HF/6-31+G(d,p), MP2/6-31+G(d,p),12 and B3LYP/6-311++G(d,p)13 levels were further performed on all the minima.
To obtain an estimation of the solvation effects on the relative stability of the most relevant minima, single-point calculations were conducted on the optimized structures using a SCRF model. Specifically, the Polarizable Continuum Model (PCM) developed by Tomasi and co-workers14 was used to describe chloroform and water as solvents. The PCM method represents the polarization of the liquid by a charge density appearing on the surface of the cavity created in the solvent. This cavity is built using a molecular shape algorithm. PCM calculations were performed in the framework of the B3LYP/6-31+G(d,p) level using the standard protocol and considering the dielectric constants of chloroform (ε = 4.9) and water (ε = 78.4) to obtain the free energies of solvation (ΔGsolv) of the minimum energy conformations. Within this context, it should be emphasized that previous studies indicated that solute geometry relaxations in solution and single-point calculations on the optimized geometries in the gas phase give almost identical ΔGsolv values.15 The conformational free energies in solution (ΔGChl and ΔGwater) at the B3LYP/6-31+G(d,p) level were then estimated using the classical thermodynamics scheme, i.e. by adding the ΔGsolv and ΔGgp values.
Results and Discussion
Complete geometry optimizations and frequency calculations led to the characterization of 17 and 21 minimum energy conformations in the gas phase for Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe, respectively. They are listed in Tables 1 and 2, where the conformation adopted by the peptide backbone and the cyclohexane ring, as well as the relative energy (ΔEgp) and free energy (ΔGgp) calculated at the B3LYP/6-31+G(d,p) level are indicated for each minimum. Perczel's nomenclature16 has been used to identify the different peptide backbone conformations.
Table 1.
Backbone dihedral angles,a,b relative energyc in the gas phase (ΔEgp), and relative free energyc in the gas phase (ΔGgp) for the minimum energy conformations of Ac-c-l-c6Phe-NHMe characterized at the B3LYP/6-31+G(d,p) level. The arrangement adopted by the peptide backbone and the cyclohexane ringd is indicated.
| # | ω0 | ϕ | ψ | ω | backbone | cyclohexane | ΔEgp | ΔGgp |
|---|---|---|---|---|---|---|---|---|
| 1 | −175.3 | −71.0 | 35.2 | 172.9 | γL | chair I | 0.0e | 0.0f |
| 2 | −173.6 | −73.6 | 67.4 | −176.0 | γL | chair II | 2.6 | 2.7 |
| 3 | −174.6 | −73.5 | 46.7 | 176.1 | γL | twist | 3.1 | 3.4 |
| 4 | 174.5 | 71.9 | −68.5 | 175.0 | γD | chair II | 3.7 | 4.1 |
| 5 | 171.5 | 46.9 | −13.7 | 177.4 | γD | chair I | 4.9 | 4.7 |
| 6 | 161.1 | −63.7 | 162.0 | −177.7 | εL | chair I | 5.1 | 3.8 |
| 7 | 177.4 | −178.2 | 179.8 | −178.3 | βL | chair I | 5.6 | 4.9 |
| 8 | 166.2 | 59.3 | 40.2 | −175.5 | αD | chair II | 5.7 | 5.4 |
| 9 | −168.5 | −61.8 | −35.9 | 175.6 | αL | chair II | 6.1 | 5.3 |
| 10 | 166.8 | 167.4 | −27.1 | −171.9 | δD | chair I | 7.9 | 7.7 |
| 11 | −165.4 | 32.9 | −124.0 | 175.5 | εD | chair I | 8.0 | 7.5 |
| 12 | −170.9 | −166.4 | 31.5 | 171.5 | δL | twist | 8.6 | 7.5 |
| 13 | 169.9 | 56.1 | 42.4 | −175.0 | αD | twist | 9.3 | 9.0 |
| 14 | −174.4 | −142.7 | 110.5 | −174.1 | δL | chair II | 9.7 | 7.7 |
| 15 | 165.3 | −61.5 | 150.8 | −177.3 | εL | twist | 9.8 | 8.8 |
| 16 | 169.5 | −171.3 | −46.1 | −174.1 | δD | twist | 10.3 | 9.7 |
| 17 | 178.6 | −146.0 | −68.6 | 178.3 | δD | twist | 13.9 | 12.5 |
Table 2.
Backbone dihedral angles,a,b relative energyc in the gas phase (ΔEgp) and relative free energyc in the gas phase (ΔGgp) for the minimum energy conformations of Ac-t-l-c6Phe-NHMe characterized at the B3LYP/6-31+G(d,p) level. The arrangement adopted by the peptide backbone and the cyclohexane ringd is indicated.
| # | ω0 | ϕ | ψ | ω | backbone | cyclohexane | ΔEgp | ΔGgp |
|---|---|---|---|---|---|---|---|---|
| 1 | −175.2 | −73.5 | 52.2 | 178.4 | γL | chair IV | 0.0e | 0.0f |
| 2 | 179.7 | −179.2 | 175.2 | −178.6 | βL | chair IV | 2.7 | 2.5 |
| 3 | 177.6 | 71.4 | −58.2 | −178.7 | γD | chair III | 3.5 | 3.1 |
| 4 | −169.7 | −170.6 | 29.9 | 171.7 | δL | chair IV | 4.1 | 3.7 |
| 5 | 169.5 | 177.2 | −30.9 | −173.2 | δD | chair IV | 4.3 | 3.8 |
| 6 | 168.1 | 53.0 | −16.9 | −177.6 | γD | chair IV | 5.9 | 5.5 |
| 7 | 167.1 | −59.6 | 131.7 | −177.7 | εL | chair III | 6.0 | 5.3 |
| 8 | 164.6 | 48.1 | 42.5 | −174.4 | αD | chair IV | 6.2 | 6.3 |
| 9 | 175.7 | −69.2 | 89.5 | −172.5 | γL | chair III | 6.9 | 6.1 |
| 10 | −167.3 | 58.0 | −123.9 | 175.8 | εD | chair III | 7.4 | 6.3 |
| 11 | −170.0 | −61.6 | −37.5 | 176.7 | αL | chair III | 7.4 | 6.7 |
| 12 | −176.2 | −54.3 | 72.4 | 177.6 | γL | twist | 9.3 | 9.4 |
| 13 | 177.3 | 72.8 | −54.2 | −176.0 | γD | twist | 9.3 | 9.4 |
| 14 | −176.2 | 176.2 | 164.0 | 175.0 | βL | chair III | 10.3 | 10.1 |
| 15 | 179.9 | −172.1 | 159.7 | 174.3 | βL | twist | 11.5 | 11.2 |
| 16 | 176.8 | −68.8 | 85.7 | −172.3 | γL | twist | 13.2 | 12.5 |
| 17 | −172.4 | 179.4 | −47.5 | −172.8 | δD | twist | 13.3 | 12.7 |
| 18 | −171.0 | −168.7 | 32.4 | 172.2 | δL | twist | 13.5 | 12.4 |
| 19 | −169.1 | −61.7 | −38.0 | 178.3 | αL | twist | 13.6 | 12.7 |
| 20 | 164.5 | −61.8 | 149.3 | 177.8 | εL | twist | 14.4 | 13.0 |
| 21 | −163.0 | 59.5 | −166.7 | −177.5 | εD | twist | 14.6 | 14.4 |
Ac-cl-c6Phe-NHMe
The 17 minimum energy conformations characterized for this compound in the gas phase are distributed within a relative energy interval of 13.9 kcal/mol (Table 1). Eight of them present ΔEgp values lower than 6 kcal/mol, and are depicted in Figure 2. The contribution of the remaining minima in Table 1 to the conformational equilibrium of Ac-c-l-c6Phe-NHMe is expected to be negligible not only in the gas phase but also in solution, given that solute-solvent interactions typically mean a stabilization of several kcal/mol.
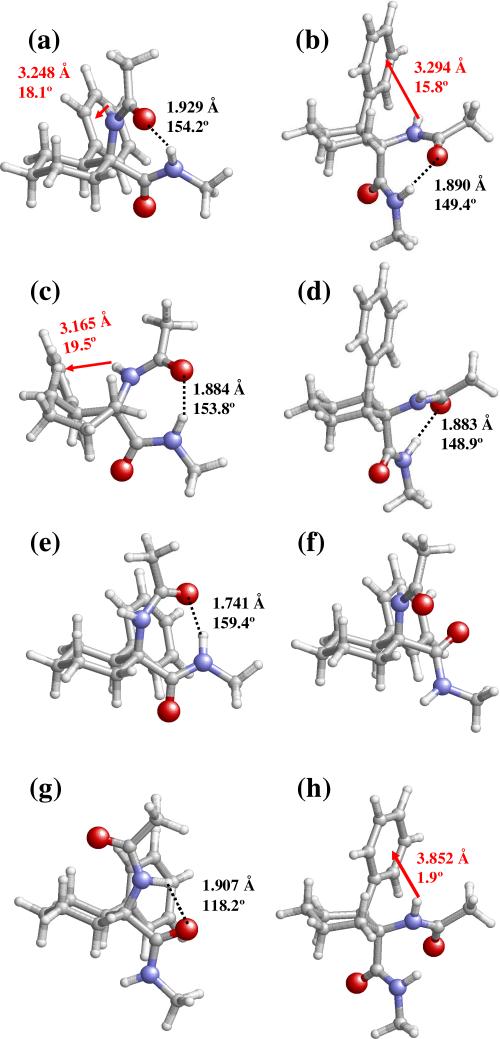
Figure 2.
Selected minimum energy conformations characterized for Ac-c-l-c6Phe-NHMe at the B3LYP/6-31+G(d,p) level: (a) 1; (b) 2; (c) 3; (d) 4; (e) 5; (f) 6; (g) 7; and (h) 8. Conformational parameters are provided in Table 1. Intramolecular N–H···O hydrogen bonds and N–H···π interactions are indicated by black dashed lines and red arrows, respectively. The geometrical parameters correspond to the d(H···O) and <N–H···O (in Å and degrees, respectively) for hydrogen bonds and to dH···Ph and θ (in Å and degrees, respectively; see text for definition) for N–H···π interactions.
The lowest energy minimum of Ac-c-l-c6Phe-NHMe (1) adopts a γL backbone arrangement (inverse γ-turn or equatorial C7 conformation), in which the terminal acetyl CO and methylamide NH sites form an intramolecular hydrogen bond [d(H···O)= 1.929 Å, <N–H···O= 154.2°] defining a seven-membered cycle (Figure 2a). In this conformation, the phenyl substituent and the amino group of cis-c6Phe lie in close proximity, thus allowing the formation of a weak attractive interaction of the N–H···π type. The geometry of this interaction is defined by the distance between the amide hydrogen and the center of the aromatic system (dH···Ph= 3.248 Å) and the angle formed by the N–H bond and the phenyl ring plane (θ= 18.1°). The ability of the π electron density of aromatic systems to interact with proton donors has long been recognized,17 and more recently has been identified as a stabilizing factor of peptide and protein structures.18 Importantly, interactions of this type have been observed experimentally in small peptides containing c3Phe4f and c6Phe,4e and characterized theoretically for the former residue.4c
The same γL backbone conformation is found in minima 2 and 3, which are stabilized by similar hydrogen bonding and NH-aromatic interactions (Figures 2b and 2c). Indeed, the three γL structures in Table 1 differ mainly in the shape of the cyclohexane ring. The global minimum presents a chair of type I (Figure 1b), which is expected to be that preferred by Ac-c-l-c6Phe-NHMe according to the axial/equatorial arrangement of the substituents and the tendency of bulky groups to avoid axial positions because they are known to produce a higher steric hindrance with the rest of the cyclohexane system. In chair I, the bulky phenyl ring is equatorially located and only one of the three cyclohexane substituents (–NHCOMe) occupies an axial position. In minimum 2, the six-membered ring assumes the alternate chair arrangement (II, Figure 1b), that bears the phenyl and –CONHMe groups in axial whereas the amino moiety occupies an equatorial position. The less favorable orientation of the cyclohexane substituents in this case is associated to an energy penalty of 2.6 kcal/mol in terms of ΔEgp. It is highly remarkable that the six-membered ring in minimum 3 adopts a twist conformation, that is, a slightly distorted boat. The high stability of this structure, which is only 0.5 kcal/mol above minimum 2, may be related to the presence of two substituents –among which is the sterically more demanding one (the phenyl group)– in pseudoequatorial positions, and the non-existence of steric conflicts between the peptide backbone and either the cyclohexane moiety or the phenyl substituent.
The next two minima, 4 (Figure 2d) and 5 (Figure 2e), also exhibit a hydrogen bond linking the terminal acetyl CO and methylamide NH groups and closing the seven-membered cycle typical of a γ-turn. However, in this case, the sign of the (φ,ψ) dihedral angles corresponds to a γD conformation (axial C7 or classical γ-turn). Inversion of the γ-turn type results in the disruption of the interaction between the aromatic π orbitals and the cis-c6Phe NH site. Moreover, only chair arrangements of the cyclohexane ring are found to be compatible with this backbone conformation. Surprisingly enough, the most stable γD minimum (4) presents a type II chair, with the bulky phenyl group located axially. The a priori unexpected higher stability observed for this conformer is due to the fact that chair I in 5 brings in close proximity the acetyl oxygen atom of the –NHCOMe peptide fragment and the aromatic ring, thus introducing a very strong steric and electronic repulsion. To minimize this repulsive interaction, the peptide backbone geometry in minimum 5, (φ,ψ) = (47,–14), is highly distorted with respect to that corresponding to a standard γD conformation, (φ,ψ) ≈ (70,–60).
The unfavorable interactions between the –NHCOMe oxygen and the phenyl substituent for a chair I-shaped cyclohexane are not present when the peptide backbone assumes semi-extended or fully-extended structures characterized by large ψ values. Thus, minima 6 and 7 combine, respectively, an εL (also called polyproline II) or a βL (C5) conformation with a chair I disposition for the cyclohexane ring. In contrast, none of these backbone arrangements seem to be compatible with chair II (Table 1). Minimum 6 (Figure 2f) lacks stabilizing interactions either of the hydrogen bond or N–H···π type, whereas a weak intramolecular hydrogen bond [d(H···O)= 1.907 Å, <N–H···O= 118.2°] connecting the amide functions is present in 7 (Figure 2g). It is interesting to note that 6 presents a ΔGgp value considerably low (3.8 kcal/mol), and is the fourth most stable minima –instead of the sixth– in terms of relative free energy.
The last conformer in Figure 2 (8, Figure 2h) exhibits a ΔEgp value of 5.7 kcal/mol and falls into the left-handed α-helix region (αD) of the Ramachandran map. This backbone conformation combined with a cyclohexane chair II arrangement allows the formation of a stabilizing interaction between the π cloud of the axially-oriented phenyl ring and the NH group of cis-c6Phe. Although not included in Figure 2, a minimum with an αL backbone geometry and very close in energy (9, Table 1) was also characterized. Notably enough, none of the α-helical minima located present a cyclohexane accommodating the alternate chair conformation.
Ac-t-l-c6Phe-NHMe
Table 2 describes the most relevant geometrical parameters corresponding to the 21 minimum energy conformations characterized for the trans-c6Phe derivative in the gas phase, which show ΔEgp and ΔGgp values of up to 14.6 and 14.4 kcal/mol, respectively. The 8 most stable minima are displayed in Figure 3.
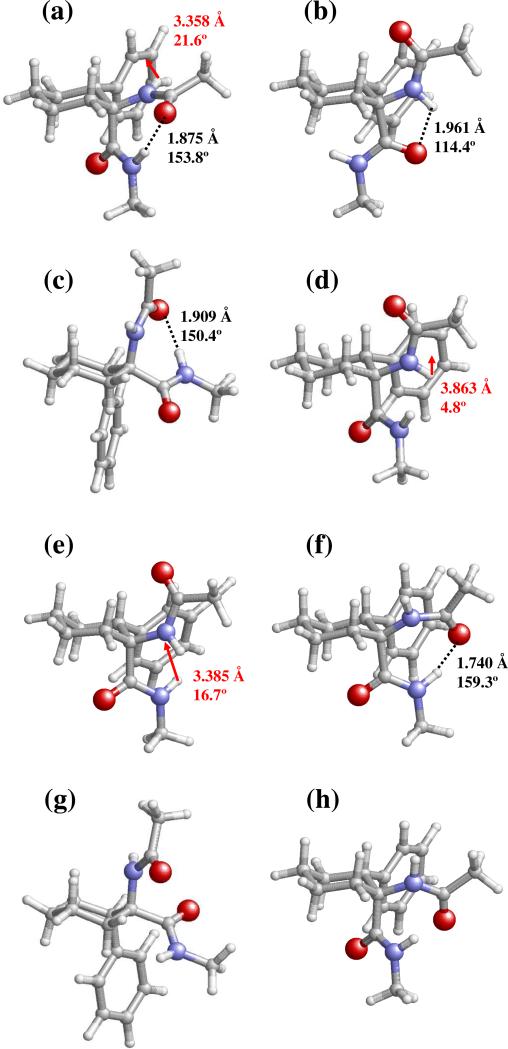
Figure 3.
Selected minimum energy conformations characterized for Ac-t-l-c6Phe-NHMe at the B3LYP/6-31+G(d,p) level: (a) 1; (b) 2; (c) 3; (d) 4; (e) 5; (f) 6; (g) 7; and (h) 8. Conformational parameters are provided in Table 2. Intramolecular N–H···O hydrogen bonds and N–H···π interactions are indicated by black dashed lines and red arrows, respectively. The geometrical parameters correspond to the d(H···O) and <N–H···O (in Å and degrees, respectively) for hydrogen bonds and to dH···Ph and θ (in Å and degrees, respectively; see text for definition) for N–H···π interactions.
As observed before for the cis-c6Phe derivative, the global minimum of Ac-t-l-c6Phe-NHMe (1, Figure 3a) combines a γL backbone conformation and a cyclohexane ring assuming the most favorable chair arrangement, namely that with the bulky aromatic substituent equatorially located (chair IV, Figure 1b). This structure is stabilized by the intramolecular hydrogen bond typical of the γ-turn conformation and by an NH-aromatic interaction, with geometrical parameters similar to those described for the global minimum of cis-c6Phe.
Other energy minima exhibiting a γL backbone and differing from the global minimum in the cyclohexane shape were also located for Ac-t-l-c6Phe-NHMe (Table 2). However, in this case, reversal of the chair type from IV to III results in a destabilization of 6.9 kcal/mol (minimum 9). Also a six-membered ring with a twist arrangement was found to be compatible with the γL structure for this compound, but at a high energy cost (minima 12 and 16). This is in sharp contrast to that described before for Ac-c-l-c6Phe-NHMe. Thus, although cis- and trans-c6Phe present global minima with high structural similarity, their response to changes in the cyclohexane conformation for this backbone disposition is notably different. The reason for this distinct behavior lies in the relative orientation of the phenyl ring, which for the trans derivative is next to the carboxyl terminus. Thus, the chair III arrangement combined with a γL backbone conformation (minimum 9) results in a high proximity between the carbonyl oxygen of trans-c6Phe and the aromatic substituent.
A parallel situation, although less marked, is found when comparing the minima exhibiting a γD structure for Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe. Thus, for the latter compound, changing the most favorable cyclohexane arrangement for this backbone (chair III, minimum 3) to other shapes results in an energy increase of at least 2.4 kcal/mol. In comparison, for the cis derivative, the two alternate chairs are separated by an energy gap of 1.2 kcal/mol for the same γD conformation. Interestingly, for both compounds, this backbone type is more stable when combined with the chair bearing the aromatic substituent in axial (II for cis-c6Phe and III for trans-c6Phe).
Another remarkable feature in Table 2 is that three different conformational regions are visited by the minima with ΔEgp below 5 kcal/mol, namely γ, β and δ. In comparison, only the former appears in Table 1 for the same energy limit. This indicates that the presence of the phenyl substituent produces a less severe restriction of the peptide backbone flexibility for the trans compound, at least, in the gas phase. A similar trend was observed for the c3Phe4c and c5Phe4a residues investigated before, indicating that the introduction of a phenyl group in Acnc to give the corresponding cnPhe analogue produces more intense conformational constraints when the aromatic substituent is located in the neighborhood of the amino moiety (cis-cnPhe).
The location of δ minima for the trans-c6Phe derivative at about 4 kcal/mol (minima 4 and 5) is noteworthy, since this type of peptide backbone geometry was not detected among the energy minima of the cnPhe residues studied before.4a,c Although minima with a δ structure are also present in Table 1 for cis-c6Phe, their relative energy is much higher (7.9 kcal/mol and above). The same holds true for the fully-extended conformation (β). Indeed, minimum 2 in Table 2 exhibits the intramolecularly hydrogen-bonded five-membered ring [d(H···O)= 1.961 Å, <N–H···O= 114.4°] typical of a β backbone conformation, and is destabilized with respect to the global minimum by only 2.7 kcal/mol. In comparison, the most stable structure with a β backbone found for Ac-c-l-c6Phe-NHMe is 5.6 kcal/mol less stable than the lowest energy minimum. This distinct behavior must be ascribed to the different steric hindrance produced between the cyclohexane ring (the axial hydrogen atoms attached to the γ and γ’ carbons, mainly) and the –NHCOMe or –CONHMe peptide fragments when the latter are axially located (as occurs in the chairs of the most stable β conformers of cis- c6Phe and trans-c6Phe, respectively) and adopt a fully-extended conformation, that is, φ and ψ angles close to 180°.
Influence of the thermodynamic corrections, the quantum mechanical method and the basis set on the relative stabilities
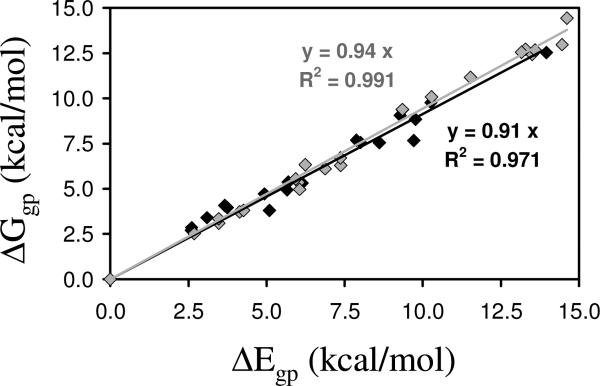
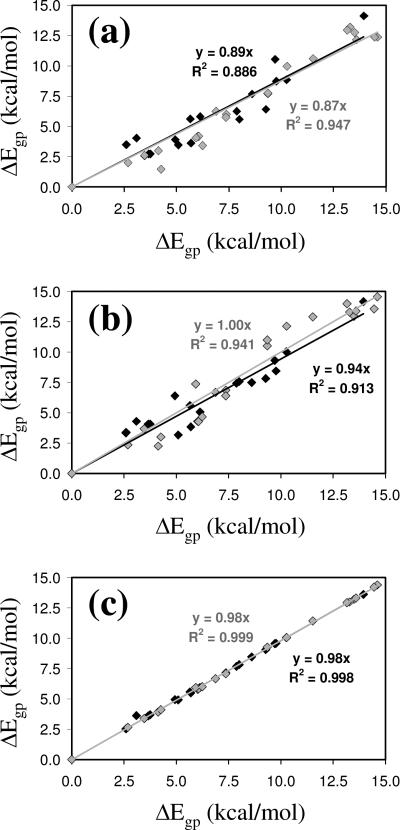
Figure 4 represents ΔEgp vs. ΔGgp for the minimum energy conformations of Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe at the B3LYP/6-31+G(d,p) level of theory (Tables 1 and 2). As can be seen, there is a strong correlation between the two parameters, the incorporation of ZPVE, thermal, and entropic corrections leading to a systematic reduction of the ΔEgp values. Thus, ΔGgp is on average 7% lower than ΔEgp, with an average reduction of 9% and 6%, respectively, for the cis and trans derivatives.
Figure 4.
Graphical representation of ΔEgp vs. ΔGgp calculated at the B3LYP/6-31+G(d,p) level for Ac-c-l-c6Phe-NHMe (black diamonds) and Ac-t-l-c6Phe-NHMe (grey diamonds). The regressions (y= c·x) and correlation coefficients (R2) are displayed for each compound.
Tables 3 and 4 compare the ΔEgp values calculated for the compounds under study at different theoretical levels: B3LYP/6-31+G(d,p), MP2/6-31+G(d,p), HF/6-31+G(d,p) and B3LYP/6-311++G(d,p), with molecular geometries optimized at the B3LYP/6-31+G(d,p) level in all cases. Comparison of the results obtained using the B3LYP and MP2 methods combined with the 6-31+G(d,p) basis set (Figure 5a) reveals an excellent agreement. Thus, the ΔEgp values provided by the former method are overestimated by about 11–13% only. The difference between these two methods should be mainly attributed to the N–H···π interaction, which is better described at the MP2 level, because the strength of interactions involving the π cloud of aromatic systems is typically underestimated by the B3LYP functional.15 A very significant concordance is also displayed by the ΔEgp values calculated using the B3LYP and HF methods combined with the 6-31+G(d,p) basis set (Figure 5b). On the other hand, the negligible influence of the size of the basis set is reflected in Figure 5c, which compares the ΔEgp values obtained using the B3LYP functional combined with the 6-31+G(d,p) and 6-311++G(d,p) basis sets. The overall of these results indicates that the B3LYP/6-31+G(d,p) theoretical level is suitable for the evaluation of energies in the gas phase.
Table 3.
Relative energy in the gas phasea (ΔEgp) calculated at different theoretical levelsb for the minimum energy conformations of Ac-c-l-c6Phe-NHMe.
| 6-31+G(d,p) |
6-311++G(d,p) | |||
|---|---|---|---|---|
| # | B3LYP | MP2 | HF | B3LYP |
| 1 | 0.0c | 0.0d | 0.0e | 0.0f |
| 2 | 2.6 | 3.5 | 3.3 | 2.5 |
| 3 | 3.1 | 4.0 | 4.3 | 3.6 |
| 4 | 3.7 | 2.7 | 4.1 | 3.6 |
| 5 | 4.9 | 3.9 | 6.4 | 4.9 |
| 6 | 5.1 | 3.5 | 3.2 | 4.9 |
| 7 | 5.6 | 5.6 | 5.6 | 5.6 |
| 8 | 5.7 | 3.6 | 3.8 | 5.5 |
| 9 | 6.1 | 5.8 | 5.1 | 5.9 |
| 10 | 7.9 | 6.2 | 7.4 | 7.7 |
| 11 | 8.0 | 7.8 | 5.6 | 7.6 |
| 12 | 8.6 | 7.7 | 7.5 | 8.5 |
| 13 | 9.3 | 6.4 | 7.8 | 9.1 |
| 14 | 9.7 | 10.5 | 9.3 | 9.6 |
| 15 | 9.8 | 8.7 | 8.4 | 9.6 |
| 16 | 10.3 | 8.9 | 10.0 | 10.0 |
| 17 | 13.9 | 14.1 | 14.1 | 13.6 |
In kcal/mol.
Energies at the MP2/6-31+G(d,p), HF/6-31+G(d,p) and B3LYP/6-311++G(d,p) levels were derived from single point calculations on B3LYP/6-31+G(d,p) geometries.
E=−883.014845 a.u.
E=−880.353232 a.u.
E=−877.425595 a.u.
E=−883.196342 a.u.
Table 4.
Relative energy in the gas phasea (ΔEgp) calculated at different theoretical levelsb for the minimum energy conformations of Ac-t-l-c6Phe-NHMe.
| # | 6-31+G(d,p) |
6-311++G(d,p) | ||
|---|---|---|---|---|
| B3LYP | MP2 | HF | B3LYP | |
| 1 | 0.0c | 0.0d | 0.0e | 0.0f |
| 2 | 2.7 | 2.0 | 2.3 | 2.6 |
| 3 | 3.5 | 2.6 | 3.7 | 3.4 |
| 4 | 4.1 | 3.0 | 2.2 | 3.9 |
| 5 | 4.3 | 1.5 | 3.0 | 4.2 |
| 6 | 5.9 | 4.1 | 7.3 | 5.9 |
| 7 | 6.0 | 4.2 | 4.3 | 5.8 |
| 8 | 6.2 | 6.0 | 3.4 | 4.7 |
| 9 | 6.9 | 6.7 | 6.2 | 6.7 |
| 10 | 7.4 | 7.1 | 6.0 | 6.9 |
| 11 | 7.4 | 5.7 | 6.4 | 7.1 |
| 12 | 9.3 | 7.7 | 10.5 | 9.2 |
| 13 | 9.3 | 7.7 | 11.0 | 9.3 |
| 14 | 10.3 | 9.9 | 12.1 | 10.0 |
| 15 | 11.5 | 10.6 | 12.9 | 11.4 |
| 16 | 13.2 | 12.9 | 14.0 | 12.9 |
| 17 | 13.3 | 13.2 | 13.3 | 13.0 |
| 18 | 13.5 | 12.7 | 12.9 | 13.2 |
| 19 | 13.6 | 12.1 | 13.4 | 13.3 |
| 20 | 14.4 | 12.4 | 13.6 | 14.2 |
| 21 | 14.6 | 12.3 | 14.6 | 14.4 |
In kcal/mol.
Energies at the MP2/6-31+G(d,p), HF/6-31+G(d,p) and B3LYP/6-311++G(d,p) levels were derived from single point calculations on B3LYP/6-31+G(d,p) geometries.
E=−883.014189 a.u.
E=−880.351683 a.u.
E=−877.424807 a.u.
E=−883.195613 a.u.
Figure 5.
Graphical representation of ΔEgp calculated at the B3LYP/6-31+G(d,p) level vs. ΔEgp predicted at the (a) MP2/6-31+G(d,p), (b) HF/6-31+G(d,p) and (c) B3LYP/6-311++G(d,p) levels for Ac-c-l-c6Phe-NHMe (black diamonds) and Ac-t-l-c6Phe-NHMe (grey diamonds). The regressions (y= c·x) and correlation coefficients (R2) are displayed for each compound.
Influence of the solvent
The influence of chloroform and water on the conformational preferences of the c6Phe derivatives was evaluated using the PCM method. The conformational free energy in a given solvent was approximated by adding ΔGsolv to the best estimation of ΔGgp, which is abbreviated as ΔGgp,* and was determined by combining the ΔEgp value calculated at the MP2/6-31+G(d,p) level with the thermodynamic corrections obtained at the B3LYP/6-31+G(d,p) level. The values of ΔGgp,* and the conformational free energies in chloroform and aqueous solutions (ΔGChl and ΔGwater, respectively) for all the minimum energy conformations of Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe are given in Tables 5 and 6, respectively. In general, the solvent plays a stabilizing role, which is reflected by a reduction of the relative free energies interval. Specifically, for Ac-c-l-c6Phe-NHMe, the ΔGgp,* interval is wider than the ΔGChl and ΔGwater ones by 2.5 and 2.1 kcal/mol, respectively. For Ac-t-l-c6Phe-NHMe, this feature is detected in chloroform solution only (1.6 kcal/mol). It is also interesting to note that the ΔGgp,* value for some conformers is significantly lower than that of ΔGgp provided in Tables 1 and 2.
Table 5.
Relative conformational free energiesa at 298 K for the minimum energy conformations of Ac-c-l-c6Phe-NHMe in the gas phase (ΔGgp,*),b chloroform solution (ΔGChl) and aqueous solution (ΔGwater). The solvation free energiesa in chloroform and aqueous solutions [ΔGsolv(Chl) and ΔGsolv(water), respectively] are also given.
| # | ΔGgp,* | ΔGsolv(Chl) | ΔGChl | ΔGsolv(water) | ΔGwater |
|---|---|---|---|---|---|
| 1 | 0.0c | 2.8 | 0.6 | 4.7 | 5.5 |
| 2 | 3.6 | 2.5 | 3.9 | 4.5 | 8.9 |
| 3 | 4.3 | 3.1 | 5.2 | 5.5 | 10.6 |
| 4 | 3.1 | 1.0 | 2.0 | 1.2 | 5.2 |
| 5 | 3.7 | 2.0 | 3.5 | 1.5 | 6.0 |
| 6 | 2.2 | 0.0 | 0.0 | −2.2 | 0.8 |
| 7 | 4.9 | 0.3 | 3.0 | −1.5 | 4.2 |
| 8 | 3.3 | −1.0 | 0.1 | −4.1 | 0.0 |
| 9 | 5.0 | 0.9 | 3.7 | −0.5 | 5.3 |
| 10 | 6.1 | 1.4 | 5.3 | −0.4 | 6.5 |
| 11 | 5.1 | 0.4 | 3.3 | −0.5 | 5.4 |
| 12 | 6.6 | 1.3 | 5.7 | 0.2 | 7.6 |
| 13 | 6.2 | −1.4 | 2.6 | −5.0 | 2.0 |
| 14 | 8.5 | 0.5 | 6.8 | −0.6 | 8.7 |
| 15 | 7.8 | 1.0 | 6.6 | −0.4 | 8.2 |
| 16 | 8.4 | −1.4 | 4.8 | 1.1 | 10.3 |
| 17 | 12.7 | −0.3 | 10.2 | −4.6 | 8.9 |
In kcal/mol.
ΔGgp,* corresponds to the best estimation of ΔGgp, which was obtained by combining the ΔEgp value calculated at the MP2/6-31+G(d,p) level with the thermodynamic corrections obtained at the B3LYP/6-31+G(d,p) level.
G= −880.039927 a.u.
Table 6.
Relative conformational free energiesa at 298 K for the minimum energy conformations of Ac-t-l-c6Phe-NHMe in the gas phase (ΔGgp,*),b chloroform solution (ΔGChl) and aqueous solution (ΔGwater). The solvation free energiesa in chloroform and aqueous solutions [ΔGsolv(Chl) and ΔGsolv(water), respectively] are also given.
| # | ΔGgp,* | ΔGsolv(Chl) | ΔGChl | ΔGsolv(water) | ΔGwater |
|---|---|---|---|---|---|
| 1 | 0.0c | 3.3 | 1.0 | 5.3 | 6.2 |
| 2 | 1.8 | 1.3 | 0.8 | 1.7 | 4.4 |
| 3 | 2.2 | 1.0 | 0.9 | 0.5 | 3.6 |
| 4 | 2.6 | 1.7 | 1.9 | −0.7 | 2.7 |
| 5 | 1.0 | 1.5 | 0.2 | 0.4 | 2.3 |
| 6 | 3.7 | 1.4 | 2.8 | 0.0 | 4.6 |
| 7 | 3.5 | −0.3 | 0.9 | −2.1 | 2.3 |
| 8 | 3.5 | −1.2 | 0.0 | −4.4 | 0.0 |
| 9 | 5.5 | 0.6 | 3.8 | −0.8 | 5.6 |
| 10 | 4.9 | 0.9 | 3.5 | 0.3 | 6.1 |
| 11 | 5.1 | −1.3 | 1.5 | −5.5 | 0.5 |
| 12 | 7.7 | 1.2 | 6.6 | 1.1 | 9.7 |
| 13 | 7.8 | 0.5 | 5.9 | 0.5 | 9.1 |
| 14 | 9.7 | 1.3 | 8.7 | 2.1 | 12.7 |
| 15 | 10.2 | 1.3 | 9.2 | 2.5 | 13.6 |
| 16 | 12.3 | 0.2 | 11.5 | −0.8 | 10.2 |
| 17 | 13.1 | 0.6 | 11.4 | −1.5 | 12.5 |
| 18 | 11.7 | 0.2 | 9.6 | −0.9 | 11.7 |
| 19 | 11.2 | −1.8 | 7.1 | −6.7 | 5.4 |
| 20 | 10.9 | −1.1 | 7.5 | −3.4 | 8.4 |
| 21 | 12.2 | −0.5 | 9.3 | 0.2 | 13.2 |
In kcal/mol.
ΔGgp,* corresponds to the best estimation of ΔGgp, which was obtained by combining the ΔEgp value calculated at the MP2/6-31+G(d,p) level with the thermodynamic corrections obtained at the B3LYP/6-31+G(d,p) level.
G= −880.038710 a.u.
Furthermore, solvation induces important changes in the stability order of the different conformers. Thus, 6 and 8 become the most favored structures for the cis-c6Phe derivative in chloroform (Table 5). Although the relative stability of 1 decreases significantly in this organic solvent (ΔGChl= 0.6 kcal/mol), it is still significantly populated at room temperature. Indeed, the populations of 6, 8 and 1 in chloroform according to a Boltzmann distribution are 44.3%, 37.3% and 15.5%, respectively. The stability of 8 increases with the polarity of the environment, and it becomes the lowest energy minimum in aqueous solution, with an estimated population of 77.3%. Conformer 6 is unfavored by 0.8 kcal/mol in water and accounts for 20.0% of the population, while the 15 remaining minima contribute by 2.7% only. Thus, the conformation preferred by Ac-c-l-c6Phe-NHMe moves from the γ-turn in the gas phase to structures lacking an intramolecular hydrogen bond, namely polyproline II and α-helix, in condensed phases. It should be noted that conformers with the six-membered ring arranged in twist are destabilized by more than 4.5 kcal/mol in solution, with 13 being the only exception. This conformer exhibits ΔGChl= 2.6 kcal/mol and ΔGwater= 2.0 kcal/mol, which correspond to an estimated population of 0.5% and 2.6% in chloroform and water, respectively.
The data in Table 6 reveal that the conformational flexibility of Ac-t-l-c6Phe-NHMe is greatly increased by the presence of chloroform. Thus, seven structures are predicted to present significant populations in this organic solvent, namely 8 (36.6%), 5 (26.1%), 2 (9.3%), 3 (8.2%), 7 (8.0%), 1 (6.9%), and 11 (3.0%). In comparison, only 8 (66.7%) and 11 (29.7%) are expected to be significantly populated (≥ 3.0%) in aqueous solution. Accordingly, almost all types of peptide backbone arrangements are accessible to Ac-t-l-c6Phe-NHMe in chloroform, whereas only the α-helical structure (either of the αL or αD type) is expected to be present in aqueous solution. Regarding the cyclohexane ring, although the structures exhibiting a twist arrangement are, in general, stabilized by the solvent, they present ΔGChl and ΔGwater values above 5.4 kcal/mol, and their populations remain, therefore, negligible at room temperature.
It should be mentioned that the X-ray crystalline structures of small peptides incorporating the c6Phe residues4b,e actually show conformations corresponding to the α-helical region (occasionally, with some distortion induced by a contiguous proline), which is in agreement with the conformational preferences predicted for the two compounds under study in aqueous solution. In the solid state, the amide moieties of the peptide backbone may interact with the complementary CO and NH sites of neighboring molecules and, as a consequence, intra- and intermolecular hydrogen bonding compete, as happens in the presence of a solvent able to form hydrogen bonds with the peptide.
It is also noteworthy that the great influence exerted by the environment on the conformational preferences of the c6Phe derivatives under study differs significantly from the behavior observed before for other cnPhe residues. Thus, solvation was found to alter the conformational profiles of c3Phe4c and c5Phe4a from a quantitative point of view but not qualitatively and, indeed, the most populated conformations for these residues in the gas-phase were also found to be preferred in aqueous solution.
Comparison with the unsubstituted Ac6c residue
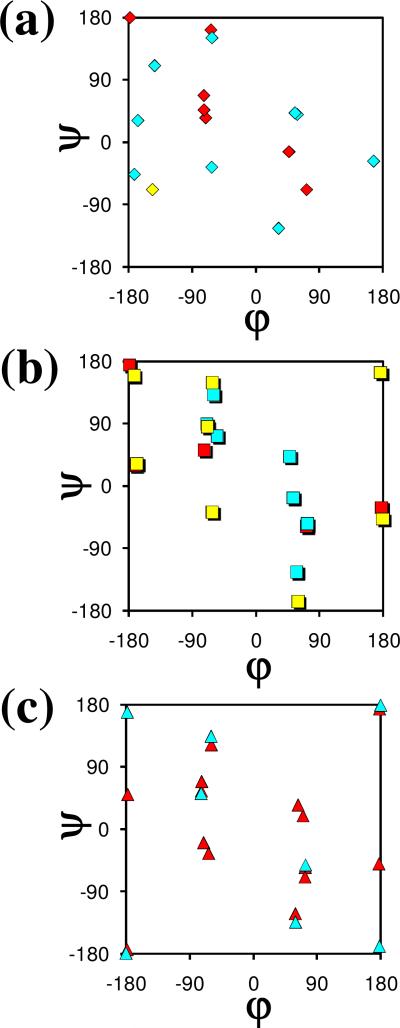
Figure 6 represents a Ramachandran map comparing the distribution of the minimum energy conformations found for Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe in the present work (Tables 1 and 2) and those characterized before for the analogous Ac6c derivative, Ac-Ac6c-NHMe, at the same level of theory.6 A color code has been used to identify the stability range of the different minima, which have been classified into three categories: ΔGgp < 5 kcal/mol (red), 5 kcal/mol ≤ ΔGgp < 10 kcal/mol (blue) and ΔGgp ≥ 10 kcal/mol (yellow).
Figure 6.
Comparison between the minimum energy conformations predicted for (a) Ac-c-l-c6Phe-NHMe, (b), Ac-t-l-c6Phe-NHMe and (c) Ac-Ac6c-NHMe at the B3LYP/6-31+G(d,p) level. Color code: red, ΔGgp < 5 kcal/mol; blue, 5 kcal/mol ≤ ΔGgp < 10 kcal/mol; yellow, ΔGgp ≥ 10 kcal/mol. For the Ac6c derivative, both the (φ,ψ) and (–φ,–ψ) positions are indicated for each minimum.
The incorporation of the phenyl side group, either in cis or in trans with respect to the amino terminus, produces a drastic reduction in the conformational diversity accessible to the peptide main chain. Thus, for Ac-Ac6c-NHMe, four different types of backbone arrangements (namely, γ, α, ε, and β, with the L and D forms being energetically indistinguishable) were found within a ΔGgp interval of 2.1 kcal/mol above the global minimum.6 In contrast, only the γL conformation appears below this energy level for the two c6Phe derivatives (Tables 1 and 2). The effect is particularly pronounced in the case of the cis compound, for which the most stable conformer with a peptide backbone other than γL exhibits a ΔGgp value of 3.8 kcal/mol.
The destabilization induced by the aromatic substituent is clearly seen when comparing the minimum energy structures located in the α-helix region. The most stable minimum of this type found for Ac-Ac6c-NHMe6 presents the cyclohexane ring in a chair conformation with the –NHCOMe substituent axially oriented and shows a ΔGgp value of 1.6 kcal/mol. In comparison, the most stable α-helical minima characterized for Ac-c-l-c6Phe-NHMe (Table 1) and Ac-t-l-c6Phe-NHMe (Table 2) display ΔGgp values of 5.4 and 6.3 kcal/mol, respectively. Moreover, in the Ac6c derivative, reversal of the cyclohexane chair leads to another α-helical minimum 1.9 kcal/mol higher in energy. In contrast, only one chair is compatible with each type of α-helical backbone arrangement for the c6Phe-containing compounds.
Minima exhibiting a twist-shaped cyclohexane ring were characterized for some backbone types in Ac6c, with the α-helix not among these.6 In contrast, such twist conformations are found to be compatible with the α-helix for both cis- and trans-c6Phe. Moreover, in the case of the cis compound, a twist minimum was characterized at a ΔGgp value as low as 3.4 kcal/mol (Table 1), whereas the most stable twist arrangement for Ac6c exhibits a ΔGgp value close to 6 kcal/mol.6
The incorporation of the phenyl substituent produces other effects, such as the modification of the geometry of minima with an ε backbone conformation. Minima of this type were characterized for Ac-Ac6c-NHMe6 with a chair-shaped cyclohexane at (φ,ψ) angles near (–60,120), or the equivalent (60,–120). When a phenyl group is incorporated in cis with the amino moiety, repulsive interactions arise between the aromatic group and the carbonyl oxygen of either the –NHCOMe or the –CONHMe substituent, depending on the εD or εL backbone type, respectively. To alleviate these, the φ or ψ angle, respectively, deviates by about 30° from the value observed in Ac6c. Accordingly, the εD and εL minima in Ac-c-l-c6Phe-NHMe with a cyclohexane ring arranged as a chair appear at (33,–124) and (–64,162), respectively (Table 1).
Conclusions
Quantum mechanical calculations have been used to characterize the intrinsic conformational preferences of Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe, incorporating the cyclohexane analogues of phenylalanine that bear the aromatic substituent in a cis or a trans orientation, respectively, relative to the amino group. A total of 17 and 21 energy minima have been found and characterized using B3LYP/6-31+G(d,p) calculations for Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe, respectively. Single point calculations at the MP2/6-31+G(d,p), HF/6-31+G(d,p), and B3LYP/6-311++G(d,p) levels were also performed. In the gas phase, the only significantly populated structures at room temperature for both compounds are those exhibiting a γL backbone conformation. Comparison with the results previously reported for the unsubstituted cyclohexane residue (Ac6c) provides evidence for the ability of the aromatic group to interact with the peptide backbone not only sterically but also electronically through the aromatic π orbitals. Moreover, the additional phenyl group exerts a strong influence on the conformational equilibrium of the cyclohexane ring, which, in turn, affects the peptide backbone arrangement. As a consequence, the incorporation of the aromatic group in Ac6c induces a severe restriction of the peptide backbone flexibility, which, in the gas phase, is less intense for the trans than for the cis c6Phe analogue.
Solvation effects have been considered through a SCRF method and have been shown to alter significantly the conformational preferences of the c6Phe derivatives under study. Thus, in aqueous solution, structures lacking any intramolecular hydrogen bond, such as polyproline II and α-helix, are preferred. It should be noted that the conformational preferences predicted in this environment are in agreement with the experimental data available from X-ray diffraction structures of small peptides containing the c6Phe residues investigated.
Supplementary Material
Acknowledgements
Gratitude is expressed to the Centre de Supercomputació de Catalunya (CESCA) for computational facilities. Financial support from the Ministerio de Educación y Ciencia - FEDER (project CTQ2007-62245), Gobierno de Aragón (research group E40) and Generalitat de Catalunya (research group 2009 SGR 925) is gratefully acknowledged. Support for the research of C.A. was received through the prize “ICREA Academia” for excellence in research funded by the Generalitat de Catalunya. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract number N01-CO-12400. The content of this publication does not necessarily reflect the view of the policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Supporting Information Available
Coordinates and energy of the minimum energy conformations characterized for Ac-c-l-c6Phe-NHMe and Ac-t-l-c6Phe-NHMe. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Toniolo C, Formaggio F, Kaptein B, Broxterman QB. Synlett. 2006:1295. [Google Scholar]; b Venkatraman J, Shankaramma SC, Balaram P. Chem. Rev. 2001;101:3131. doi: 10.1021/cr000053z. [DOI] [PubMed] [Google Scholar]; c Toniolo C, Crisma M, Formaggio F, Peggion C. Biopolymers (Pept. Sci.) 2001;60:396. doi: 10.1002/1097-0282(2001)60:6<396::AID-BIP10184>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]; d Karle IL. Biopolymers (Pept. Sci.) 2001;60:351. doi: 10.1002/1097-0282(2001)60:5<351::AID-BIP10174>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; e Kaul R, Balaram P. Bioorg. Med. Chem. 1999;7:105. doi: 10.1016/s0968-0896(98)00221-1. [DOI] [PubMed] [Google Scholar]; f Karle IL. Acc. Chem. Res. 1999;32:693. [Google Scholar]; g Benedetti E. Biopolymers (Pept. Sci.) 1996;40:3. doi: 10.1002/(sici)1097-0282(1996)40:1<3::aid-bip2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; h Toniolo C, Benedetti E. Macromolecules. 1991;24:4004. [Google Scholar]
- 2.Chakrabarti P, Pal D. Prog. Biophys. Mol. Biol. 2001;76:1. doi: 10.1016/s0079-6107(01)00005-0. [DOI] [PubMed] [Google Scholar]
- 3.Lasa M, Cativiela C. Synlett. 2006:2517. references therein. [Google Scholar]
- 4.a Casanovas J, Jiménez AI, Cativiela C, Nussinov R, Alemán C. J. Org. Chem. 2008;73:644. doi: 10.1021/jo702107s. [DOI] [PubMed] [Google Scholar]; b Lasa M, Jiménez AI, Zurbano MM, Cativiela C. Tetrahedron Lett. 2005;46:8377. [Google Scholar]; c Alemán C, Jiménez AI, Cativiela C, Pérez JJ, Casanovas J. J. Phys. Chem. B. 2002;106:11849. [Google Scholar]; d Gomez-Catalan J, Jiménez AI, Cativiela C, Perez JJ. J. Pept. Res. 2001;57:435. doi: 10.1034/j.1399-3011.2001.00840.x. [DOI] [PubMed] [Google Scholar]; e Jiménez AI, Cativiela C, Gómez-Catalán J, Pérez JJ, Aubry A, París M, Marraud M. J. Am. Chem. Soc. 2000;122:5811. [Google Scholar]; f Jiménez AI, Cativiela C, Aubry A, Marraud M. J. Am. Chem. Soc. 1998;120:9452. [Google Scholar]
- 5.a Zanuy D, Jiménez AI, Cativiela C, Nussinov R, Alemán C. J. Phys. Chem. B. 2007;111:3236. doi: 10.1021/jp065025k. [DOI] [PubMed] [Google Scholar]; b Crisma M, Toniolo C, Royo S, Jiménez AI, Cativiela C. Org. Lett. 2006;8:6091. doi: 10.1021/ol062600u. [DOI] [PubMed] [Google Scholar]; c Crisma M, De Borggraeve WM, Peggion C, Formaggio F, Royo S, Jiménez AI, Cativiela C, Toniolo C. Chem. Eur. J. 2006;12:251. doi: 10.1002/chem.200500865. [DOI] [PubMed] [Google Scholar]; d Jiménez AI, Ballano G, Cativiela C. Angew. Chem. Int. Ed. 2005;44:396. doi: 10.1002/anie.200461230. [DOI] [PubMed] [Google Scholar]; e Royo S, De Borggraeve WM, Peggion C, Formaggio F, Crisma M, Jiménez AI, Cativiela C, Toniolo C. J. Am. Chem. Soc. 2005;127:2036. doi: 10.1021/ja043116u. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Ropero F, Zanuy D, Casanovas J, Nussinov R, Alemán C. J. Chem. Inf. Mod. 2008;48:333. doi: 10.1021/ci700291x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemán C, Zanuy D, Casanovas J, Cativiela C, Nussinov R. J. Phys. Chem. B. 2006;110:21264. doi: 10.1021/jp062804s. [DOI] [PubMed] [Google Scholar]
- 8.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Jr., Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, C. Strain M, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision B.02. Gaussian, Inc.; Pittsburgh PA: 2003. [Google Scholar]
- 9.Becke AD. J. Chem. Phys. 1993;98:1372. [Google Scholar]
- 10.Lee C, Yang W, Parr RG. Phys. Rev. B. 1993;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 11.Frich MJ, Pople JA, Krishnam R, Binkley JS. J. Chem. Phys. 1984;80:3264. [Google Scholar]
- 12.Møller C, Plesset MS. Phys. Rev. 1934;46:618. [Google Scholar]
- 13.McLean AD, Chandler GS. J. Chem. Phys. 1980;72:5639. [Google Scholar]
- 14.a Miertus S, Scrocco E, Tomasi J. Chem. Phys. 1981;55:117. [Google Scholar]; b Miertus S, Tomasi J. Chem. Phys. 1982;65:239. [Google Scholar]; c Tomasi J, Persico M. Chem. Phys. 1994;94:2027. [Google Scholar]; d Tomasi J, Mennucci B, Cammi R. Chem. Rev. 2005;105:2999. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
- 15.a Hawkins GD, Cramer CJ, Truhlar DG. J. Phys. Chem. B. 1998;102:3257. [Google Scholar]; b Jang YH, Goddard WA, III, Noyes KT, Sowers LC, Hwang S, Chung DS. J. Phys. Chem. B. 2003;107:344. [Google Scholar]; c Iribarren JI, Casanovas J, Zanuy D, Alemán C. Chem. Phys. 2004;302:77. [Google Scholar]
- 16.Perczel A, Angyan JG, Kajtar M, Viviani W, Rivail J-L, Marcoccia J-F, Csizmadia IG. J. Am. Chem. Soc. 1991;113:6256. [Google Scholar]
- 17.a Kopple KD, Marr DH. J. Am. Chem. Soc. 1967;89:6193. doi: 10.1021/ja01000a035. [DOI] [PubMed] [Google Scholar]; b Robinson DR, Jencks WP. J. Am. Chem. Soc. 1965;87:2470. doi: 10.1021/ja01089a029. [DOI] [PubMed] [Google Scholar]
- 18.a Gil AM, Buñuel E, Jiménez AI, Cativiela C. Tetrahedron Lett. 2003;44:5999. [Google Scholar]; b Halab L, Lubell WD. J. Am. Chem. Soc. 2002;124:2474. doi: 10.1021/ja012442w. [DOI] [PubMed] [Google Scholar]; c Tóth G, Murphy RF, Lovas S. J. Am. Chem. Soc. 2001;123:11782. doi: 10.1021/ja011245u. [DOI] [PubMed] [Google Scholar]; d Tóth G, Watts CR, Murphy RF, Lovas S. Proteins: Struct. Funct. Genet. 2001;43:373. doi: 10.1002/prot.1050. [DOI] [PubMed] [Google Scholar]; e Steiner T, Koellner G. J. Mol. Biol. 2001;305:535. doi: 10.1006/jmbi.2000.4301. [DOI] [PubMed] [Google Scholar]; f Steiner T, Schreurs AMM, Kanters JA, Kroon J. Acta Cryst., Sect. D. 1998;54:25. doi: 10.1107/s0907444997007981. [DOI] [PubMed] [Google Scholar]; g Worth GA, Wade RC. J. Phys. Chem. 1995;99:17473. [Google Scholar]; h Mitchell JBO, Nandi CL, McDonald IK, Thornton JM. J. Mol. Biol. 1994;239:315. doi: 10.1006/jmbi.1994.1370. [DOI] [PubMed] [Google Scholar]; i Flocco MM, Mowbray SL. J. Mol. Biol. 1994;235:709. doi: 10.1006/jmbi.1994.1022. [DOI] [PubMed] [Google Scholar]; j Mitchell JBO, Nandi CL, Ali S, McDonald IK, Thornton JM. Nature. 1993;366:413. [Google Scholar]; k Singh J, Thornton JM. J. Mol. Biol. 1990;211:595. doi: 10.1016/0022-2836(90)90268-Q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.