Abstract
A growing body of data suggests that hyperactivation of the immune system has been implicated in the pathophysiology of major depressive disorder (MDD). Several pro-inflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) have been found to be significantly increased in patients with MDD. This review focuses on these two cytokines based on multiple lines of evidence from genetic, animal behaviour, and clinical studies showing that altered levels of serum TNF-α and IL-1 are associated with increased risk of depression, cognitive impairments, and reduced responsiveness to treatment. In addition, recent findings have shown that centrally expressed TNF-α and IL-1 play a dual role in the regulation of synaptic plasticity. In this paper, we review and critically appraise the mechanisms by which cytokines regulate synaptic and neural plasticity, and their implications for the pathophysiology and treatment of MDD. Finally, we discuss the therapeutic potential of anti-inflammatory-based approaches for treating patients with severe mood disorders. This is a promising field for increasing our understanding of the mechanistic interaction between the immune system, synaptic plasticity, and antidepressants, and for the ultimate development of novel and improved therapeutics for severe mood disorders.
Keywords: Bipolar disorder, cytokines, depression, inflammation, synaptic plasticity, treatment
Introduction
Cytokines are small pleiotropic proteins previously discovered in the context of cellular activation and cell-to-cell communication in the immune system. Cytokines can be viewed as either ‘pro-inflammatory’ or ‘anti-inflammatory’, depending on the sum total of their effects on target cells. Although the presence and activity of cytokines in the brain was discovered more than a decade ago, their role in physiological and pathological brain functions remains to be fully elucidated. Early studies of the role of cytokines in the brain suggested that their expression and activity was induced in response to infection, head trauma, ischaemia, stroke, or various neurodegenerative diseases (Lacroix & Rivest, 1998; Licinio, 1997; Pitossi et al. 1997; Rivest et al. 2000). However, the notion that inflammatory cytokines are only expressed in the brain in response to pathological stimuli has recently been challenged by emerging data indicating that the proinflammatory cytokines interleukin-1 (IL-1), IL-18, and tumour necrosis factor-alpha (TNF-α) are expressed in normal brain and also play an active role in cellular events that induce structural changes at the synaptic level (reviewed in Pickering et al. 2005; Tonelli & Postolache, 2005).
These recently discovered cytokine functions in the brain, and the novel molecular relationship between immunity and neural activity, are of particular relevance to patients suffering from psychiatric or neurological diseases. Notably, patients with depressive disorders have elevated levels of pro-inflammatory cytokines, suggesting a potential link between depressive illness and activation of the inflammatory response (Anisman et al. 1999; Kim et al. 2007; Maes et al. 1999; Muller & Ackenheil, 1998; Nassberger & Traskman-Bendz, 1993; Sluzewska, 1999; Tsao et al. 2006; Tuglu et al. 2003). In addition, depressive disorders have frequently been observed in association with peripheral inflammatory cytokine activation in several medical conditions, including viral infections, rheumatoid arthritis, cancer, and neurodegenerative diseases (Miller & Raison, 2006; Raison et al. 2006; Wichers & Maes, 2002).
Relatedly, increasing pre-clinical and clinical studies have shown that mood disorders such as major depressive disorder (MDD) and bipolar disorder (BPD), which have historically been viewed as neurochemical disorders, are associated with structural and functional impairments of synaptic plasticity in various regions of the central nervous system (CNS) (reviewed in Schloesser et al. 2008). Overlap between the molecular actions of synaptic plasticity and those targeted by antidepressants provides further evidence for a mechanistic convergence between the two phenomena. Here, synaptic plasticity refers to the cellular processes that result in lasting changes in the efficacy of neuro-transmission. More specifically, synaptic plasticity refers to both the changes in number of synapses and the variability of the strength of a signal transmitted through a synapse.
Given recent data showing elevated proinflammatory cytokine levels in MDD and animal models of stress, this review evaluates the potential role of cytokine-mediated impairments of synaptic plasticity in mood disorders, with a special emphasis on TNF-α and IL-1. Recent findings from a variety of genetic, animal behaviour, and clinical studies show that increased levels of serum TNF-α and IL-1 correlate with ‘sickness behaviour’, increased risk of MDD and/ or reduced responsiveness to standard antidepressant treatment. In addition, the finding that centrally expressed TNF-α and IL-1 play a ‘double-edged sword’ role in regulating synaptic plasticity raises the possibility that it is maintaining the intricate balance between physiological and pathological levels of these cytokines that is key to the pathogenesis of mood disorders. Finally, we discuss potential therapeutic strategies and targets for anti-cytokine therapy in MDD.
Pro-inflammatory cytokines in the normal brain
It has been well-established that peripherally produced cytokines can access the brain and thus affect brain function via several routes, including (1) entry through leaky regions in the blood–brain barrier, such as the circumventricular organs; (2) binding to cytokine-specific carrier molecules expressed on brain endothelium, and (3) activation of vagal afferent fibres that transmit cytokine signals to specific brain nuclei – such as the nucleus of the solitary tract – which then serves as a relay station to other brain nuclei, including the paraventricular nucleus in the hypothalamus (reviewed in Raison et al. 2006; Schiepers et al. 2005). Interestingly, accumulating evidence suggests that the pro-inflammatory cytokines TNF-α, IL-1, and IL-6, as well as interferons and their receptors, are constitutively expressed in various brain regions (Table 1). However, it is worth mentioning that not all studies have detected the expression or bioactivity of proinflammatory cytokines in the CNS (Cunningham et al. 1992; Fontana et al. 1984; Gabellec et al. 1996; Gayle et al. 1998; Holmin et al. 1997; Hunt et al. 1992; Medana et al. 1997; Parnet et al. 1994; Tchelingerian et al. 1993; Turnbull et al. 1997; van Dam et al. 1998). In the context of this review, it is important to note that TNF-α and IL-1 share similar signal transduction pathways, leading to nuclear factor kappa B (NF-κB) activation. This, in turn, is believed to represent a point of convergence for signalling pathways involved in normal neuronal function and synaptic plasticity (Grilli & Memo, 1999), and may suggest a potential role for constitutive central cytokine production in neuronal development and neuroplasticity.
Table 1.
Expression profile and signalling pathways of selected pro-inflammatory cytokines in the normal brain
| Cytokine | Primary localization of cytokine | Primary localization of cytokine receptor |
Associated signalling cascades |
|---|---|---|---|
| TNF-α | Neurons in hypothalamus, caudal raphe nuclei (Breder et al. 1993; Churchill et al. 2008); astrocytes (Chung & Benveniste, 1990; Lieberman et al. 1989) | TNFR1 and TNFR2 in neurons and glia in the cortex, hippocampus, thalamus (Boka et al. 1994; Tchelingerian et al. 1993) | TNFR1 (via FADD) caspase 8 and caspase 3 pathwayTNFR1 and TNFR2 (via TRAF2) – JNK, p38MAPK and NF-κB pathways (Aggarwal, 2003) |
| IL-1 family (IL-1α, IL-1β, IL-18) | Glial cells in cerebal cortex and hypothalamus (Breder et al. 1993; Vitkovic et al. 2000); Neurons in hypothalamus (Friedman, 2001; Rettori et al. 1994; Yasuhara et al. 1997) | IL-1RI and IL-1RII in neurons in hippocampus, hypothalamus and dentate gyrus (Cunningham et al. 1992; Parnet et al. 1994) | IL-1RI (via IRAK) – NF-κB pathwaysIL-1Rs – JNK, p42/44 MAPK, p38MAPK pathways (Vitkovic et al. 2000) |
| IL-6 | Neurons in hippocampus and cortex (Gadient & Otten, 1994; Schobitz et al. 1993) astrocytes (Van Wagoner et al. 1999) | Neurons in hippocampus and cortex (Gadient & Otten, 1994; Schobitz et al. 1993) | Jak/STAT and Ras/MEK/MAPK pathways (Heinrich et al. 1998; Pizzi et al. 2004) |
| INFs (INF-α/β, INF-γ) | Neurons, astrocytes and microglia (Benveniste, 1998) | Neurons in hippocampus and cortex (Gadient & Otten, 1994; Schobitz et al. 1993) | Jak/STAT and PI3K pathways (Bartee et al. 2008; Li et al. 2007) |
FADD, Fas-associated death domain; IL-6, interleukin 6; IL-1RI, IL-1 receptor type I; IL-1RII, IL-1 receptor type II; INF, interferon; IRAK, interleukin 1 receptor-associated kinase; Jak/STAT, Janus kinases/signal transducers and activators of transcription; JNK, c-Jun N-terminal kinases/stress-activated protein kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK/extracellular signal-related kinase (ERK) kinase; NF-κB, nuclear factor kappa B; PI3K, phosphoinositide-3 kinase; TNFR1, TNF-α type I receptor; TNFR2, TNF-α type 2 receptor; TRAF2, TNFR-associated factor 2.
Regulation of synaptic plasticity by pro-inflammatory cytokines
The relative abundance of pro-inflammatory cytokines in the hippocampus suggests that they may play a role in hippocampal synaptic plasticity, which regulates learning and memory. Indeed, multiple studies have shown that cytokines, notably IL-1 and TNF-α, modulate long-term potentiation (LTP) and glutamatergic-dependent synaptic plasticity (Carlezon & Nestler, 2002; Du et al. 2004, 2007, 2008; Kendell et al. 2005; Malenka, 2003; Sun et al. 2005; Wolf et al. 2004).
Regulation of synaptic plasticity by TNF-α
Increased levels of TNF-α have been observed in several neuropathological states associated with learning and memory deficits, such as Alzheimer’s disease, leading researchers to explore TNF-α’s putative role in regulating neuroplasticity. Indeed, pathophysiological levels of TNF-α have been shown to inhibit LTP in the CA1 region, as well as the dentate gyrus of the rat hippocampus (Butler et al. 2004; Cunningham et al. 1996; Tancredi et al. 1992). LTP is a long-lasting increase in synaptic efficacy, and is thought to be an important underlying mechanism of learning and memory formation (Bliss & Collingridge, 1993). In addition, it has been shown that TNF receptor knockout mice demonstrate impaired long-term depression (LTD) in the CA1 region of the hippocampus (Albensi & Mattson, 2000). The findings relating to the effects of TNF-α on synaptic plasticity appear to have some behavioural correlates in vivo. TNF-α knockout mice showed improved performance on spatial memory and learning tasks and, conversely, TNF-α overexpressing mice were significantly impaired on spatial learning and memory tasks (Aloe et al. 1999; Golan et al. 2004).
Although most studies suggest that TNF-α has deleterious effects on synaptic plasticity, recent evidence shows that physiologically low levels of TNF-α may be important in brain development, as well as the regulation of homeostatic synaptic plasticity, namely ‘synaptic scaling’ (Golan et al. 2004; Stellwagen & Malenka, 2006). TNF-α released from glial cells in response to decreased neuronal activity increases the number of synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and thus synaptic strength, and is therefore critical for homeostatic adjustment of neuronal excitability. Interestingly, removal of TNF-α from brain slices results in weakening synapses (Beattie et al. 2002), suggesting that glially released TNF-α is important not only in increasing synaptic strength, but also in maintaining or preserving it. This TNF-α-induced AMPA receptor exocytosis has recently been shown to be mediated by activation of TNF-R1 receptors and is selective for Ca2+-permeable AMPA receptor subunits. Independent of a critical role of TNF-α in homeostatic scaling, it is important to note that this effect of TNF-α on Ca2+ homeostasis might also have implications for neuronal toxicity, especially when extracellular levels of TNF-α are high, as seen in a number of neuropathological conditions.
Although recent findings with regard to the role of TNF-α in regulating synaptic plasticity appear initially to be conflicting, it is also possible that it is precisely the delicate balance between pathophysiological and physiological levels of TNF-α that is important. Thus it is conceivable that under pathophysiological conditions, when central levels of TNF-α become elevated, LTP is likely to be inhibited, while under physiological conditions, low levels of TNF-α serve as modulators of homeostatic synaptic plasticity (Table 2).
Table 2.
Regulation of synaptic plasticity and behavioural correlates by pro-inflammatory cytokines
| Cytokine | Effect on synaptic plasticity | Behavioural correlates |
|---|---|---|
| Physiological levels of TNF-α | Up-regulation of AMPA receptor trafficking; increased synaptic strength (Beattie et al. 2002; Stellwagen et al. 2005) | ? |
| High pathological levels of TNF-α | Inhibition of LTP (Butler et al. 2004; Coogan et al. 1999; Cunningham et al. 1996; Tancredi et al. 1992) | ‘Depressive-like ’ behaviour, impaired learning and memory in animal models (Aloe et al. 1999; Dantzer, 2001; Golan et al. 2004) |
| Depressive symptoms, anxiety and memory impairments in mood disorders (Dantzer et al. 2008; Raison et al. 2006) | ||
| Physiological levels of IL-1 | Maintenance of short-term plasticity and LTP (Avital et al. 2003; Goshen et al. 2007, 2008; Yirmiya et al. 2002) | Improved hippocampal-dependent memory (Avital et al. 2003; Brennan et al. 2003; Song et al. 2003) |
| High pathological levels of IL-1 or IL-18 | Impaired LTP (Coogan et al. 1999; Curran & O’Connor, 2001; Goshen et al. 2007) | ‘Depressive-like ’ behaviour and impaired hippocampal-dependent memory in animal models (Dantzer, 2001; Dantzer et al. 2008; Gibertini et al. 1995) |
| Increased levels of IL-6 | Decreased glutamate release (D’Arcangelo et al. 2000); decreased expression of LTP (Tancredi et al. 2000) | ‘Depressive-like ’ behaviour, impaired learning and memory in animal models (Balschun et al. 2004; Bluthe et al. 1999; Heyser et al. 1997) |
| Marked cognitive disturbances and depression symptoms in MDD (Capuron et al. 2001a; Raison et al. 2006) | ||
| Increased levels of INF-α and INF-γ | Decreased dendritic AMPA receptor clustering (Vikman et al. 2001); inhibition of glutamate-mediated excitatory post-synaptic potentials and LTP (Mendoza-Fernandez et al. 2000) | Anxiety and learning deficits in animal models (Fahey et al. 2008; Myint et al. 2007) |
| Depressive symptoms and cognitive deficits in MDD (Gabbay et al. 2008; Raison et al. 2006) |
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; IL-1, interleukin 1; IL-6, interleukin 6; INF-α, interferon-alpha; INF-γ, interferon-gamma; LTP, long-term potentiation; MDD, major depressive disorder.
Regulation of synaptic plasticity by IL-1
In addition to its well-known role in immunoregulation of inflammatory processes, recent evidence suggests that IL-1 may modulate synaptic plasticity and behavioural systems. Indeed, it has been noted that pathophysiological levels of IL-1 can have detrimental effects on hippocampal-dependent memory and learning processes (Barrientos et al. 2002; Bellinger et al. 1993; Curran & O’Connor, 2001; Gibertini et al. 1995; Goshen et al. 2008; Oitzl et al. 1993; Pugh et al. 1999), while stress-induced inhibition of hippocampus-dependent conditioning can be reversed by IL-1ra, an IL-1 receptor antagonist (Maier & Watkins, 1995; Pugh et al. 1999, 2000). In accordance with these behavioural effects, IL-1 was found to impair LTP in the hippocampus (Cunningham et al. 1996; Murray & Lynch, 1998).
Although most findings to date indicate that IL-1 has deleterious effects on synaptic function and memory, recent evidence suggests that, like TNF-α, it may also be required for the physiological regulation of hippocampal plasticity. Early studies showed that LTP in the hippocampus was accompanied by a long-lasting increase in IL-1 gene expression, and that exposure to IL-1ra impairs the maintenance of LTP (Schneider et al. 1998). Furthermore, it has been shown that, in rats, administration of IL-1ra impairs memory in the water-maze and passive-avoidance paradigms, both of which are associated with hippocampal functioning. In contrast, relatively low doses of IL-1β improve avoidance memory (Brennan et al. 2003; Song et al. 2003; Yirmiya et al. 2002). Similarly, mice with a targeted deletion of IL-1R1 (IL-1rKO) display severely impaired hippocampal-dependent memory, diminished short-term plasticity, and exhibit no LTP, both in vivo and in vitro (Avital et al. 2003). Recently, an elegant series of studies by Goshen and colleagues found conclusive evidence that the involvement of IL-1 in hippocampal-dependent memory processes follows an inverted U-shaped pattern, which could explain the observed discrepancy of IL-1 effects on synaptic plasticity (Goshen et al. 2007, 2008). They demonstrated that physiological levels of IL-1 are needed for memory formation, and a slight increase in brain IL-1 levels can even improve memory; however, any deviation from the physiological range, either by excess elevation in IL-1 levels (induced by exogenously administered IL-1 or by enhanced endogenous release of IL-1) or by blockade of IL-1 signalling, results in impaired memory (Table 2).
Synaptic plasticity in the pathophysiology and treatment of MDD
Increasing pre-clinical and clinical evidence demonstrates that synaptic plasticity, a fundamental mechanism of neuronal adaptation, is altered in mood disorders, including depression, and in animal models of stress. The impairment of synaptic plasticity includes both structural and functional plasticity.
Neuronal loss and atrophy in mood disorders
Neuroimaging and post-mortem studies suggest that severe mood disorders such as MDD and BPD are associated with structural and functional impairments related to neuroplasticity in various regions of the CNS. Brain imaging and post-mortem studies show prominent neuronal and glial abnormalities in hippocampal and frontal cortex areas in patients with MDD or BPD, especially those who have experienced multiple episodes (Bielau et al. 2005; MacQueen et al. 2003; Ongur et al. 1998; Rajkowska, 2000, 2002; Rajkowska et al. 2001; Rajkowska & Miguel-Hidalgo, 2007; Sheline, 2000). In addition, decreased gene expression for astrocytic specific proteins, glutamate transporter, glutamine synthesis, and key oligodendrocyte- and myelin-related genes have also been observed in the frontal cortex tissue of patients with MDD or BPD (Choudary et al. 2005; Tkachev et al. 2003; Uranova et al. 2004). Similarly, multiple studies in rodents and non-human primates demonstrate that exposure to stress can alter processes or number of neurons (reviewed in Duman, 2004; Warner-Schmidt & Duman, 2006). Various behavioural stress paradigms or long-term exposure to high levels of glucocorticoids induce neuronal atrophy in hippocampus, decrease glial proliferation and alter glial cell glutamate metabolism (Banasr & Duman, 2008; Cook & Wellman, 2004) (reviewed in Banasr & Duman, 2007; Manji & Duman, 2001). Stress can cause a decrease of up to 25% in the number of glial fibrillary acidic protein (GFAP)-positive cells in hippocampus and this number is highly correlated with reduced hippocampal volume (Czeh et al. 2006).
Impairments of functional synaptic plasticity in mood disorders
While the precise contribution of such perturbations to the network changes in brain function is difficult to infer, it is conceivable that morphological and/or changes in neuronal and glial function related to stress may contribute to the pathophysiology of mood disorders. Hippocampal synaptic plasticity, as modelled by LTP, is widely believed to represent an important component mechanism of hippocampus-dependent memory formation (Malenka, 2003). It is therefore striking that chronic or severe stress has been shown to disrupt hippocampus-dependent memory in experimental animals (reviewed in Sapolsky, 2003). Furthermore, specific impairments of hippocampus-dependent explicit memory are also seen after treating human subjects with glucocorticoids (de Quervain et al. 2000; Newcomer et al. 1999) and after stress (reviewed in Shors, 2006). Several independent studies have demonstrated that sufficiently severe stress can impair LTP and facilitate LTD in the rodent hippocampus (reviewed in Connor & Leonard, 1998; Kim & Diamond, 2002). Stress can affect synaptic plasticity via a variety of mechanisms, including glutamatergic and serotonergic-dependent mechanisms, and glucocorticoid receptor-dependent initiation of transcription and translation (Shakesby et al. 2002; Xu et al. 1998). Consistent with the involvement of these mechanisms in stress modification of plasticity, antagonists of N-methyl-d-aspartate (NMDA) receptors, glucocorticoid receptors, or serotonin uptake enhancers prevent LTP blocking by stress.
Interestingly, multiple clinical and experimental studies indicate that stress and depression are also associated with increased circulating concentrations of TNF-α and IL-1 (reviewed in Connor & Leonard, 1998), which can impair synaptic plasticity and cognitive processes and contribute to progression of depressive disorders. Indeed, patients with MDD exhibit prominent deficits in explicit memory (Zakzanis et al. 1998), a cognitive capacity that depends on the hippocampus and medial temporal lobe (Cavanagh et al. 2002; Clark et al. 2002; Eastwood & Harrison, 2001; Squire et al. 2004). Notably, several signalling pathways involved in neuronal plasticity have been demonstrated to be impaired in patients with mood disorders or in animal models of stress. Decreased expression of molecular markers of synaptic plasticity, including GAP-43, synapsins and synaptophysins have been demonstrated in the post-mortem brains of patients with BPD (Benowitz & Perrone-Bizzozero, 1991; Vawter et al. 2002). Pre-clinical studies have also indicated that the expression of critical molecules involved in the regulation of synaptic plasticity, such as adenosine monophosphate (cAMP) response element-binding protein (CREB), brain-derived neurotrophic factor (BDNF), and Bcl-2 is reduced in response to stress (reviewed in Schloesser et al. 2008; Zarate et al. 2006).
The effect of antidepressants on synaptic plasticity
If the effects of stress or mood disorders on the mechanisms of synaptic plasticity contribute to the pathophysiology of MDD, then antidepressant treatments might be expected to affect the same mechanisms. Indeed, several studies have demonstrated that antidepressants affect LTP in specific brain regions, such as the dentate gyrus and CA1 area of hippocampus. In the dentate gyrus, both chronic electroconvulsive therapy (ECT) and chemical antidepressant treatment increase LTP (Levkovitz et al. 2001; Stewart & Reid, 2000). Recent studies also suggest that chronic administration of a selective serotonin reuptake inhibitor (SSRI) or an atypical antidepressant (tianeptine) increases LTP and blocks the stress-induced impairment of LTP and enhancement of LTD in the CA1 region (Holderbach et al. 2007; Vouimba et al. 2006). Chronic SSRI administration similarly affects hippocampal–prefrontal cortex circuits, reversing stress-induced impairment of LTP and enhancement of LTD (Rocher et al. 2004).
In addition, several studies demonstrate that key signalling components, including glutamatergic receptors, BDNF, and Bcl-2, all of which are important regulators of synaptic plasticity, also serve as major targets for antidepressants and are required for the cellular and behavioural actions of antidepressant treatments. NMDA antagonists, such as MK-801 and AP-7, have antidepressant effects in animal models of depression and in animals exposed to stress (reviewed in Manji et al. 2003). There is also evidence that memantine, a high-affinity NMDA receptor antagonist, has a rapid antidepressant effect in patients with severe depression (Zarate et al. 2006). Pre-clinical studies have also shown that modulation of AMPA receptors by AMPA receptor potentiators (ampakines) enhances mitogen-activated protein kinase (MAPK) activation and BDNF expression, and exerts an antidepresant effect in animal models of depression and in animals exposed to chronic mild stress (reviewed in Manji et al. 2003). Finally, in very preliminary clinical studies, ampakines appear to have beneficial effects on learning and memory (Goff et al. 2001). Furthermore, riluzole and lamotrigine, both of which are glutamatergic modulators with anticonvulsant properties, increase the surface expression of the AMPA subunits GluR1 and GluR2 (Du et al. 2007). Recent studies have shown that riluzole stimulates the synthesis of growth factors including BDNF (Mizuta et al. 2001). Consistent with the evidence that modulating the glutamergic system may be key to the mechanism of antidepressants, one open-label study found that riluzole had significant antidepressant effects in patients with severe depression (Zarate et al. 2004a). In addition, several pre-clinical and clinical studies have implicated neurotrophic factors as targets of standard antidepressant treatment (reviewed in Tanis et al. 2007). Notably, pramipexole, which up-regulates Bcl-2 levels in several brain areas, had antidepressant effects in one double-blind, placebo-controlled trial of patients with bipolar II depression (Zarate et al. 2004b). Together, these studies indicate that chronic antidepressant treatments can regulate intracellular signalling pathways involved in regulating neuroplasticity and reverse impairments of synaptic plasticity and cellular resilience. These changes may be particularly important in understanding the therapeutic effectiveness of these drugs.
Involvement of cytokines in the pathogenesis of depressive disorders
In view of recent data supporting the role of proinflammatory cytokines in the regulation of synaptic plasticity, and emerging data suggesting that synaptic plasticity is impaired in mood disorders, it is conceivable that activation of the immune system network may be related to at least some aspects of the complex pathophysiology of depressive disorders. However, it is beyond the scope of this paper to review in detail the burgeoning literature demonstrating that depressive disorders are pro-inflammatory states. Here we briefly summarize some of the most salient findings; the interested reader is referred to several outstanding papers on the topic (Dantzer et al. 2008; Maes, 1994; McNally et al. 2008; Miller & Raison, 2006; Raison et al. 2006).
Cytokine-induced ‘sickness behaviour’ in animal models
Emerging evidence implicates hyperactivation of the immune system resulting in increased TH1 cytokines in the aetiology of depressive disorders (Maes et al. 1995a, b; Sedgwick & Czerkinsky, 1992). For instance, several animal studies have shown that administration of cytokines, such as IL-1 or activation of macrophages and other inflammatory immune cells by systemic lipopolysaccharide (LPS) treatment, provokes behavioural symptoms collectively referred to as ‘sickness behaviour’ (Dantzer, 2001; Goshen et al. 2008; Kent et al. 1992; Larson & Dunn, 2001; Maier & Watkins, 1998). Motivation and some cognitive functions may also be affected (Dantzer, 2001; Larson & Dunn, 2001). Mice lacking the enzyme required to synthesize IL-1 have reduced ‘sickness behaviour’ and lower expression of neurotoxic and inflammatory mediator genes in the brain after peripheral endotoxin injection (Mastronardi et al. 2007). In addition, deletion of either TNF-α receptor 1 (TNFR1) or TNFR2 genes resulted in antidepressant-like effects (Simen et al. 2006).
Interestingly, chronic stress induced depressive-like symptoms concomitantly with an increase in IL-1 expression in hippocampus, but mice with a deletion of the IL-1 receptor or with restricted overexpression of IL-1 antagonist did not display stress-induced behavioural or neuroendocrine changes (Goshen et al. 2008). Further support for the role of immune system activation in the pathogenesis of depressive disorders comes from studies noting that the antidepressants desipramine and fluoxetine reduce the inflammatory reaction in ovalbumin-sensitized rats in the LPS murine model of autoimmunity (Roumestan et al. 2007).
Psychiatric adverse effects associated with cytokine immunotherapy
Interestingly, the findings from animal studies showing that cytokines play a potential role in the development of depression-like behaviours appear to correlate with clinical studies. There is increasing evidence that immunotherapy with IL-2 or IFN-α is often associated with marked cognitive disturbances and neurovegetative symptoms such as fatigue, sleep disturbances, irritability, appetite suppression, and depressed mood that correlate with elevated serum levels of IFN-α, IL-6, IL-8, and IL-10 (Bonaccorso et al. 2001, 2002; Capuron et al. 2001a, b; Dieperink et al. 2000, 2003). In addition, in healthy human volunteers, depression, anxiety, and memory impairment are associated with immune activation by the bacterial endotoxin LPS, and are correlated with serum IL-1 and TNF-α levels induced by that treatment (Yirmiya et al. 2000).
The incidence of depressive disorders associated with cytokine therapy is highly variable, ranging from 0% to 45% in different studies. The reasons for these variations are probably related to the disease being treated, the cytokine being used and its dose, as well as assessment measures and psychiatric history (de Beaurepaire, 2002). However, in most cases, the depressive symptoms can be treated effectively with antidepressants.
Clinical evidence for immune activation in MDD
Psychological stress is a common risk factor for the development of MDD, and most initial episodes of MDD are preceded by an identifiable stressor (Kendler et al. 2000). Consistent with the notion that stress might provide a link between MDD and inflammation, emerging pre-clinical and clinical evidence indicate that acute and chronic stress elevates levels of proinflammatory cytokines, such as IL-1 and TNF-α and activates their signalling pathways in the periphery and CNS (Deinzer et al. 2004; Goebel et al. 2000; Madrigal et al. 2002; O’Connor et al. 2003). Further support for the cytokine hypothesis comes from clinical studies in patients with MDD and BPD who present with a significant rise in serum levels of proinflammatory cytokines, such as TNF-α, IL-1 IL-6, IL-12, soluble IL-6R, IL-2, soluble IL-2R, IL-1ra, and IFN-α (Hestad et al. 2003; Kim et al. 2007; Kubera et al. 2000; Maes, 1994; O’Brien et al. 2006; Raison et al. 2006; Sluzewska, 1999). Recently, another study demonstrated that, compared to healthy controls, the expression of inflammatory genes – including TNF-α, IL-1, and IL-6 – was increased in the monocytes of a large proportion of individuals with BPD as well as the offspring of BPD patients (Padmos et al. 2008). In addition gene expression microarray studies have shown that several receptors for immune genes, such as interferon α/β receptor, IL-8 receptor, and interferon c-inducible protein 16 (IFI-16) were found to be differentially regulated in the frontal cortex of patients with BPD (Bezchlibnyk et al. 2001; Iwamoto et al. 2004). Interestingly, IFI-16 exerts its immunomodulatory effects through regulation of p53 activity, a key tumour suppressor protein necessary in the signalling cascade activated by TNF-α (Asefa et al. 2004; Hofseth et al. 2004). It is notable that another severe psychiatric disorder, schizophrenia, has also been associated with increased inflammatory response and elevated levels of pro-inflammatory cytokines (reviewed in Muller & Schwarz, 2006).
Recently it has been shown that patients with MDD appear to have an imbalance between pro- and anti-inflammatory cytokines, which can be attenuated following treatment with the antidepressants fluoxetine, sertraline, or paroxetine (Kim et al. 2007; Kubera et al. 2000; Sutcigil et al. 2007; Taler et al. 2007). Other recent studies found that MDD patients with abnormal allelic variants of the genes for IL-1 and TNF-α and higher levels of TNF-α showed a reduced responsiveness to antidepressant treatment (Eller et al. 2008; Fertuzinhos et al. 2004; Jun et al. 2003; Rosa et al. 2004).
A glial–cytokine relationship in MDD
Several pre-clinical and clinical studies also indicate a potential key role for excitotoxicity and microglial activation in the aetiology of MDD. Activation of the immune system has been observed in patients with MDD, resulting in increased levels of circulating pro-inflammatory-cytokines. Specifically, pro-inflammatory cytokines can contribute to glutamate neurotoxicity in multiple ways: (1) directly, via activation of the kynurenine pathway in microglia and increased production of quinolinic acid and glutamate release; (2) indirectly, via decreasing glial glutamate transporter activity leading to reduced glutamate removal from the extracellular space; and (3) by inducing long-term activation of microglia to release TNF-α and IL-1 in a positive feedback manner (reviewed in McNally et al. 2008). For instance, riluzole, a glutamatergic modulator with neuroprotective, plasticity-enhancing, and antidepressant properties, enhances glutamate clearance by astrocytes and prevents decrease in glial metabolism (Banasr & Duman, 2008). Furthermore, antidepressants have been shown to inhibit INF-α-induced microglia production of IL-6 and nitric oxide (Hashioka et al. 2007), suggesting that inhibiting brain inflammation may represent a novel mechanism of action of antidepressants. Inflammation-mediated imbalance of glutamatergic neurotransmission appears to be similarly implicated in schizophrenia (Muller & Schwarz, 2006), suggesting that immune-mediated glutamatergic disturbance might be a component of the pathophysiology of psychiatric illnesses associated with severe cognitive impairments.
Cytokines as potential therapeutic targets in mood disorders
Therapy with standard antidepressants
More direct support for the role of pro-inflammatory cytokines in regulating synaptic plasticity in the pathogenesis of MDD comes from studies wherein antidepressant drugs from two different pharmacological classes induced changes in TNF-α expression and function in the brain. Both acute and chronic treatment with the tricyclic antidepressant (TCA) desipramine depletes neuron-localized TNF-α mRNA and protein in brain regions implicated in mood expression (Ignatowski et al. 1997; Nickola et al. 2001). Recently, it was reported that the SSRIs sertraline and paroxetine inhibited TNF-α secretion, leading to the attenuation of pro-inflammatory activity (Taler et al. 2007, 2008). In addition, it has been shown that administration of the TCAs desipramine and amitriptyline, as well as the SSRI zimelidine decreased TNF-α levels to facilitate norepinephrine release (Reynolds et al. 2005). Furthermore, facilitation of noradrenergic neurotransmission induced by decreased levels of TNF-α in the brain is key to the efficacy of desipramine. This effect appears to be shared by other types of antidepressant drugs; chronic adminstration of the TCA amitriptyline or the SSRI zimelidine transformed TNF-α regulation of norepinephrine release to facilitation, an effect that occurs in association with α2-adrenergic receptor activation (Nickola et al. 2001; Reynolds et al. 2004). Collectively, these data demonstrate that dissimilar antidepressants regulate TNF-α levels in the brain, thus ultimately modifying noradrenergic and possibly serotonergic and dopaminergic neurotransmission, and provide further evidence for the role of TNF-α-induced modulation of synaptic plasticity in the mechanism of antidepressant action.
Anti-cytokine therapy
Both pre-clinical and clinical studies have demonstrated that antidepressants can inhibit the production and/or release of pro-inflammatory cytokines and stimulate the production of anti-inflammatory cytokines, suggesting that reductions in inflammation might contribute to treatment response (Hestad et al. 2003; Kenis & Maes, 2002; Lanquillon et al. 2000; Tuglu et al. 2003). These observations also raise the possibility that inhibiting pro-inflammatory cytokine signalling is a potential strategy for treating depressive disorders, especially in patients with evidence of increased inflammatory activity before therapy, who might be less likely to respond to conventional agents.
Indeed, cytokine antagonists appear to have anti-depressant-like effects, even in the absence of an immune challenge. For example, intracerebroventricular administration of IL-1ra in rodents prevents memory deficits following the psychological stress of social isolation (Pugh et al. 1999), and intracerebroventricularly administered antibodies to TNF-α have antidepressant effects in the forced swim test (Reynolds et al. 2004). In humans, administration of TNF-α blockers such as etanercept (Enbrel®; Amgen, USA) and infliximab (Remicade®; Johnson and Johnson, USA) has been found to attenuate the depressive symptoms that accompany immune system activation in psoriasis (Dantzer, 1999; Krishnan et al. 2007; Tyring et al. 2006; Yirmiya, 2000). In addition, inhibition of the production of pro-inflammatory cytokines, such TNF-α and IL-1 by celexocib induced a rapid antidepressant response and prevented cognitive decline in patients with MDD and BPD (Muller et al. 2006; Nery et al. 2008). Although the putative antidepressant effects of anti-cytokine therapy have not yet been fully illustrated, it is not unreasonable to assume that antagonism of cytokine function may represent a novel target in the treatment of depressive disorders. Alternatively, because it has been shown that imbalance between pro-and anti-inflammatory cytokines might be involved in the pathogenesis of depressive disorders it is possible that anti-inflammatory cytokines with a rather broad spectrum of action (e.g. IL-4 and IL-10) may also be useful anti-cytokine therapies.
Concluding remarks
The present review seeks to bridge the gap between recent findings that implicate both impairments in synaptic plasticity and increased levels of proinflammatory cytokines in patients with mood disorders. As this paper has explored, we propose that cytokine-induced impairments in synaptic plasticity may underlie at least some aspects of the complex pathophysiology of MDD based on the evidence that: (1) elevation of brain cytokine levels is necessary and sufficient to induce depressive symptoms and neuro-endocrine changes in animal models of depression; (2) increased levels of brain cytokines have been shown to impair synaptic plasticity both at morphological and functional levels; and (3) cytokine-induced modulation of neurotransmission and synaptic plasticity plays an important role in the mechanism of antidepressant action and the efficacy of antidepressant treatment.
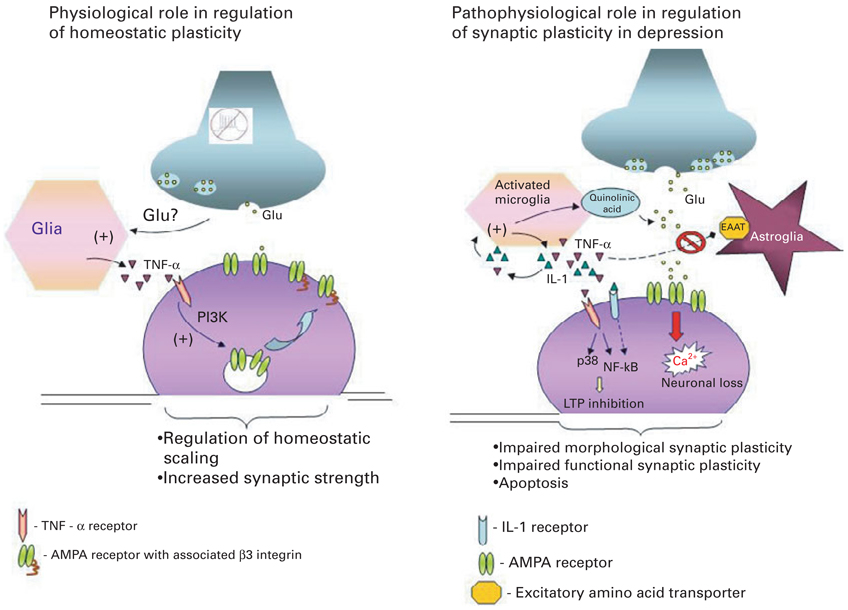
It is important to note that although the increased levels of cytokines seen in patients with MDD are detrimental to neuroplasticity, physiological levels of pro-inflammatory cytokines are essential for normal brain development and homeostatic regulation of synaptic scaling (Avital et al. 2003; Beattie et al. 2002; Goshen et al. 2007, 2008; Stellwagen & Malenka, 2006). These two conflicting pieces of evidence suggest that it is the disturbance of this intricate equilibrium between physiological and pathophysiolgical levels of cytokines in the brain that affects synaptic plasticity and plays a critical role in the pathophysiology of MDD (Fig. 1).
Fig. 1.
Dual role of pro-inflammatory cytokines in regulating synaptic plasticity. The diagram on the left depicts the critical role of constitutively expressed TNF-α in regulation of homeostatic synaptic plasticity in the normal brain. Decreased neuronal activity and consequently reduced glutamate release from axons is sensed by glia, which triggers release of TNF-α. TNF-α activates neuronal TNF-α receptors type I (TNFR1) leading to activation of the phosphoinositide-3 kinase (PI3K) pathway and up-regulation of specific adhesion molecule-β3 integrin, which in turn triggers α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor insertion to the membrane and increases synaptic strength. The diagram on the right depicts the various signalling cascades initiated by high pathophysiological levels of pro-inflammatory cytokines in the brain by activated microglia, which might underlie at least some aspects of the pathophysiology of depression. (1) TNF-α and IL-1 trigger production of quinolinic acid and release of glutamate by microglia; (2) TNF-α and IL-1 inhibit glutamate removal by astrocytes, leading to excess extracellular glutamate and neurotoxicity; (3) TNF-α acts via TNFR1 to up-regulate membrane expression of Ca-permeable AMPA receptor subunits, thus leading to increased Ca2+ influx and neuronal death; (4) TNFR1 activation coupled to activation of p38 and NF-κB pathways inhibits the early and late phases of LTP. These effects of pathophysiological levels of pro-inflammatory cytokines on synaptic plasticity at both morphological and functional levels might underlie the cognitive disturbances and impairments of memory seen in patients with depression.
In the context of this review, it is important to note that despite the accumulating evidence in support of the cytokine hypothesis of MDD, several studies have found only a weak association, or no association, between inflammation and the development of depression when factors such as body mass index, gender, and personality were taken into account (Brambilla & Maggioni, 1998; Carpenter et al. 2004; Miller et al. 2003; Rothermundt et al. 2001). In addition, some otherwise positive studies failed to find a correlation between inflammation and the severity of depressive symptoms (Hestad et al. 2003), or found disparate and occasionally opposing correlations for different pro-inflammatory mediators (Miller et al. 2002, 2005; Pollmacher et al. 2002).
Another issue that remains to be elucidated is whether pro-inflammatory cytokines, released peripherally upon immune system activation, play a causal role in the onset of MDD, or whether they represent an immunological side-effect of this disease. Indeed, due to the associative nature of the studies investigating the relationship between immune activation, cytokines, and MDD it is unclear whether the activation of the immune system observed in depressed patients precedes or follows the onset of depressive disorders (reviewed in Dantzer et al. 2008; Raison et al. 2006). However, the findings that cytokine therapy is often accompanied by adverse psychiatric events, which disappear when cytokine treatment ends or antidepressant treatment begins, suggest a potentially causal role for pro-inflammatory cytokines in the aetiology and pathophysiology of mood disorders; the observation that anti-cytokine treatment produces an antidepressant response in patients with MDD and BPD lends further credence to this notion.
Future research will need to determine the clinical effects of cytokine antagonists on the pathophysiological and psychological features of mood disorders. In order to elucidate the functional role of cytokine-induced alterations of synaptic plasticity in the pathophysiology of MDD, it will also be important to identify the effects of cytokine antagonists and cytokine synthesis inhibitors on neuroplasticity, both at the morphological and functional level. This is a promising field for increasing our understanding of the mechanistic interaction between the immune system, synaptic plasticity, and antidepressants, and for the ultimate development of novel and improved therapeutics for severe mood disorders.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH), Department of Health and Human Services (DHHS). This work was completed under the auspices of the Intramural Program of the NIMH. Dr Manji is now at Johnson and Johnson PRD. We thank Ioline Henter for outstanding editorial assistance.
Footnotes
Statement of Interest
None.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nature Reviews. Immunology. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Aloe L, Properzi F, Probert L, Akassoglou K, Kassiotis G, Micera A, Fiore M. Learning abilities, NGF and BDNF brain levels in two lines of TNF-alpha transgenic mice, one characterized by neurological disorders, the other phenotypically normal. Brain Research. 1999;840:125–137. doi: 10.1016/s0006-8993(99)01748-5. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Interleukin-1 beta production in dysthymia before and after pharmacotherapy. Biological Psychiatry. 1999;46:1649–1655. doi: 10.1016/s0006-3223(99)00211-5. [DOI] [PubMed] [Google Scholar]
- Asefa B, Klarmann KD, Copeland NG, Gilbert DJ, Jenkins NA, Keller JR. The interferon-inducible p200 family of proteins: a perspective on their roles in cell cycle regulation and differentiation. Blood Cells, Molecules and Diseases. 2004;32:155–167. doi: 10.1016/j.bcmd.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB Journal. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS and Neurological Disorders Drug Targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biological Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behavioural Brain Research. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Bartee E, Mohamed MR, McFadden G. Tumor necrosis factor and interferon: cytokines in harmony. Current Opinion in Microbiology. 2008;11:378–383. doi: 10.1016/j.mib.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Research. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Perrone-Bizzozero NI. The relationship of GAP-43 to the development and plasticity of synaptic connections. Annals of the New York Academy of Sciences. 1991;627:58–74. doi: 10.1111/j.1749-6632.1991.tb25914.x. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Cytokine actions in the central nervous system. Cytokine & Growth Factor Reviews. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk YB, Wang JF, McQueen GM, Young LT. Gene expression differences in bipolar disorder revealed by cDNA array analysis of post-mortem frontal cortex. Journal of Neurochemistry. 2001;79:826–834. doi: 10.1046/j.1471-4159.2001.00628.x. [DOI] [PubMed] [Google Scholar]
- Bielau H, Trubner K, Krell D, Agelink MW, Bernstein HG, Stauch R, Mawrin C, Danos P, Gerhard L, Bogerts B, Baumann B. Volume deficits of subcortical nuclei in mood disorders A postmortem study. European Archives of Psychiatry and Neurological Sciences. 2005;255:401–412. doi: 10.1007/s00406-005-0581-y. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neuroscience Letters. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. Journal of Affective Disorders. 2002;72:237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M, Almerighi C, Levrero M, Egyed B, Bosmans E, Meltzer HY, Maes M. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Research. 2001;105:45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatrica Scandinavica. 1998;97:309–313. doi: 10.1111/j.1600-0447.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB. Distribution and characterization of tumor necrosis factor-alpha-like immunoreactivity in the murine central nervous system. Journal of Comparative Neurology. 1993;337:543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Beck KD, Servatius RJ. Low doses of interleukin-1beta improve the leverpress avoidance performance of Sprague-Dawley rats. Neurobiology of Learning and Memory. 2003;80:168–171. doi: 10.1016/s1074-7427(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–326. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosomatic Medicine. 2001a;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001b;26:797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends in Neurosciences. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Heninger GR, Malison RT, Tyrka AR, Price LH. Cerebrospinal fluid interleukin (IL)-6 in unipolar major depression. Journal of Affective Disorders. 2004;79:285–289. doi: 10.1016/S0165-0327(02)00460-3. [DOI] [PubMed] [Google Scholar]
- Cavanagh JT, Van Beck M, Muir W, Blackwood DH. Case-control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. British Journal of Psychiatry. 2002;180:320–326. doi: 10.1192/bjp.180.4.320. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proceedings of the National Academy of Sciences USA. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. Journal of Immunology. 1990;144:2999–3007. [PubMed] [Google Scholar]
- Churchill L, Rector DM, Yasuda K, Fix C, Rojas MJ, Yasuda T, Krueger JM. Tumor necrosis factor alpha: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Zola SM, Squire LR. Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. Journal of Neuroscience. 2002;22:4663–4669. doi: 10.1523/JNEUROSCI.22-11-04663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor TJ, Leonard BE. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sciences. 1998;62:583–606. doi: 10.1016/s0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- Coogan AN, O’Neill LA, O’Connor JJ. The P38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1beta on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neuroscience Letters. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Wada E, Carter DB, Tracey DE, Battey JF, De Souza EB. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. Journal of Neuroscience. 1992;12:1101–1114. doi: 10.1523/JNEUROSCI.12-03-01101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran B, O’Connor JJ. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience. 2001;108:83–90. doi: 10.1016/s0306-4522(01)00405-5. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Tancredi V, Onofri F, D’Antuono M, Giovedi S, Benfenati F. Interleukin-6 inhibits neurotransmitter release and the spread of excitation in the rat cerebral cortex. European Journal of Neuroscience. 2000;12:1241–1252. doi: 10.1046/j.1460-9568.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokines, Stress and Depression. New York: Kluwer Academic/Plenum Publishers; 1999. Mechanisms of the behavioral effects of cytokines. [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Annals of the New York Academy of Sciences. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaurepaire R. Questions raised by the cytokine hypothesis of depression. Brain, Behavior, and Immunity. 2002;16:610–617. doi: 10.1016/s0889-1591(02)00005-3. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Deinzer R, Granrath N, Stuhl H, Twork L, Idel H, Waschul B, Herforth A. Acute stress effects on local Il-1beta responses to pathogens in a human in vivo model. Brain, Behavior, and Immunity. 2004;18:458–467. doi: 10.1016/j.bbi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: A review. American Journal of Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- Du J, Creson TK, Wu LJ, Ren M, Gray NA, Falke C, Wei Y, Wang Y, Blumenthal R, Machado-Vieira R, et al. The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. Journal of Neuroscience. 2008;28:68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, Einat H, Manji HK. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. Journal of Neuroscience. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Jr, Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biological Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Research Bulletin. 2001;55:569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Fahey B, Barlow S, Day JS, O’Mara SM. Interferon-alpha-induced deficits in novel object recognition are rescued by chronic exercise. Physiology & Behavior. 2008;95:125–129. doi: 10.1016/j.physbeh.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos SM, Oliveira JR, Nishimura AL, Pontual D, Carvalho DR, Sougey EB, Otto PA, Zatz M. Analysis of IL-1alpha, IL-1beta, and IL-1RA [correction of IL-RA] polymorphisms in dysthymia. Journal of Molecular Neuroscience. 2004;22:251–256. doi: 10.1385/jmn:22:3:251. [DOI] [PubMed] [Google Scholar]
- Fontana A, Weber E, Dayer JM. Synthesis of interleukin 1/endogenous pyrogen in the brain of endotoxin-treated mice: a step in fever induction? Journal of Immunology. 1984;133:1696–1698. [PubMed] [Google Scholar]
- Friedman WJ. Cytokines regulate expression of the type 1 interleukin-1 receptor in rat hippocampal neurons and glia. Experimental Neurology. 2001;168:23–31. doi: 10.1006/exnr.2000.7595. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ. Immune system dysregulation in adolescent major depressive disorder. Journal of Affective Disorders. 2008 doi: 10.1016/j.jad.2008.07.022. Published online 13 September 2008. doi:10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellec MM, Griffais R, Fillion G, Haour F. Interleukin-1 receptors type I and type II in the mouse brain: kinetics of mRNA expressions after peripheral administration of bacterial lipopolysaccharide. Journal of Neuroimmunology. 1996;66:65–70. doi: 10.1016/0165-5728(96)00021-5. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain Research. 1994;637:10–14. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Flynn MC, Plata-Salaman CR. Lipopolysaccharide (LPS)- and muramyl dipeptide (MDP)-induced anorexia during refeeding following acute fasting: characterization of brain cytokine and neuropeptide systems mRNAs. Brain Research. 1998;795:77–86. doi: 10.1016/s0006-8993(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain, Behavior, and Immunity. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Mills PJ, Irwin MR, Ziegler MG. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosomatic Medicine. 2000;62:591–598. doi: 10.1097/00006842-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, Johnson SA, Lynch G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. Journal of Clinical Psychopharmacology. 2001;21:484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Golan H, Levav T, Mendelsohn A, Huleihel M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cerebral Cortex. 2004;14:97–105. doi: 10.1093/cercor/bhg108. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Molecular Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Grilli M, Memo M. Nuclear factor-kappaB/Rel proteins: a point of convergence of signalling pathways relevant in neuronal function and dysfunction. Biochemical Pharmacology. 1999;57:1–7. doi: 10.1016/s0006-2952(98)00214-7. [DOI] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, Monji A, Kato T, Sawada M, McGeer PL, Kanba S. Antidepressants inhibit interferon-gamma-induced microglial production of IL-6 and nitric oxide. Experimental Neurology. 2007;206:33–42. doi: 10.1016/j.expneurol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochemical Journal. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad KA, Tonseth S, Stoen CD, Ueland T, Aukrust P. Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. Journal of ECT. 2003;19:183–188. doi: 10.1097/00124509-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proceedings of the National Academy of Sciences USA. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofseth LJ, Hussain SP, Harris CC. p53: 25 years after its discovery. Trends in Pharmacological Sciences. 2004;25:177–181. doi: 10.1016/j.tips.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Holderbach R, Clark K, Moreau JL, Bischofberger J, Normann C. Enhanced long-term synaptic depression in an animal model of depression. Biological Psychiatry. 2007;62:92–100. doi: 10.1016/j.biopsych.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Holmin S, Schalling M, Hojeberg B, Nordqvist AC, Skeftruna AK, Mathiesen T. Delayed cytokine expression in rat brain following experimental contusion. Journal of Neurosurgery. 1997;86:493–504. doi: 10.3171/jns.1997.86.3.0493. [DOI] [PubMed] [Google Scholar]
- Hunt JS, Chen HL, Hu XL, Chen TY, Morrison DC. Tumor necrosis factor-alpha gene expression in the tissues of normal mice. Cytokine. 1992;4:340–346. doi: 10.1016/1043-4666(92)90076-4. [DOI] [PubMed] [Google Scholar]
- Ignatowski TA, Noble BK, Wright JR, Gorfien JL, Heffner RR, Spengler RN. Neuronal-associated tumor necrosis factor (TNF alpha): its role in noradrenergic functioning and modification of its expression following antidepressant drug administration. Journal of Neuroimmunology. 1997;79:84–90. doi: 10.1016/s0165-5728(97)00107-0. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Kakiuchi C, Bundo M, Ikeda K, Kato T. Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders. Molecular Psychiatry. 2004;9:406–416. doi: 10.1038/sj.mp.4001437. [DOI] [PubMed] [Google Scholar]
- Jun TY, Pae CU, Hoon H, Chae JH, Bahk WM, Kim KS, Serretti A. Possible association between-G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean population. Psychiatric Genetics. 2003;13:179–181. doi: 10.1097/00041444-200309000-00008. [DOI] [PubMed] [Google Scholar]
- Kendell SF, Krystal JH, Sanacora G. GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opinion on Therapeutic Targets. 2005;9:153–168. doi: 10.1517/14728222.9.1.153. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the ‘kindling’ hypothesis. American Journal of Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. International Journal of Neuropsychopharmacology. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends in Pharmacological Sciences. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2007;31:1044–1053. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Cella D, Leonardi C, Papp K, Gottlieb AB, Dunn M, Chiou CF, Patel V, Jahreis A. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. British Journal of Dermatology. 2007;157:1275–1277. doi: 10.1111/j.1365-2133.2007.08205.x. [DOI] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Zieba A, Dudek D, Nowak G, Maes M. Plasma levels of interleukin-6, interleukin-10, and interleukin-1 receptor antagonist in depression: comparison between the acute state and after remission. Polish Journal of Pharmacology. 2000;52:237–241. [PubMed] [Google Scholar]
- Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. Journal of Neurochemistry. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain, Behavior, and Immunity. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Grisaru N, Segal M. Transcranial magnetic stimulation and antidepressive drugs share similar cellular effects in rat hippocampus. Neuropsychopharmacology. 2001;24:608–616. doi: 10.1016/S0893-133X(00)00244-X. [DOI] [PubMed] [Google Scholar]
- Li H, Gade P, Xiao W, Kalvakolanu DV. The interferon signaling network and transcription factor C/EBP-beta. Cellular & Molecular Immunology. 2007;4:407–418. [PMC free article] [PubMed] [Google Scholar]
- Licinio J. Central nervous system cytokines and their relevance for neurotoxicity and apoptosis. Journal of Neural Transmission (Suppl.) 1997;49:169–175. doi: 10.1007/978-3-7091-6844-8_18. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proceedings of the National Academy of Sciences USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Maes M. Cytokines in major depression. Biological Psychiatry. 1994;36:498–499. doi: 10.1016/0006-3223(94)90652-1. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, Desnyder R. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. Journal of Affective Disorders. 1995a;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995b;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- Maes M, Van Bockstaele DR, Gastel A, Song C, Schotte C, Neels H, DeMeester I, Scharpe S, Janca A. The effects of psychological stress on leukocyte subset distribution in humans: evidence of immune activation. Neuropsychobiology. 1999;39:1–9. doi: 10.1159/000026552. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Research. 1995;695:279–282. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Malenka RC. The long-term potential of LTP. Nature Reviews. Neuroscience. 2003;4:923–926. doi: 10.1038/nrn1258. [DOI] [PubMed] [Google Scholar]
- Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacology Bulletin. 2001;35:5–49. [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff KD, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biological Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Mastronardi C, Whelan F, Yildiz OA, Hannestad J, Elashoff D, McCann SM, Licinio J, Wong ML. Caspase 1 deficiency reduces inflammation-induced brain transcription. Proceedings of the National Academy of Sciences USA. 2007;104:7205–7210. doi: 10.1073/pnas.0701366104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectrums. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- Medana IM, Hunt NH, Chaudhri G. Tumor necrosis factor-alpha expression in the brain during fatal murine cerebral malaria: evidence for production by microglia and astrocytes. American Journal of Pathology. 1997;150:1473–1486. [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C. Interferon-alpha inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain Research. 2000;885:14–24. doi: 10.1016/s0006-8993(00)02877-8. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL. Cytokines, p38 MAP kinase and the pathophysiology of depression. Neuropsychopharmacology. 2006;31:2089–2090. doi: 10.1038/sj.npp.1301032. [DOI] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain, Behavior, and Immunity. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosomatic Medicine. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. American Journal of Cardiology. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Kuno S. Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neuroscience Letters. 2001;310:117–120. doi: 10.1016/s0304-3940(01)02098-5. [DOI] [PubMed] [Google Scholar]
- Muller N, Ackenheil M. Psychoneuroimmunology and the cytokine action in the CNS: implications for psychiatric disorders. Progress in Neuropsychopharmacology and Biological Psychiatry. 1998;22:1–33. doi: 10.1016/s0278-5846(97)00179-6. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotoxicity Research. 2006;10:131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Molecular Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. Journal of Neuroscience. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint AM, O’Mahony S, Kubera M, Kim YK, Kenny C, Kaim-Basta A, Steinbusch HW, Leonard BE. Role of paroxetine in interferon-alpha-induced immune and behavioural changes in male Wistar rats. Journal of Psychopharmacology. 2007;21:843–850. doi: 10.1177/0269881107077165. [DOI] [PubMed] [Google Scholar]
- Nassberger L, Traskman-Bendz L. Increased soluble interleukin-2 receptor concentrations in suicide attempters. Acta Psychiatrica Scandinavica. 1993;88:48–52. doi: 10.1111/j.1600-0447.1993.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, Bowden CL, Soares JC. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Human Psychopharmacology. 2008;23:87–94. doi: 10.1002/hup.912. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Archives of General Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Nickola TJ, Ignatowski TA, Reynolds JL, Spengler RN. Antidepressant drug-induced alterations in neuron-localized tumor necrosis factor-alpha mRNA and alpha(2)-adrenergic receptor sensitivity. Journal of Pharmacology and Experimental Therapeutics. 2001;297:680–687. [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder : focus on acutely ill patients. Journal of Affective Disorders. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Research. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schobitz B, de Kloet ER. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Research. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmos RC, Hillegers MH, Knijff EM, Vonk R, Bouvy A, Staal FJ, de Ridder D, Kupka RW, Nolen WA, Drexhage HA. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Archives of General Psychiatry. 2008;65:395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- Parnet P, Amindari S, Wu C, Brunke-Reese D, Goujon E, Weyhenmeyer JA, Dantzer R, Kelley KW. Expression of type I and type II interleukin-1 receptors in mouse brain. Brain research. Molecular Brain Research. 1994;27:63–70. doi: 10.1016/0169-328x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Pickering M, Cumiskey D, O’Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Experimental Physiology. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Pitossi F, del Rey A, Kabiersch A, Besedovsky H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. Journal of Neuroscience Research. 1997;48:287–298. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Sarnico I, Boroni F, Benarese M, Dreano M, Garotta G, Valerio A, Spano P. Prevention of neuron and oligodendrocyte degeneration by interleukin-6 (IL-6) and IL-6 receptor/IL-6 fusion protein in organotypic hippocampal slices. Molecular and Cellular Neurosciences. 2004;25:301–311. doi: 10.1016/j.mcn.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines - do they affect human brain functions? Brain, Behavior, and Immunity. 2002;16:525–532. doi: 10.1016/s0889-1591(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Johnson JD, Martin D, Rudy JW, Maier SF, Watkins LR. Human immunodeficiency virus-1 coat protein gp120 impairs contextual fear conditioning: a potential role in AIDS related learning and memory impairments. Brain Research. 2000;861:8–15. doi: 10.1016/s0006-8993(99)02445-2. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behavioural Brain Research. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biological Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Cell pathology in mood disorders. Seminars in Clinical Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS & Neurological Disorders Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V, Belova N, Kamat A, Lyson K, Gimeno M, McCann SM. Blockade by interleukin-1-alpha of nitricoxidergic control of luteinizing hormone-releasing hormone release in vivo and in vitro. Neuroimmunomodulation. 1994;1:86–91. doi: 10.1159/000097095. [DOI] [PubMed] [Google Scholar]
- Reynolds JL, Ignatowski TA, Sud R, Spengler RN. Brain-derived tumor necrosis factor-alpha and its involvement in noradrenergic neuron functioning involved in the mechanism of action of an antidepressant. Journal of Pharmacology and Experimental Therapeutics. 2004;310:1216–1225. doi: 10.1124/jpet.104.067835. [DOI] [PubMed] [Google Scholar]
- Reynolds JL, Ignatowski TA, Sud R, Spengler RN. An antidepressant mechanism of desipramine is to decrease tumor necrosis factor-alpha production culminating in increases in noradrenergic neurotransmission. Neuroscience. 2005;133:519–531. doi: 10.1016/j.neuroscience.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proceedings of the Society for Experimental Biology and Medicine. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cerebral Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Rosa A, Peralta V, Papiol S, Cuesta MJ, Serrano F, Martinez-Larrea A, Fananas L. Interleukin-1beta (IL-1beta) gene and increased risk for the depressive symptom-dimension in schizophrenia spectrum disorders. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2004;124B:10–14. doi: 10.1002/ajmg.b.20074. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Fenker J, Gutbrodt H, Peters M, Kirchner H. Different immune patterns in melancholic and non-melancholic major depression. European Archives of Psychiatry and Clinical Neuroscience. 2001;251:90–97. doi: 10.1007/s004060170058. [DOI] [PubMed] [Google Scholar]
- Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, Jaffuel D, Mathieu M. Anti-inflammatory properties of desipramine and fluoxetine. Respiratory Research. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochemical Research. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:110–133. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proceedings of the National Academy of Sciences USA. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobitz B, Van Den Dobbelsteen M, Holsboer F, Sutanto W, De Kloet ER. Regulation of interleukin 6 gene expression in rat. Endocrinology. 1993;132:1569–1576. doi: 10.1210/endo.132.4.8462455. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Czerkinsky C. Detection of cell-surface molecules, secreted products of single cells and cellular proliferation by enzyme immunoassay. Journal of Immunological Methods. 1992;150:159–175. doi: 10.1016/0022-1759(92)90075-5. [DOI] [PubMed] [Google Scholar]
- Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. Journal of Neuroscience. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biological Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Significant life events and the shape of memories to come: a hypothesis. Neurobiology of Learning and Memory. 2006;85:103–115. doi: 10.1016/j.nlm.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biological Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Sluzewska A. Indicators of immune activation in depressed patients. Advances in Experimental Medicine and Biology. 1999;461:59–73. doi: 10.1007/978-0-585-37970-8_4. [DOI] [PubMed] [Google Scholar]
- Song C, Phillips AG, Leonard B. Interleukin 1 beta enhances conditioned fear memory in rats: possible involvement of glucocorticoids. European Journal of Neuroscience. 2003;18:1739–1743. doi: 10.1046/j.1460-9568.2003.02886.x. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. Journal of Neuroscience. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology. 2000;148:217–223. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. Journal of Neuroscience. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clinical and Developmental Immunology 2007. 2007:26438. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taler M, Bar M, Korob I, Lomnitski L, Baharav E, Grunbaum-Novak N, Weizman A, Gil-Ad I. Evidence for an inhibitory immunomodulatory effect of selected antidepressants on rat splenocytes: possible relevance to depression and hyperactive-immune disorders. International Immunopharmacology. 2008;8:526–533. doi: 10.1016/j.intimp.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Taler M, Gil-Ad I, Lomnitski L, Korov I, Baharav E, Bar M, Zolokov A, Weizman A. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. European Neuropsychopharmacology. 2007;17:774–780. doi: 10.1016/j.euroneuro.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D’Antuono M, Cafe C, Giovedi S, Bue MC, D’Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. Journal of Neurochemistry. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D’Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, Eusebi F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neuroscience Letters. 1992;146:176–178. doi: 10.1016/0304-3940(92)90071-e. [DOI] [PubMed] [Google Scholar]
- Tanis KQ, Newton SS, Duman RS. Targeting neurotrophic/growth factor expression and signaling for antidepressant drug development. CNS & Neurological Disorders Drug Targets. 2007;6:151–160. doi: 10.2174/187152707780363276. [DOI] [PubMed] [Google Scholar]
- Tchelingerian JL, Quinonero J, Booss J, Jacque C. Localization of TNF alpha and IL-1 alpha immunoreactivities in striatal neurons after surgical injury to the hippocampus. Neuron. 1993;10:213–224. doi: 10.1016/0896-6273(93)90312-f. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurological Research. 2005;27:679–684. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Chen CC, Bai CH, Wu SR. Cytokines and serotonin transporter in patients with major depression. Progress in Neuropsychopharmacology and Biological Psychiatry. 2006;30:899–905. doi: 10.1016/j.pnpbp.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Pitossi FJ, Lebrun JJ, Lee S, Meltzer JC, Nance DM, del Rey A, Besedovsky HO, Rivier C. Inhibition of tumor necrosis factor-alpha action within the CNS markedly reduces the plasma adrenocorticotropin response to peripheral local inflammation in rats. Journal of Neuroscience. 1997;17:3262–3273. doi: 10.1523/JNEUROSCI.17-09-03262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis : double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophrenia Research. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- van Dam AM, Poole S, Schultzberg M, Zavala F, Tilders FJ. Effects of peripheral administration of LPS on the expression of immunoreactive interleukin-1 alpha, beta, and receptor antagonist in rat brain. Annals of the New York Academy of Sciences. 1998;840:128–138. doi: 10.1111/j.1749-6632.1998.tb09557.x. [DOI] [PubMed] [Google Scholar]
- Van Wagoner NJ, Oh JW, Repovic P, Benveniste EN. Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. Journal of Neuroscience. 1999;19:5236–5244. doi: 10.1523/JNEUROSCI.19-13-05236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Thatcher L, Usen N, Hyde TM, Kleinman JE, Freed WJ. Reduction of synapsin in the hippocampus of patients with bipolar disorder and schizophrenia. Molecular Psychiatry. 2002;7:571–578. doi: 10.1038/sj.mp.4001158. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Owe-Larsson B, Brask J, Kristensson KS, Hill RH. Interferon-gamma-induced changes in synaptic activity and AMPA receptor clustering in hippocampal cultures. Brain Research. 2001;896:18–29. doi: 10.1016/s0006-8993(00)03238-8. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C. ‘Inflammatory’ cytokines: neuromodulators in normal brain? Journal of Neurochemistry. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5:375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47 Suppl. 1:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proceedings of the National Academy of Sciences USA. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara O, Matsuo A, Terai K, Walker DG, Berger AE, Akiguchi I, Kimura J, McGeer PL. Expression of interleukin-1 receptor antagonist protein in post-mortem human brain tissues of Alzheimer’s disease and control cases. Acta Neuropathologica. 1997;93:414–420. doi: 10.1007/s004010050633. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Depression in medical illness: the role of the immune system. Western Journal of Medicine. 2000;173:333–336. doi: 10.1136/ewjm.173.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmacher T. Illness, cytokines, and depression. Annals of the New York Academy of Sciences. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiology of Learning and Memory. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1998;11:111–119. [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK. An open-label trial of riluzole in patients with treatment-resistant major depression. American Journal of Psychiatry. 2004a;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, Charney DS, Manji HK. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biological Psychiatry. 2004b;56:54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biological Psychiatry. 2006;59:1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]