Abstract
According to “Wolff’s Law”, bone is deposited and reinforced at areas of greatest stress. From a clinical perspective, this “law” is supported by the strong association between bone density and physical activity. From a mechanistic standpoint, however, the law presents a challenge to scientists seeking to understand how osteocytes and osteoblasts sense the mechanical load. In the 1960’s, collagen piezoelectricity was invoked as a potential mechanism by which osteocytes could detect areas of greater stress but piezoelectricity diminished in importance as more compelling mechanisms, such as streaming potential, were identified. In addition, accumulating evidence for the role of fluid-related shear stress in osteocyte’s mechanosensory function has made piezoelectricity seemingly more obsolete in bone physiology. This review critically evaluates the role of collagen piezoelectricity (if any) in Wolff’s Law – specifically, the evidence regarding its involvement in strain-generated potentials, existing alternate mechanisms, the present understanding of bone mechanosensation, and whether piezoelectricity serves an influential role within the context of this newly proposed mechanism. In addition to reviewing the literature, this review generates several hypotheses and proposes future research to fully address the relevance of piezoelectricity in bone physiology.
Introduction
In the late 19th century, the German anatomist and surgeon, Julius Wolff, proposed that trabecular bone oriented itself in a direction that aligned with the principle stress experienced by the bone. While Dr. Wolff largely focused on trabecular bone particularly that of the proximal femur, the idea that bone is a dynamic organ adaptive to its mechanical environment has been generalized, over time, to all bone including compact portion of bone and to non-extremity axial bone.1 This generalization may be inappropriate as this “law” is a likely oversimplification of an organ as complex as bone. However, the gist of “Wolff’s Law”, namely bone’s ability to adapt to mechanical stresses, has largely been accepted by clinicians. For instance, clinicians recognize that astronauts return with weaker bone after a long mission, while weightlifters possess increased bone density in response to their training.
How the bone is capable of responding to mechanical environment and specifically how osteocytes and osteoblasts can perceive forces remain unanswered. In the 1960’s, collagen piezoelectricity was invoked as a potential mechanism by which osteocytes could detect areas of greater stress. According to this theory, applied stress generated local potential gradients along the collagen fiber and thus provided a local stimulus for bone-generating cells.2–4 This theory initially generated substantial enthusiasm but diminished in importance as more compelling mechanisms, such as streaming potential and fluid-generated shear stress, were described. As a result, in recent literature, collagen piezoelectricity hardly enters into the discussion of bone physiology. This review takes a renewed look at collagen piezoelectricity and considers recent developments and advances in bone physiology and mechanics. It reviews the evidence against collagen piezoelectricity as a mediator for bone regulation, the appeal for more compelling mechanisms such as strain-generated potentials, our present understanding of bone mechanosensation, and whether piezoelectricity serves an influential role within the context of our recent understanding of bone physiology. This review also generates several hypotheses by which collagen piezoelectricity may still be germane to bone growth and proposes future research to fully address the relevance of piezoelectricity to bone physiology.
Strain-Generated Potentials in Bone
In the 1960’s (and possibly earlier), investigators observed that mechanical strain of compact bone generated electrical potential differences along the lateral and longitudinal axes of bone.5 This discovery aroused substantial interest in the scientific community - driven with the hope that elucidating the underlying mechanism of this phenomenon may somehow clarify how bone is selectively deposited at areas of stress. Anecdotal reports of electromagnetic field-related healing of bony fractures may have further fueled the interest in this area. The strain generated potential (SGP) was attributed to two main mechanisms: piezoelectricity and streaming potential.
Piezoelectricity was identified as the primary mechanism for SGP in dry bone and was ascribed to the non-centrosymmetric nature of collagen. For wet bone, however, the role for piezoelectricity was less clear. Based on multiple levels of experimental findings, streaming potential was increasingly accepted as the primary drive for strain-generated potential in wet bone. First, the relaxation times for SGP in wet bone ranged from 0.1 to 3.0 seconds, considerably longer than what was expected for piezoelectricity. The charges generated by piezoelectricity undergo rapid relaxation and, by classic dielectric measurements, relaxation time constants were approximately 0.5 to 50 µs.6
Secondly, alterations in ionic strength and viscosity of bone fluid yielded changes in SGP most consistent with a streaming potential mechanism. For example, in 1983, Pienkowski used whole bovine tibia and a four point bending apparatus to test the SGP and relaxation times as a function of sodium chloride concentration and viscosity.6 Both step loading and 1 Hz sinusoidal stress were used. Pienkowski found that the SGP amplitude decreased as the NaCl concentration of the soaking solution was increased. Above a certain concentration, the SGP polarity even reversed. Interestingly, over three orders of magnitude of NaCl concentration, the relaxation times remained constant. For viscosity, the SGP diminished with increasing solution viscosity while the relaxation time increased linearly. These findings were highly supportive of a streaming potential mechanism and are explained, in part, by the following streaming potential relationship6:
where ζ is the zeta potential; ε the dielectric permittivity; ΔP the pressure gradient imposed across a sample; σ the solution conductivity; and η the solution viscosity. As made clear by this relationship, the streaming potential diminishes with increasing solution viscosity and conductivity (conductivity acts doubly by reducing the zeta potential as well). Although not explicitly stated in this equation, the streaming potential relaxation time would also be expected to increase with increasing fluid viscosity since streaming potential, unlike piezoelectricity, is mediated by fluid flow. The reversal in SGP polarity associated with the greater concentrations of sodium chloride was explained by surface adsorption of positive ions (likely Na+) that led to an eventual shift towards a positive zeta potential.
For piezoelectricity, the stress generated voltage is expressed by this following relationship7:
Where dijk is a third rank piezoelectric tensor; L, sample thickness; B, load applied to the sample; and t, time. If a piezoelectric mechanism were to predominate in bone, changes in solution viscosity and conductivity should not have altered the SGP amplitude. However, in Pienkowski’s study, the viscosity and conductivity did indeed affect SGP amplitude. In addition, increasing the solution conductivity should have decreased the SGP relaxation time considerably, but in this same study, the SGP relaxation time remained constant through three orders of magnitude in conductivity.
This evidence against piezoelectricity was corroborated by other studies, and, as a consequence, piezoelectricity fell out of favor as an explanatory mechanism for SGP’s in wet bone. Streaming potential became widely accepted and generally considered more germane to the physiologic conditions of bone where the solid matrix is bathed in fluid. As will be discussed later in this review, however, the prospect of a combined effect from piezoelectricity and streaming potential has never, to this author’s knowledge, been considered in wet bone or in the context of live bone physiology.
Bone Anatomy and Physiology
By weight, compact bone is 35% organic, composed mostly of type 1 collagen along with a small percentage of noncollagenous proteins. Sixty five percent of bone is composed of inorganic minerals, predominantly hydroxyapatite with small amounts of various impurities such as citrate, fluoride and magnesium. Within compact bone, there exists a well developed vascular system that runs mostly parallel to the long axis of the bone. The blood vessels lie in their own channels called Haversian canals and bone is concentrically layered around each canal. Collectively, the concentric layers of bone and the canal form a unit called a Haversian system or osteon. Between each concentric layer, a ring of dark elliptical spots called lacunae are present where, normally, osteocytes are embedded. Osteocytes have as many as 80 long processes that radiate out about 15 µm from the cell body in every direction.8 These processes are located in small channels (~ 200 nm radius) called canaliculi that interconnect adjacent osteocytes. These canaliculi eventually connect with a Haversian canal. Processes communicate bidrectionally with those of other osteocytes via gap junctions – forming a complex connected cellular network that includes cells within the periosteal and endosteal membranes. This complex network is believed to formulate a nervous-like system that coordinates bone metabolism and production.9
Canaliculi are considered the life lines that permit nutrients, oxygen and wastes to be exchanged between the blood vessels within the Haversian canal and the osteocytes. Based on recent theoretical and cell culture studies, the canaliculi may also serve an important mechanosensory role by providing the channels through which osteocytes can sense fluid shear forces.10, 11 Osteocytes (mature, terminally differentiated osteoblasts) are generally recognized as the mechanosensing cell within the bone.
In vivo studies have shown that uniaxial strain in bone reaches a maximum of only 0.3%. A mere 0.15% bending strain is sufficient to recruit osteoblasts to the bone surface.12 Yet, in in vitro studies, much higher deformation of approximately 1–3% strain is needed to obtain a cellular response in osteocyte cells13 – leading many to suggest that an additional process, beyond the actual deformation of the cells, is necessary to stimulate the osteocytes in bone. To address this discrepancy, the canalicular fluid-flow hypothesis was proposed where the canaliculi and lacunae fill the role of pressure transducers.10, 14 When the bone is loaded, interstitial fluid within the lacuna and canaliculi is squeezed through the thin layer of non-mineralized matrix surrounding the cell bodies and cell processes, toward the Haversian or Volkmann channels. The fluid flow mobilizes the cell surface glycocalyx and initiates a biochemical process for osteogenesis.
Based on Biot’s poroelastic theory, simulations have calculated the fluid shear stress over the cell process’ surface to be approximately 0.8–3.0 Pa.14 This should be sufficient for cell response since endothelial cells are capable of responding to fluid shear stress as low as 0.5 Pa.15 The pulsating fluid flow and in vivo strain increase levels of intracellular Ca2+ and protein kinase C which in turn stimulate release of nitric oxide (NO) and prostaglandin-E2 (PGE2), both potent anabolic regulators of bone growth.16, 17 Both strain magnitude and strain rate are considered particularly important stimuli. In the moving, living body, strain frequencies typically range from 0 to >60 Hz.8 Skeletal muscles contract at much higher frequencies (15–60 Hz) than walking – although 15–30 Hz generates only 4% of magnitude deformation of strains of 0–15 Hz.18 The optimal response at lower frequencies may imply that fluid flow/streaming potential is important in mediating the response since these mechanisms operate at similar time scales.
Role for Streaming Potential and Piezoelectricity
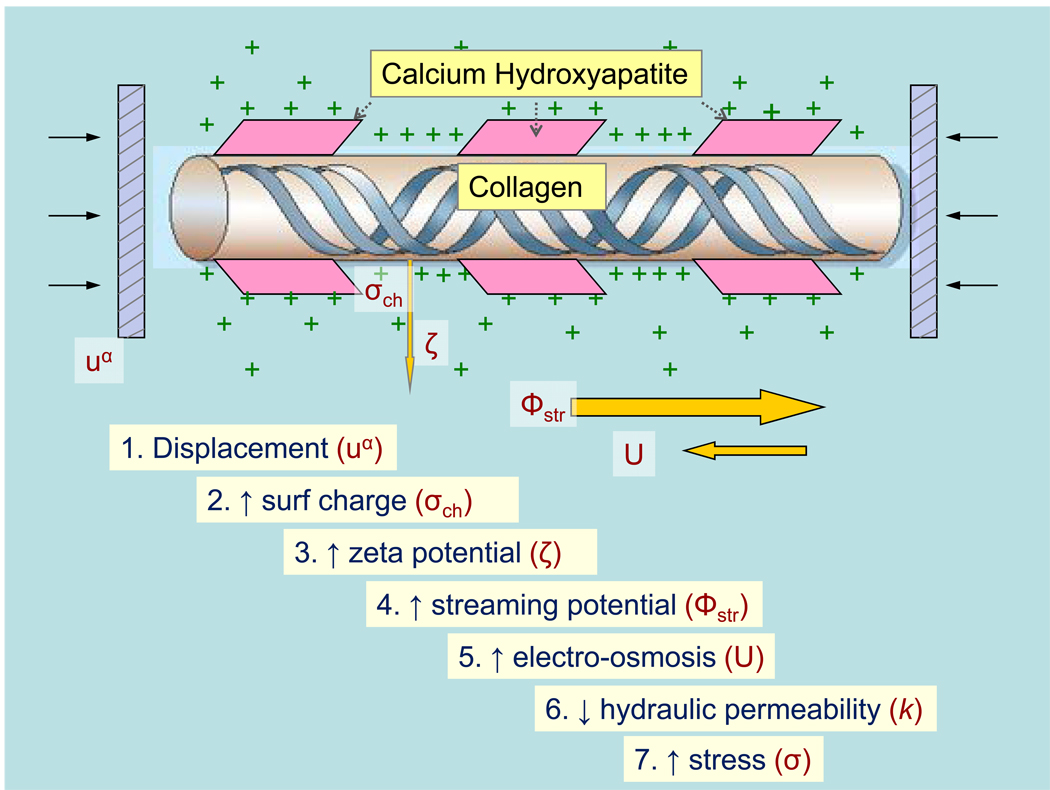
With this recent understanding of bone mechanosensation, is there any room for implicating streaming potential or piezoelectricity mechanisms? Does the importance of fluid shear stress obviate the importance of bone electrokinetics altogether? Based on our understanding of fixed charges on poroelastic properties and fluid flow, these electrokinetic mechanisms may rather play an integral role within this new bone mechanosensation framework. For instance, in an open circuit model of tissue compression, an increase in fixed charge density at the bony matrix generates a greater zeta potential, increased streaming potential, a corresponding increase in electroosmosis, a decrease in the ‘open circuit’ hydraulic permeability, and thus increased dynamic stiffness of the tissue (Figure 1).19, 20 This may generate, based on the local fixed charge density, differences in the mechanical load sensed by the canalicular system and thus the fluid flow around the osteocyte processes. Moreover, through Donnan potential equilibrium, fixed charges can modify the stable state fluid content of the bone and thus the amount available for transfer from the collagen-hydroxyapatite microporosity to the lacuna-canalicular system where the osteocytes reside. Iatridis, in 2003, eloquently demonstrated the importance of charge density on net fluid flow through a vertebral disc during dynamic compression.21 Using a poroelastic model integrated with electrical and chemical forces along with finite element modeling, he was able to show that healthy discs with intact GAG fixed charges maintained a larger total fluid content in the presence of dynamic compression compared to degenerate discs with lower fixed charge densities.
Figure 1.
Theoretical effects of collagen piezoelectricity on bone stiffness under open-circuit conditions. Displacement of bone generates surface charges that indirectly contribute to greater stress and thus greater bone stiffness.
If streaming potential has the ability to modulate stiffness and fluid flow in the bone, how about piezoelectricity? Why should piezoelectricity even enter into the conversation of mechanosensation, when its role in SGP’s is suspect and its existence in physiologic conditions remains in doubt? This manuscript proposes a hypothesis regarding piezoelectricity that has not been explored or even discussed in the past literature of bone – namely, the idea that piezoelectricity acts not in isolation, but in conjunction with streaming potential mechanisms. By changing the surface charge with mechanical stress, the piezoelectricity of collagen may influence the magnitude of the zeta potential and thus the streaming potential during compression. It may thus indirectly modify the stiffness and the fluid dynamics of bone. Past studies have evaluated the relevance of piezoelectricity to biology by investigating its solitary effects or comparing its influence to that of other competing mechanisms. This novel perspective of piezoelectricity, however, requires a reevaluation of the literature to assess the scientific and clinical feasibility of this hypothesis. The remainder of this review will aim to do this and will additionally suggest future investigations to further ascertain the validity of the hypothesis.
Exploring Collagen Piezoelectricity
In many ways, collagen within compact bone is ideally suited to execute a piezoelectric process. Unlike cartilage, bone possesses collagen that is highly oriented and patterned. The collagen structure provides the collective, cohesive response to mechanical loading needed for a physiologically significant effect, if any were to exist. Stresses such as tension or compression generate modifications in not only one, but multiple similarly oriented collagen fibers to evoke changes that are not only physiologically substantive but also orientationally specific.
Furthermore, calcium hydroxyapatite lends to an environment highly conducive to piezoelectric effects. Mineral crystals display remarkably high elastic moduli compared to biological molecules. This ensures that loads placed onto the bone are transmitted across large spatial scales while permitting the collagen fibers to mechanically respond locally. With smaller elastic moduli, collagen fibers will likely bear the greatest strain of all the molecules within the solid matrix and thus generate the needed deformation required for a piezoelectric effect. In addition, the hydroxyapatite restricts access of water to collagen, permitting the piezoelectric mechanisms observed in dry bone to occur even in the wet state. This dehydrating effect of calcium hydroxyapatite is supported by a number of physical observations: fully calcified bone is found to be more closely packed than decalcified bone, collagen in decalcified bone shrink upon heating while collagen in calcified bone do not, and permittivity of decalcified bone is higher than that of calcified bone because of higher adsorption of water.22 Ultimately, the calcium hydroxyapatite may not simply be a passive component for collagen piezoelectricity but rather play an important facilitating role.
The appeal for collagen piezoelectricity within bone also extends to physiologic areas. While the fluid shear stress mechanism is strongly supported by data and anatomic considerations, it alone cannot account for bone’s complex response to loading. For example, within the fluid shear framework, how can osteocytes differentiate the varied types of bone stresses: axial compression vs. bending vs. twisting vs. shear? Microscopic evaluation of bone reveals that the architecture of collagen and Haversian systems are uniquely suited to resist these different stresses.23 Certainly, an intrinsic system must be in place for the forces to be detected and for such complex architectures to be designed accordingly. In this regard, collagen is a highly appealing candidate. Its structured orientation and its anisotropic piezoelectric property provide the bone with the means for selective response of varying stresses. It confers the osteocytes the much needed global point of reference.
As much as the appeal for collagen piezoelectricity exists, this proposition remains all speculative unless supporting evidence from the literature exists. For a collagen piezoelectric mechanism to be deemed feasible, three important components of the hypothesis should be demonstrated: (1) collagen is an important contributor to the zeta potential in wet bone (2) there is actual evidence of piezoelectricity, separate from streaming potential, occurring in wet bone, and (3) the piezoelectric response is clinically consistent and physiologically significant.
Collagen and Zeta Potential
A number of experiments have demonstrated that the zeta potential in bone is largely determined by collagen and not the mineral content of bone. Otter et al, in 1988, took three different samples - whole bone, demineralized bone (hydroxyapatite removed), and anorganic bone (collagen removed by either boiling or Na hypochlorite treatment) - and measured their streaming potential and calculated their corresponding zeta potential.24 The zeta potential of whole bone and demineralized bone were statistically identical, while the zeta potential of anorganic samples (collagen eliminated) was dramatically smaller than both samples containing collagen. Indeed, the lower zeta potential in anorganic samples was equal in magnitude to zeta potentials calculated for synthetic hydroxyapatite alone. Furthermore, the streaming potential sign inversions observed with increasing concentrations of sodium did not occur unless the organic content of bone was included.25 This implied that the streaming potential inversions, and thus the streaming potential measures themselves, observed in past studies were attributed in large part to collagen. Importantly, collagen’s ability to affect bone’s zeta potential is a necessary prerequisite for our piezoelectric-streaming potential mechanism to hold true: surface charges generated from piezoelectric-related processes will do little if other molecules simply overpower the changes. Because collagen is a major contributor to the zeta potential, piezoelectric-related changes can influence the zeta potential and thus the streaming potential.
Existence of Piezoelectricity in Bone
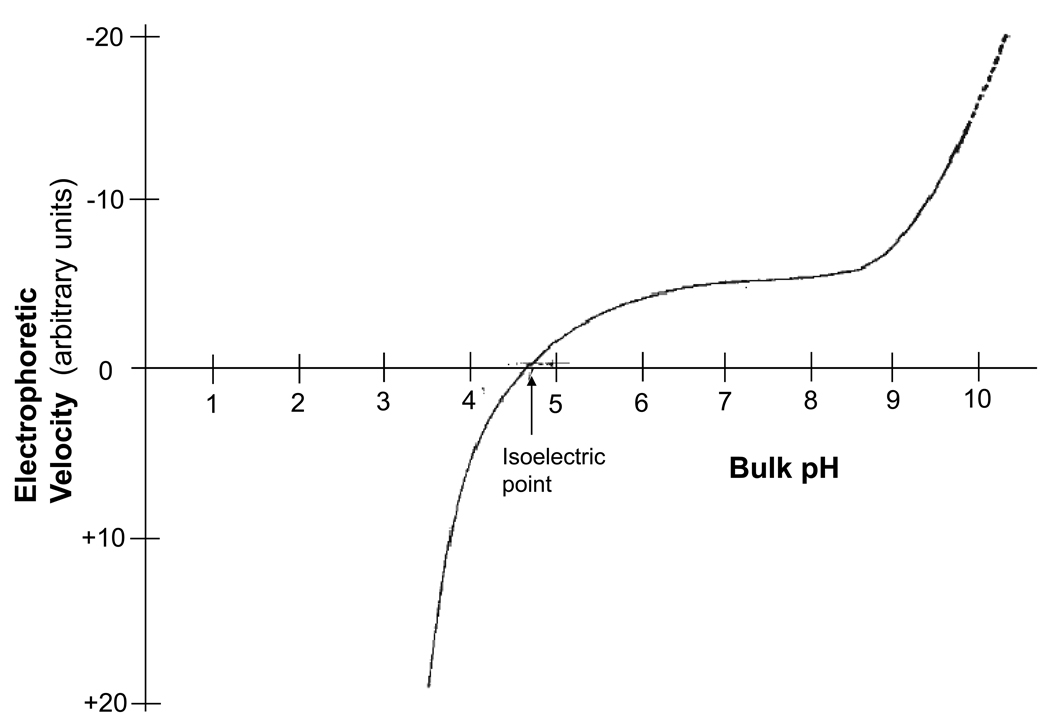
However, simply because piezoelectricity can induce changes in streaming potential does not necessarily mean that such mechanisms actually occur. For additional support of the piezoelectric-streaming hypothesis, there must be evidence for genuine piezoelectric activity in bone collagen. For this, evidentiary support exists on a number of levels. In 1968, Anderson took a human Achilles tendon and applied an impulse tensile force to the opposite ends of the tendon by dropping an attached mass of 2–10 kg for a fixed distance of 1 inch.26 The strain generated potential was measured by imbedding two Ag/AgCl electrodes 3 cm apart while varying the pH across a range from 2 to 12. The tendons were subsequently dried and ground up into 20 µm particles and submitted for electrophoretic analyses. The close correlation between the SGP and electrophoretic velocity relationships to pH was interpreted as strong evidence for a streaming potential mechanism, since electrophoretic velocity is similarly dependent on the zeta potential (See Figure 2A and 2B). Additionally, the SGP reached zero amplitude at a pH of 4.7, which closely coincided with the isoelectric point of collagen. This, again, suggested that streaming potential and not piezoelectricity was responsible for the SGP. Closer evaluation of the data, however, shows a discrepancy in magnitude at physiologic pH 5 to 11. In the electrophoresis graph, a stable plateau in amplitude is observed at physiologic pH while the SGP amplitude steadily rises as the pH is increased. This discrepancy cannot be fully ascribed to streaming potential alone since the SGP and electrophoresis should theoretically mirror each other if that were the case. Could this dissimilarity be attributed to an altogether different, unaccounted mechanism?
Figure 2.
2A: Strain generated potential of tendon as a function of pH (adapted from Anderson JC 196826). 2B: Electrophoretic velocity of tendon as a function of pH (also adapted from Anderson JC 196826)
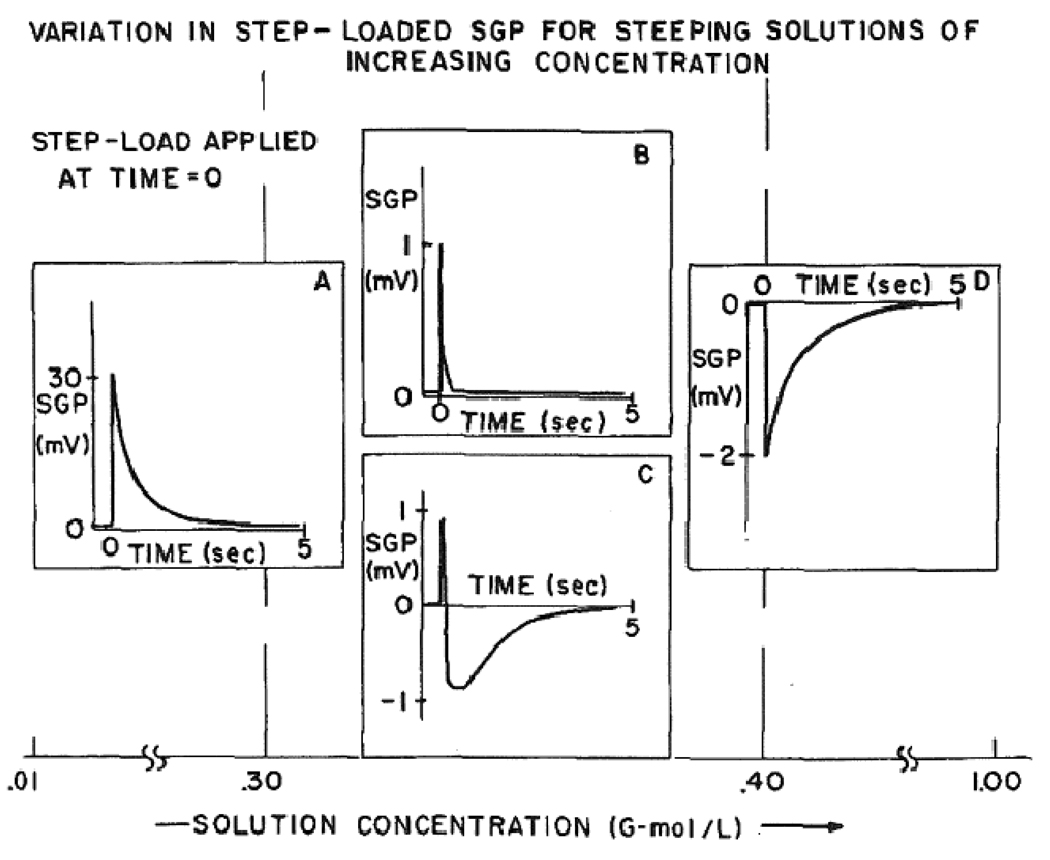
For possible answers to this question, a study published by Pollack in1984 provides additional insights.27 Similar to other past studies, Pollack noted a reversal in the zeta potential as the concentration of potassium chloride in the bony fluid was increased. Interestingly, in the vicinity of the “cross-over” region where the zeta potential reversed polarities, reproducible SGP waves in bone were observed. These SGP waves are presented in Figure 3B and 3C. Compared to SGP waves at non-zero zeta values, these “cross-over” waveforms exhibited smaller amplitudes and shorter relaxation times (in the millisecond compared to second range). On occasion, these short SGP waves were accompanied by another waveform with longer relaxation constants (see Figure 3C).
Figure 3.
3A–D: Different SGP waveforms of bone as a function of KCl concentration. B, C represent waveforms obtained at the zeta “cross-over” region (taken from Pollack SR 198427).
Based on the short relaxation times and the absence of a standing zeta potential, these small amplitude spikes can be reasonably ascribed to piezoelectricity. The subsequent, longer lasting deflections of SGP (in Figure 3C) can be explained by an accompanying streaming potential induced by the temporarily formed surface charges. Certainly, the similarity in relaxation pattern and time (~ over 5 secs) with the non-“crossover” waveforms make this explanation appealing. The absence of a streaming-like waveform in Figure 3B may be attributed to the anisotropic properties of bone: recording electrodes placed off the axis of fluid flow (normally occurring along the longitudinal axis of the osteon) can easily miss any ongoing streaming potential activities. On an additional note, the amplitude of the piezoelectric-related streaming potential may be an underestimate. The conditions at which the crossover waveforms occur likely involve high ionic concentrations. This may cause the zeta potential and the corresponding streaming potential to be much smaller than would be expected under physiologic conditions. The lower SGP amplitude in Figure 3D (higher ionic concentrations) supports this possibility.
More recent support for the existence of bone piezoelectricity comes from a recent 2004 study involving a Piezoresponse Force Microscope (PFM).28 The PFM is a modified Atomic Force Microscope where an ac current is introduced to the probe tip and the corresponding tissue displacement is recorded by the cantilever. The displacement theoretically reflects a converse piezoelectric effect where an electrical field induces mechanical changes within the bone. In this particular study, Halperin et al evaluated human tibial and humerus bone. The bone samples were cut either transversely (90 degrees to the diaphyseal axis) or longitudinally (parallel to the diaphyseal axis) and were further divided into two groups – wet bone and dry bone samples (desiccated using a 4 month drying process). For each bone sample, the PFM tip (50 nm radius) was placed on the uncoated surface of the samples while a 1.0 kHz ac voltage in the range of 0–14 volts was applied. The recorded tip displacements were used to calculate the piezoelectric coefficient constant. For transverse cuts of the bone, the tip was placed around the Haversian canal on the tips of the collagen fibers. A linear relationship between applied voltage and piezoresponse was observed. Interestingly, the piezoelectric response did not differ between wet or dry samples or between geographical locations (based on radial distance from the Haversian medullary canal). The piezoelectric coefficients were approximately 7.6 – 8.5 pC/N in value. Zero piezoresponse was observed in all longitudinal cut samples (tip placed on the sides of the collagen molecules).
Though only d33 coefficients were measured, these piezoresponse changes suggest that piezoelectricity may indeed exist for wet bone. The results cannot be readily ascribed to the phenomena of current generated stress predicted by poroelastic theory as the tip was too small and the electrical field penetration too short for a macroscopic application such as poroelastic theory to hold true. Moreover, the electrical field oscillated at a frequency of 1.0 kHz - too rapid for substantive fluid shifts to occur. As seen in Grodzinsky’s electrokinetic studies of cartilage, current generated stress quickly approached zero at frequencies as low as 1.0 Hz. While the PFM results may be partially rationalized by Maxwell stresses (where the electrical field generated by the collagen surface charge interacts with the electrical field from the PFM tip), the similarity in piezoresponse between wet and dry samples cannot be readily explained.
Physiologic Significance of Collagen Piezoelectricity
The ultimate test for piezoelectricity comes from its ability to affect physiologic and clinical processes in vivo. Does the piezoelectric-related surface charge generate changes in streaming potential sufficient to alter physiologic functions in biological systems (in this case, bone)? Based on our limited understanding of bone electromechanics, however, this question cannot be readily answered. The next logical question then becomes: is the existing evidence consistent with the clinical context and is the magnitude of effect within the range of biological feasibility?
If the piezoelectric surface charges were to contribute sufficiently to streaming potential, then collagen should ideally be designed with piezoelectric constants greatest at the lateral axes of the fiber where it can optimally modify the zeta potential and thus the streaming potential along the longitudinal axis of bone. In other words, the piezoelectric generated charges should ideally be greatest at axes 1 and 2 for it to contribute to streaming potentials along axis 3. Based on SGP's of wet and dry bone, this appears to be true. In 1970, Anderson found the piezoelectric constants of dry bone to be29:
where dij is defined by the following relationship: P is the electric polarization; T, mechanical stress; Axis 1 is aligned anterior to posterior; Axis 2, lateral; and Axis 3, longitudinal parallel to the long axis of the bone. The collagen is assumed to align parallel to the long axis of the bone (axis 3). Note: shear components were not evaluated in the study. In this particular sample, piezoelectric constants, d21 ,d23 , and d11 had the greatest values, consistent with the requisite that generated charges be largest at the sides of the collagen fiber. The same bone sample was subsequently immersed in saline solution and the SGP measured:
The two noticeably larger piezoelectric coefficients are d31 and d32 . Assuming preferential fluid flow along axis 3 induced by pressure, the greatly increased d31 may be traced to the greater d21 and d11 dry piezoelectric coefficients that help generate the corresponding zeta potential. The lower increase in d32 can be attributed to the smaller d21 and d22 dry piezoelectric constants. Of course, this assumes that the piezoelectric constants for dry bone can be extended to the wet condition. Evidence for the water shielding properties of calcium hydroxyapatite suggests that there is some basis for this assumption. While Anderson’s results are indicative, they represent a single case and cannot be used as proof of a piezoelectric-mediated streaming potential in bone.
The clinical implications for collagen piezoelectricity in bone are intimated, to some extent, by Noris-Suarez’s work in 2007.30 In this study, demineralized type I collagen was obtained from rabbit cortical bone and subject to bending deformation by inserting the bone in a radial plastic tube. The deformation assured considerable stress, sufficient to elicit polarizing piezoelectric effects. The bone was subsequently bathed in continually flowing physiologic fluid and evaluated for precipitation of hydroxyapatite crystals over a periods of weeks and with the use of Scanning Electron Microscopy. Investigators focused on the compressed and tensed portion of collagen. Undeformed cortical bones were used as controls. Interestingly, the compressed internal surface of the collagen attracted calcium ions which led to subsequent nucleation and crystallization of hydroxyapatite. Within a span of 3 weeks, statistically significant increase in calcium hydroxyapatite precipitate was observed in the compressed portion of the collagen compared to undeformed collagen, while the tensed portion of the collagen showed no difference in precipitate compared to undeformed controls. The authors reasoned, based on past studies, that compression of collagen caused the dipole of collagen to reorganize and yielded negative charges on the surface thereby initiating calcium adsorption. Tension of collagen, on the other hand, led to the formation of positive charges on the surface.
This information provides insights into the physiologic function of piezoelectricity, specifically in its role as a modulator of bone stiffness. According to several studies, the bony matrix has a negative zeta potential of approximately −5 mV under physiologic conditions. Because compression of collagen generates negative charges on the surface, the zeta potential should be augmented with bone compression. As a result, compression may lead to greater streaming potential, increased electroosmosis, reduced effective hydraulic permeability, and thus increased dynamic stiffness of bone. The converse is likely true for tensed bone where a positive surface charge attenuates the zeta potential. This may lead to reduced dynamic stiffness of bone. From a clinical standpoint, this differential response is sensible. The bone stiffens in response to compression and loosens with tension – thus helping to not only form an adaptable solid matrix but also regulate the pressure sensed by the lacuna-canalicular system. Moreover, collagen generates different piezoelectric charges based on the type and intensity of stress applied. Such differential effects can help the bone to possess a highly selective and complex system of pressure sensing and response.
To ascertain whether the magnitude of the piezoelectric effect is physiologically significant, we can use Frank and Grodzinsky’s electrokinetic model of confined dynamic compression.20 According to Grodzinsky’s model, the stress σ felt by a confined tissue under compression and under open circuit conditions is:
where HA is the confined compression modulus; ; uα is the displacement of the top tissue surface; and δ is tissue thickness. Of note, the ‘open-circuit’ hydraulic permeability, k, is determined by the matrix relationship
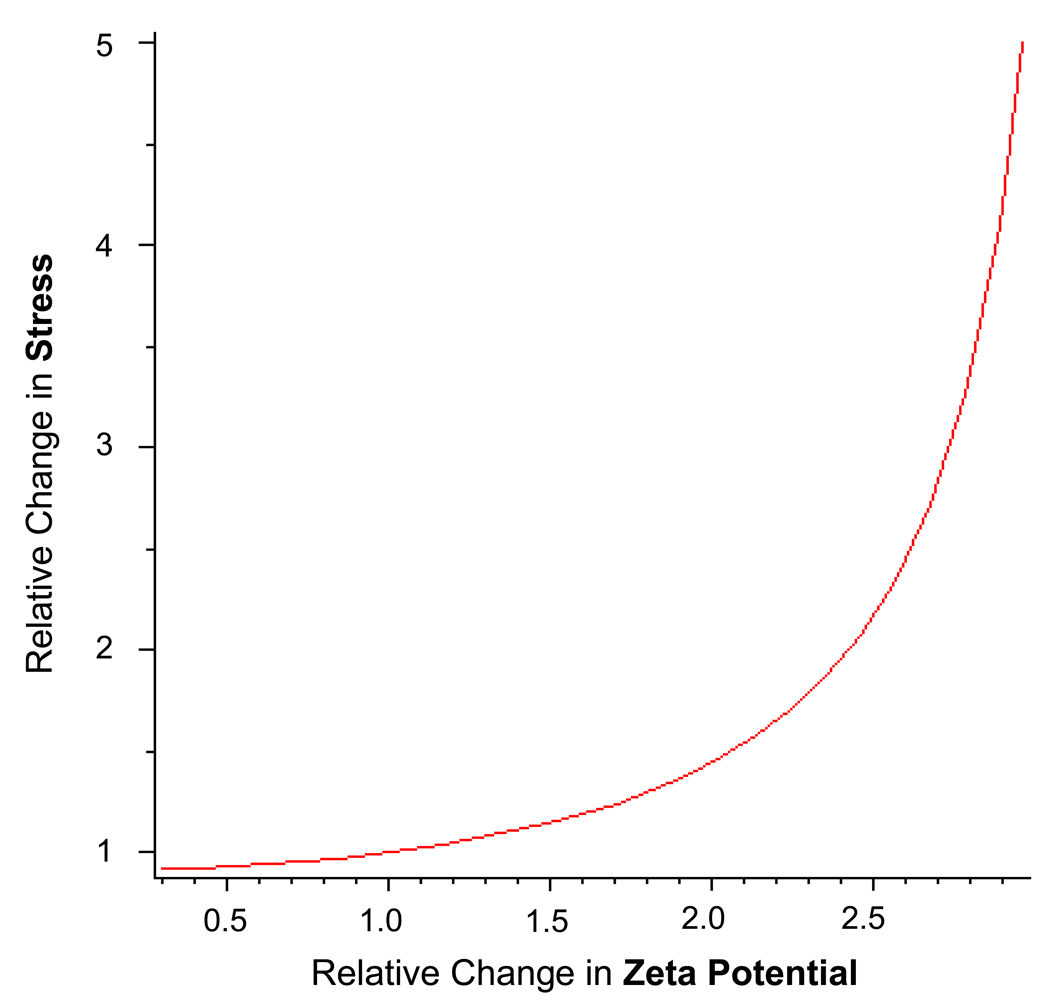
where V(z) is set to 0 to establish an open circuit condition. From this equation, the final relationship for ‘open circuit’ hydraulic permeability: is obtained. To estimate the effect of piezoelectricity on dynamic stiffness, we can use experimentally derived values and plug them into the equations above. For cortical bone, the value of the ‘close circuit’ hydraulic permeability, k11 , is estimated to be 31 (compared to for cartilage [cortical bone has smaller porosities]); electrical conductivity, k22 , of 0.1 S/m (0.7 S/m for cartilage); and electrokinetic coupling coefficients,k12 and k21, of 24 ( of cartilage [increased due to large fixed charge densities from GAG chains]). These values are specific to the collagen-hydroxyapatite matrix of cortical bone and reflect behavior at the microscopic scale (10’s µm to mm range). The Onsanger reciprocity (k12 = k21) is assumed. Using a zeta potential of −5 mV and an piezoelectric-associated augmentation of −2.5 mV (based on projections from Pollack’s streaming potential data and Anderson’s wet piezoelectric coefficients27, 29), the electrokinetic coupling coefficients k12 and k21 increase by 50% and the overall stress, σ, subsequently increases by 20% (Figure 5). When factoring in the converse changes in stress expected for bony matrix under tension, the difference in stress levels across the bone can be dramatic simply due to the changes in surface charge caused by collagen piezoelectricity. The stress differences between tension and compression conditions can dramatically influence the lacuna-canalicular fluid flow system and thus the osteocyte response to mechanical loading. Furthermore, since the surface charge generated by piezoelectric mechanisms is proportional to the applied pressure, the increase in bone stiffness (attributed to changes in surface charge) should increase with each rise in applied pressure and does so dramatically at higher surface charge/zeta potential levels (Figure 5). These calculations, of course, assume an open circuit relationship and a confined compression condition, both of which may not accurately reflect bone in a physiologic state.
Proposed Experiments
The effects of piezoelectricity on streaming potential (and thus the stiffness and fluid shifts of bone) can be readily evaluated by proposed experiments. To measure piezoelectric-generated charges under static compression, one may use Donnan equilibrium experiments to determine the fixed charge densities on collagen while the bone is immersed in saline bath. These measurements can be repeated under varying levels of compression to determine the dependence of charge density on pressure. Since Donnan equilibrium reflects net surface charge, this approach assumes that selective charges are internalized, generating a net surface charge under compression. The aforementioned Noris-Suarez’s study30 supports this assumption but will need to be confirmed. Importantly, the mechanical force associated with compression should be taken into consideration as it may affect the osmotic pressure and thus the concentration of ions within the matrix. An alternate approach would be to record streaming potentials as fluid flow is induced continuously through a statically compressed small bone sample. The strain should be small enough to ensure that the porosity of the sample is not significantly altered. In both experiments, the fixed charge densities should vary as a function of mechanical stress if piezoelectricity were to truly exist. Whether these charge effects are attributed to strictly-defined piezoelectricity or to deformation-induced changes in molecular structure should also be explored. It is possible that these two processes are one and the same or entirely distinct.
For a more dynamic assessment of collagen piezoelectricity, a zero zeta potential bone sample can be used. By adjusting either the solution pH or salt concentration, complete elimination of the standing zeta potential can be attained. Under this condition, the stress-generated potentials should theoretically reflect the exclusive contribution of piezoelectricity, barring any significant inhomogeneities of zeta potential in the sample. The magnitude of the piezoelectric-mediated streaming potential would be of particular interest. Ideally, the ionic conductivity of the solution should resemble the conductivity of physiologic fluid so as to reflect most closely the piezoelectric changes in vivo.
Finally, the dynamic stiffness of bone tissue can be assessed at different levels of static offset strain. For instance, a static offset strain of 0.1% or 0.2% can be used while an overlying dynamic strain oscillation of 0.01% is applied. As long as the static offset strain is within the linear regime of elastic moduli, the dynamic stiffness should reflect the changes in zeta potential cause by piezoelectric mechanisms. If no piezoelectric mechanisms exist, then the dynamic stiffness should remain constant despite the amount of static offset strain applied to the tissue. On the other hand, for piezoelectric tissues, the dynamic stiffness should theoretically rise as the offset strain is increased. Much like Frank and Grodzinsky’s publication in 198720, this behavior can easily be modeled. In addition to the seven poroelastic and electrokinetic equations, a piezoelectric relationship can be added to help define the now dependent variables, k12 and k21. The piezoelectric constants will need to be determined. Otherwise, the model can be a valuable means to understand the effects of piezoelectricity on bone mechanics.
Conclusion
Despite the insights provided by these proposed experiments, a number of unanswered questions will likely remain. For instance, can the changes in dynamic stiffness with respect to strain be attributed exclusively to piezoelectric-mediated streaming potential? The pores of bone are so small that they have been reported to exclude a number of small molecules, including microperoxidase32, 33 (2 nm diameter) and procion red34 (6 nm diameter). If the pores are as small as some predict, then collagen fibers are exceptionally proximal to each other (at most <10 nm) and therefore within the realm of intermolecular electrostatic interactions. Piezoelectric generated charges can lead to electrical repulsion and to subsequent rise in mechanical stiffness. To evaluate this hypothesis, a DLVO (Derjaguin, Landau, Verwey, and Overbeek) mathematical approach may be used. Another question includes the appropriateness of an open circuit model to bone. Is there a form of electrical shunt to permit circuitous flow of current in bone? Controversies may also arise as to whether fluid exchange between the three levels of fluid containment (matrix porosity, lacuna/canaliculi, and Haversian/Volkmann canals) can further influence fluid dynamics.
Additionally, how do osteoclasts register mechanical loading and how do they know when and where to resorb bone? How is the directionality of collagen fibers determined during development and aging? These questions are merely a fraction of the questions likely to be raised for a system as complex as the bone. Before we proceed with these difficult questions, however, it would be important to resolve the relevance of piezoelectricity to bone physiology. As stated in this review, the notion that piezoelectricity can influence streaming potential has never been explored in the past. Yet, this hypothesis provides possible explanations for problems that have long perplexed bone scientists. The recognition of piezoelectric effects in bone may greatly expedite scientific progress and make the complete understanding of this highly complex organ more achievable.
Figure 4.
Relationship between relative change in zeta-potential and relative change in stress/stiffness. Calculated using MAPLE 11 software (Maplesoft, Waterloo, Ontario, Canada).
Acknowledgment
This research was supported by grant number K23-AT003238 of the National Center for Complementary Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, National Institutes of Health. The study sponsor had no involvement in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: the authors have no financial or personal relationship with other people or organizations that could inappropriately influence this work.
References
- 1.Martin RB, Burr DB, Sharkey NA. Skeletal Tissue Mechanics. New York: Springer-Verlag, Inc.; 1998. [Google Scholar]
- 2.Bassett CA, Becker RO. Generation of electric potentials by bone in response to mechanical stress. Science. 1962;137:1063–1064. doi: 10.1126/science.137.3535.1063. [DOI] [PubMed] [Google Scholar]
- 3.Bassett CA, Pawluk RJ, Becker RO. Effects of Electric Currents on Bone in Vivo. Nature. 1964;204:652–654. doi: 10.1038/204652a0. [DOI] [PubMed] [Google Scholar]
- 4.Becker R, Bassett C, Bachmann C. Bioelectric factors controlling bone structure. In: Frost H, editor. Bone Biodynamics. Vol. 209. Boston: Little, Brown and Co.; 1964. [Google Scholar]
- 5.Friedenberg ZB, Brighton CT. Bioelectric potentials in bone. J Bone Joint Surg Am. 1966;48(5):915–923. [PubMed] [Google Scholar]
- 6.Pienkowski D, Pollack SR. The origin of stress-generated potentials in fluid-saturated bone. J Orthop Res. 1983;1(1):30–41. doi: 10.1002/jor.1100010105. [DOI] [PubMed] [Google Scholar]
- 7.Petrov N. On the electromechanical interaction in physiologically wet bone. Biomechanics. 1975;2:43–52. [Google Scholar]
- 8.Pearson OM, Lieberman DE. The aging of Wolff's "law": ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol. 2004 Suppl 39:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 9.Marotti G, Palumbo C. The mechanism of transduction of mechanical strains into biological signals at the bone cellular level. Eur J Histochem. 2007;51 Suppl 1:15–19. [PubMed] [Google Scholar]
- 10.Cowin SC, Moss-Salentijn L, Moss ML. Candidates for the mechanosensory system in bone. J Biomech Eng. 1991;113(2):191–197. doi: 10.1115/1.2891234. [DOI] [PubMed] [Google Scholar]
- 11.Burger EH, Klein-Nulen J. Responses of bone cells to biomechanical forces in vitro. Adv Dent Res. 1999;13:93–98. doi: 10.1177/08959374990130012201. [DOI] [PubMed] [Google Scholar]
- 12.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):397–402. [PubMed] [Google Scholar]
- 13.Murray DW, Rushton N. The effect of strain on bone cell prostaglandin E2 release: a new experimental method. Calcif Tissue Int. 1990;47(1):35–39. doi: 10.1007/BF02555863. [DOI] [PubMed] [Google Scholar]
- 14.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 15.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 16.Pavalko FM, Norvell SM, Burr DB, Turner CH, Duncan RL, Bidwell JP. A model for mechanotransduction in bone cells: the load-bearing mechanosomes. J Cell Biochem. 2003;88(1):104–112. doi: 10.1002/jcb.10284. [DOI] [PubMed] [Google Scholar]
- 17.Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349(1):1–5. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 18.Turner CH, Yoshikawa T, Forwood MR, Sun TC, Burr DB. High frequency components of bone strain in dogs measured during various activities. J Biomech. 1995;28(1):39–44. doi: 10.1016/0021-9290(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 19.Frank EH, Grodzinsky AJ. Cartilage electromechanics--I. Electrokinetic transduction and the effects of electrolyte pH and ionic strength. J Biomech. 1987;20(6):615–627. doi: 10.1016/0021-9290(87)90282-x. [DOI] [PubMed] [Google Scholar]
- 20.Frank EH, Grodzinsky AJ. Cartilage electromechanics--II. A continuum model of cartilage electrokinetics and correlation with experiments. J Biomech. 1987;20(6):629–639. doi: 10.1016/0021-9290(87)90283-1. [DOI] [PubMed] [Google Scholar]
- 21.Iatridis JC, Laible JP, Krag MH. Influence of fixed charge density magnitude and distribution on the intervertebral disc: applications of a poroelastic and chemical electric (PEACE) model. J Biomech Eng. 2003;125(1):12–24. doi: 10.1115/1.1537190. [DOI] [PubMed] [Google Scholar]
- 22.Marzec E, Kubisz L, Jaroszyk F. Dielectric studies of proton transport in air-dried fully calcified and decalcified bone. Int J Biol Macromol. 1996;18(1–2):27–31. doi: 10.1016/0141-8130(95)01052-1. [DOI] [PubMed] [Google Scholar]
- 23.Bromage TG, Goldman HM, McFarlin SC, Warshaw J, Boyde A, Riggs CM. Circularly polarized light standards for investigations of collagen fiber orientation in bone. Anat Rec B New Anat. 2003;274(1):157–168. doi: 10.1002/ar.b.10031. [DOI] [PubMed] [Google Scholar]
- 24.Otter M, Goheen S, Williams WS. Streaming potentials in chemically modified bone. J Orthop Res. 1988;6(3):346–359. doi: 10.1002/jor.1100060306. [DOI] [PubMed] [Google Scholar]
- 25.Gross D, Williams WS. Streaming potential and the electromechanical response of physiologically-moist bone. J Biomech. 1982;15(4):277–295. doi: 10.1016/0021-9290(82)90174-9. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JC, Eriksson C. Electrical properties of wet collagen. Nature. 1968;218(5137):166–168. doi: 10.1038/218166a0. [DOI] [PubMed] [Google Scholar]
- 27.Pollack SR, Salzstein R, Pienkowski D. The electric double layer in bone and its influence on stress-generated potentials. Calcif Tissue Int. 1984;36(1) Suppl 1:S77–S81. doi: 10.1007/BF02406138. [DOI] [PubMed] [Google Scholar]
- 28.Halperin C, Mutchnik S, Agronin A, et al. Piezoelectric effect in human bones studied in nanometer scale. Nano Letters. 2004;4(7):1253–1256. [Google Scholar]
- 29.Anderson JC, Eriksson C. Piezoelectric properties of dry and wet bone. Nature. 1970;227(5257):491–492. doi: 10.1038/227491a0. [DOI] [PubMed] [Google Scholar]
- 30.Noris-Suarez K, Lira-Olivares J, Ferreira AM, et al. In vitro deposition of hydroxyapatite on cortical bone collagen stimulated by deformation-induced piezoelectricity. Biomacromolecules. 2007;8(3):941–948. doi: 10.1021/bm060828z. [DOI] [PubMed] [Google Scholar]
- 31.Mak AF, Huang DT, Zhang JD, Tong P. Deformation-induced hierarchical flows and drag forces in bone canaliculi and matrix microporosity. J Biomech. 1997;30(1):11–18. doi: 10.1016/s0021-9290(96)00121-2. [DOI] [PubMed] [Google Scholar]
- 32.Ayasaka N, Kondo T, Goto T, Kido MA, Nagata E, Tanaka T. Differences in the transport systems between cementocytes and osteocytes in rats using microperoxidase as a tracer. Arch Oral Biol. 1992;37(5):363–369. doi: 10.1016/0003-9969(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Sakano A. Differences in permeability of microperoxidase and horseradish peroxidase into the alveolar bone of developing rats. J Dent Res. 1985;64(6):870–876. doi: 10.1177/00220345850640060201. [DOI] [PubMed] [Google Scholar]
- 34.Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22(2):107–117. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]