Abstract
Gamma-aminobutyric acid (GABA) is predominantly released by local interneurons in the cerebral cortex to particular subcellular domains of the target cells1,2. This suggests that compartmentalized, synapse specific action of GABA is required in cortical networks for phasic inhibition2–4. However, GABA released at the synaptic cleft diffuses to receptors outside the postsynaptic density and thus tonically activates extrasynaptic GABAA and GABAB receptors, which include subtypes of both receptor families especially sensitive to low concentrations of GABA3–7. The synaptic and extrasynaptic action of GABA is in line with idea that neurons of the brain use synaptic (or wiring) transmission and nonsynaptic (or volume) transmission for communication8,9. However, reuptake mechanisms restrict the spatial extent of extrasynaptic GABAergic effects10,11 and it was proposed that concerted action of several presynaptic interneurons or sustained firing of individual cells or increased release site density is required to reach ambient GABA levels sufficient to activate extrasynaptic receptors4,9,11–13. Here we show that individual neurogliaform cells release GABA sufficient for volume transmission within the axonal cloud and thus neurogliaform cells do not require synapses to produce inhibitory responses in the overwhelming majority of nearby neurons. Neurogliaform cells suppress connections between other neurons acting on presynaptic terminals which do not receive synapses at all in the cerebral cortex and, moreover, reach extrasynaptic, δ subunit containing GABAA (GABAAδ) receptors responsible for tonic inhibition. We reveal that GABAAδ receptors are localized to neurogliaform cells preferentially among cortical interneurons. Neurosteroids, which are modulators of GABAAδ receptors, alter unitary GABAergic effects between neurogliaform cells. In contrast to the specifically placed synapses formed by other interneurons, the output of neurosteroid sensitive neurogliaform cells represents the ultimate form of spatial unspecificity in GABAergic systems leading to long lasting network hyperpolarization combined with widespread suppression of communication in the local circuit.
Uniquely among neocortical interneurons, neurogliaform cells evoke long-lasting inhibition composed of an unusually slow GABAA receptor-mediated component and GABAB receptor mediated responses in their target neurons14–16. A distinctive feature of neurogliaform cells among cortical interneurons is the very dense axonal arborization in which presynaptic boutons on the same or neighboring collaterals can be found a couple micrometers from one another (Fig. 1)14,17. GABA can activate receptors located up to several micrometers from the release site11. We measured the density of terminals in neurogliaform (n=8) vs. basket cell (n=6) axons (421213±34289 and 78506±8423 boutons/mm3, respectively, p<0.0001) and found that a single neurogliaform axon matches the potential release site density of 5 to 6 overlapping basket cell axons. We hypothesized that the high density of neurogliaform axons could counteract transmitter reuptake mechanisms and GABA liberated from neurogliaform cells acts as volume transmitter to reach receptors placed at synaptic and nonsynaptic sites in the tissue intermingled by the axon.
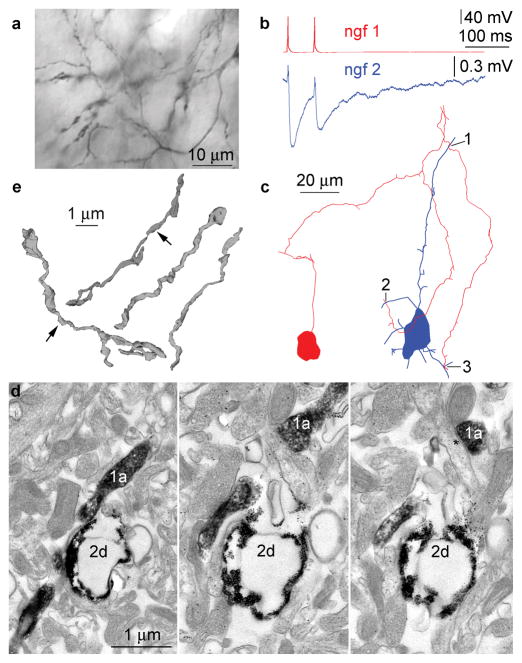
Figure 1.
Neurogliaform cells do not require direct synaptic junctions to elicit an effect on target cells. (a) Dense axonal cloud formed by a single neurogliaform cell. (b) Action potentials in neurogliaform cell 1 (ngf1, red) elicited electrical coupling potentials combined with IPSPs in neurogliaform cell 2 (ngf2, blue). (c) Route of the axon of neurogliaform cell 1 (red) cell to close appositions (1–3) with the dendrites of the neurogliaform cell 2 (blue). None of these appositions could be verified as synaptic junctions. (d) A nonsynaptic close apposition (1 on panel b) in consecutive serial ultrathin sections (1a, axon of ngf1, 2d dendrite of ngf2). The axon of neurogliaform cell 1 forms a synaptic junction (asterisk) on an unlabeled dendritic shaft. (e) Three dimensional electron microscopic reconstructions of 18 neurogliaform axonal varicosities of which only two formed synaptic junctions (arrows).
Potential volume transmission suggests a very high rate of functional coupling between neurogliaform cells and neighboring neurons. Indeed, when searching our database containing 204 simultaneously recorded pairs of neurogliaform cells and other neurons with somata located <100μM apart, we detected hyperpolarizing effects of neurogliaform cells in 178 (87%) of tested cells, a ratio unprecedented in paired recordings of cortical neurons18. In search for the morphological correlates of these connections, correlated light and electron microscopic analysis was performed on n=11 neurogliaform to interneuron pairs (n=8 rat, of which n=3 was included in19; n=3 human) and, in addition, neurogliaform to pyramid cell pairs (n=5 rat). We detected chemical synaptic junctions in only two neurogliaform to pyramid pairs supporting earlier results14, but assessment of fully available series of ultrathin sections did not encounter synaptic junctions in the remaining n=14 cell pairs when tracing neurogliaform axons along their approach to functionally coupled neurons. In these fourteen pairs, the most closely placed synapses established by the boutons of the neurogliaform cells were 2.7±1.6μM (range, 1.1–5.3μM) from the target dendrites (Fig. 1). Proving the efficacy our analysis, we confirmed the presence of gap junctions in all three pairs (presented in19) in which electrical coupling potentials were recorded in addition to IPSPs, even though ultrastructural identification of gap junctions between labeled neurons is more difficult than that of synapses (Fig. 1). Furthermore, we performed three-dimensional electron microscopic reconstructions of randomly chosen segments of neurogliaform axons. All examined axonal boutons contained synaptic vesicles, but we found only 11 synapses formed by 50 boutons suggesting that the majority (~78%) of neurogliaform axonal varicosities do not form classical synapses (Fig. 1). These results suggest that neurogliaform axons do not necessarily require a synaptic contact for eliciting inhibitory responses in target cells.
Although suspected nonsynaptic communication by neurogliaform cells is consistent with the lack of detectable synapses, positive evidence is required to prove the volume transmission hypothesis. Functional testing of single cell initiated nonsynaptic signaling is best on potential targets not contacted by synapses at all. Thus, we asked whether GABA released from neurogliaform cells, modulates axon terminals which do not receive synaptic junctions in the cerebral cortex20 but frequently express GABAB receptors13,16,21–23. We confirmed that neocortical neurogliaform cells modulate their own axon terminals via GABAB receptors similar to hippocampal neurogliaform cells16 (Supplementary Fig. 1 and data), but the modulatory action of neurogliaform cells was not limited to homosynaptic silencing of axon terminals. Heterosynaptic or paracrine effects of neurogliaform cells on axons of other neurons were also suggested by experiments in which we simultaneously recorded from three neurons consisting of a pyramidal cell to an interneuron connection (test EPSPs) and a neighboring neurogliaform cell activated 60 and 120 ms before the first and second test EPSP, respectively (Fig. 2). Alternating trials with and without the activation of neurogliaform cells were recorded at a very low frequency (once in every two minutes) to avoid activity dependent loss of neurogliaform cell output14–16. As expected from the high rate of coupling from neurogliaform cells to other neurons, the decay phase of the hyperpolarizing effect of the neurogliaform cells overlapped with the test EPSPs and corresponding input resistance changes of the neurons postsynaptic to the EPSPs were 10±5% and 4±5% measured at 60 and 120 ms after the spike in the neurogliaform cells, respectively. Accordingly, amplitudes of EPSPs and IPSPs reported below were corrected with the corresponding changes in input resistance and driving force. Switching on the action potential in the neurogliaform cells 60 ms before the spike in the pyramidal cell could not change the amplitude of the first test EPSPs (n=5; 98±4%) relative to control, i.e. when the spike in the neurogliaform cell was not elicited (Fig. 2). This indicates that tonic inhibition through GABAA receptors potentially activated by neurogliaform cells15,24 did not interfere significantly with test EPSPs apart from contributing to input resistance changes. However, the neurogliaform cells were effective in decreasing the amplitude of the second test EPSPs timed 120 ms after the spike in the neurogliaform cells to 77±5% (p<0.03) of control (Fig. 2). The differential action of neurogliaform cell output on the first and the second test EPSPs induced changes in their paired pulse ratio (from 85±20 to 69±22%, p<0.01). Moreover, neurogliaform cells, activated 120 ms prior to test IPSPs triggered by other neurogliaform cells, successfully suppressed the amplitude of test IPSPs to 74±4% (n=10; p<0.02) of control (Fig. 2). This resulted in a peculiar activation sequence dependent cross modulation of IPSP amplitudes between closely located neurogliaform cells. Thus, the experiments on test EPSPs and IPSPs showed that individual action potentials in neurogliaform cells could suppress appropriately delayed responses elicited by other neurons and these effects were enhanced by the GABA reuptake blocker NO-711 (Supplementary Fig. 2. and data). We also asked if basket cells could modulate the surrounding microcircuit similar to neurogliaform cells, but single or multiple spikes in these interneurons failed to modulate the amplitude of test connections (Supplementary Fig. 3 and data). Furthermore, we found that GABAB receptors located on presynaptic terminals25 are required and sufficient for the neurogliaform cell elicited heterosynaptic suppression of test connections and supplementary anatomical analysis showed that the modulatory effect of GABA released from neurogliaform cells appears confined to the axonal cloud (Supplementary Fig. 4 and data).
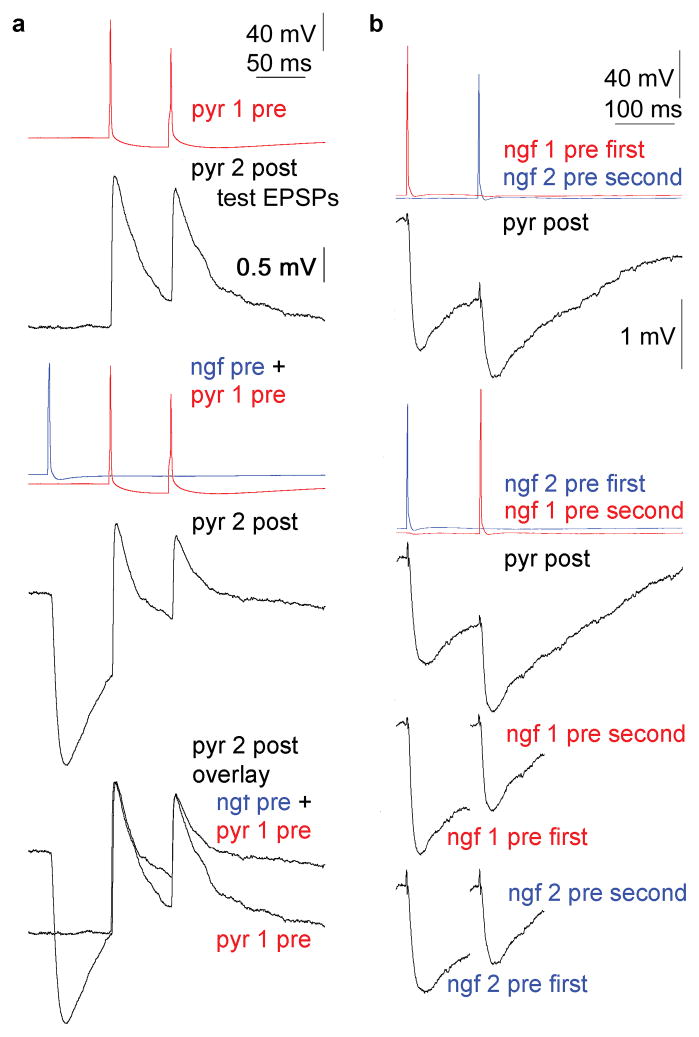
Figure 2.
(a) Single neurogliaform cells heterosynaptically modulate unitary glutamatergic connections linking other neurons. Simultaneous triple recording showing a pyramidal cell 1 (pyr1) to pyramidal cell 1 (pyr2) connection (test EPSPs) while switching the output of a neurogliaform cell on and off 60 ms before the first pyramidal spike. The neurogliaform cell suppressed the amplitude of the second, but not the first EPSPs evoked by pyramidal cell 1. (b) Activation sequence dependent cross modulation of unitary IPSP amplitudes between closely located neurogliaform cells. Top, Activation of neurogliaform cell 1 or 2 (ngf1, ngf2) followed by a spike in neurogliaform cell 2 or 1 resulted in sequential IPSPs in the postsynaptic pyramid (pyr). Bottom, Comparison of the amplitude of preceding and following IPSPs elicited by neurogliaform cell 1 or 2 indicates effective suppression of follower IPSPs.
Apart from presynaptic GABAB receptors, extrasynaptically placed GABAAδ receptors3–7 are potential targets for nonsynaptically acting GABA liberated by neurogliaform cells. Tonic inhibition mediated by GABAAδ receptors is variably present on cortical neurons26, thus, we applied immunocytochemistry which showed that neurogliaform cells are primary candidates among cortical interneurons for GABAAδ receptor expression (Supplementary Figs. 5,6 and data). Immunolabeling on electrophysiologically and anatomically identified cells confirmed that neurogliaform cells somatodendritically express GABAAδ receptors (n=9) and some (n=3) of these neurogliaform cells elicited slow IPSPs in simultaneously recorded GABAAδ receptor immunonegative interneurons (Fig. 3). Tonic inhibition mediated by GABAAδ receptors appears to be a target for stress- and ovarian steroid-derived neurosteroids 3,4,26,27 thus, these compounds should modulate the excitability of neurogliaform cells. Indeed, the average holding current necessary to clamp neurogliaform cells (n=7, but not other interneurons n=10, Supplementary Fig. 7 26) at the same holding potential was increased by 24±5pA (p<0.03) following the addition of the neurosteroid THDOC (100nM)3,4,26,27 during blockade of GABAB receptors with 40μM CGP35348 in the presence of 5μM GABA (Fig. 3). The effect of THDOC was reversed when blocking GABAA receptors with gabazine (10μM) to average holding currents 8±3pA smaller (p<0.05) than measured preceding THDOC application. Furthermore, rheobasic firing of neurogliaform cells (n=6, Fig. 3), but not other interneurons (n=8, Supplementary Fig. 7) required larger positive current injections (234±26pA) in the presence of THDOC (20nM) compared to baseline conditions (138±17pA, p<0.01; in 40μM CGP35348 and 5μM GABA) and the effect was abolished with gabazine (10 μM) indicating that GABAAδ receptors could effectively control the input-output gain3,28,29 of neurogliaform cells. Here we used low concentrations of externally added GABA mimicking ambient cortical levels similar to earlier experiments on neurosteroid modulation of tonic inhibition3,4,26,27. However, neurogliaform output could locally produce extracellular GABA levels effective on GABAAδ receptors without additional GABA. Application of THDOC (100nM) in the presence of CGP35348 (40μM) increased the half-width from 48±6ms to 59±10ms of single presynaptic action potential elicited, gabazine (10μM) sensitive IPSPs in reciprocally connected pairs of neurogliaform cells (n=5, p<0.04, Fig. 3). Neurosteroid modulation of self- and intercellular inhibition involving neurogliaform cells confirms that a neurogliaform cell is both source and target of extracellular GABA acting on tonic inhibition.
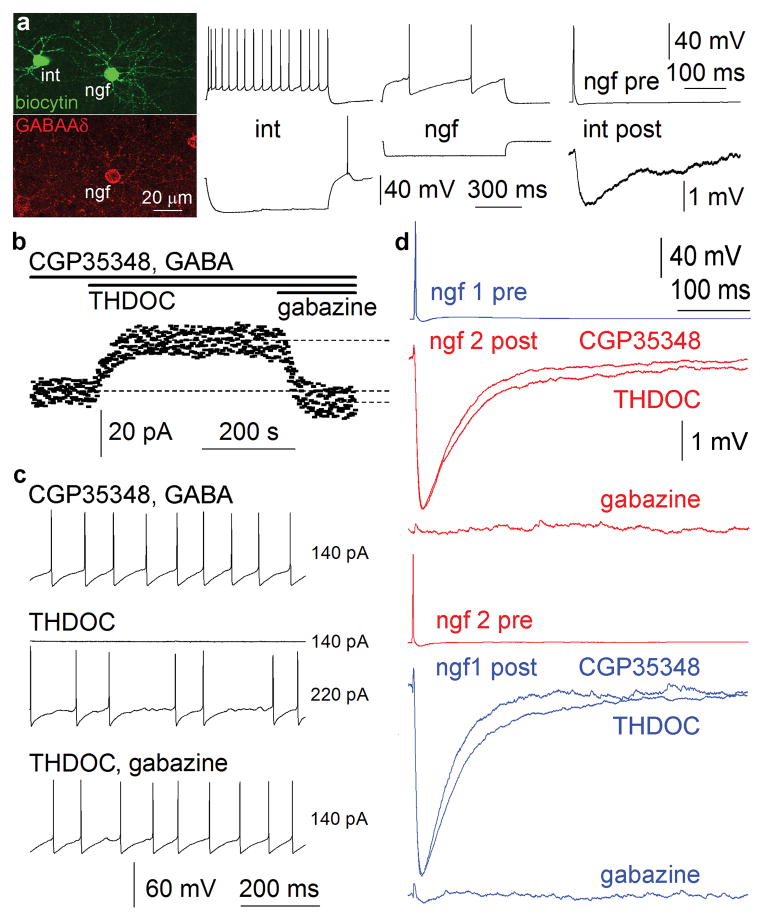
Figure 3.
Extrasynaptically placed GABAAδ receptors are localized to neurogliaform cells and targeted by GABA liberated from neurogliaform cells. (a) Left, GABAAδ receptor immunoreaction on a simultaneously recorded and biocytin filled neurogliaform cell (ngf) and postsynaptic interneuron (int). GABAAδ receptors were detected on the neurogliaform cell only. Middle, firing pattern of the interneuron and neurogliaform cell. Right, the neurogliaform cell elicited slow IPSPs in the postsynaptic GABAAδ receptor immunonegative interneuron. (b–d) Neurosteroids alter the excitability and connections of neurogliaform cells via GABAAδ receptors. (b) Average currents (dashed lines) required to hold a neurogliaform cell at the same membrane potential were different prior and following the addition of the neurosteroid THDOC (100nM) while blocking GABAB receptors with 40μM CGP35348 in the presence of 5μM GABA. The effect of THDOC was reversed when introducing gabazine (10μM) to an average holding current smaller than measured preceding THDOC application. (c) Rheobasic firing of a neurogliaform cell required larger positive current injections (220 instead of 140 pA) in the presence of THDOC (20nM) compared to baseline conditions (40μM CGP35348 and 5μM GABA) and the effect was abolished with gabazine (10 μM). (d) Application of THDOC (100nM) in the presence of CGP35348 (40μM) increased the half-width from of single presynaptic action potential elicited, gabazine (10μM) sensitive IPSPs in a reciprocally connected pair of neurogliaform cells.
Unlike other interneuron types specifically placing synapses on particular compartments of postsynaptic cells1,2, neurogliaform cells provide nonsynaptic, spatially unspecific input to the entire surface of target cells in addition to conventional synapses14. Neurogliaform cells release GABA covering their axonal field in effective concentrations and target the overwhelming majority of nearby neurons which selectively express receptors sensitive to low concentrations of the neurotransmitter on their various compartments23,25,30. Although presynaptic mechanisms producing the GABA cloud around neurogliaform axons are not understood, they might involve a unique release machinery with an unconventional calcium dependence16,22. Provided that release and reuptake mechanisms are similar, local GABA concentrations produced by distinct interneurons likely emerge at distances similar to about half the interterminal distance meaning that basket cells should be less effective than neurogliaform cells at about 3 μm from their terminals11. The spatial extent of axons14,17,19 suggest that neurogliaform cells provide means for synchronized changes in the efficacy of synaptic connections in conjunction with regulating dendritic excitability across a couple of hundred micrometers. At certain operational states of the microcircuit, solitary spikes in a single neurogliaform cell might replace the concerted action potentials of interneuron populations in modulating presynaptic terminals and postsynaptic domains expressing GABA receptors2,4,9,11–13. Neurosteroids might shift the balance among the sources for ambient GABA by lowering the contribution of neurogliaform cells with a selective increase in tonic inhibition through GABAAδ receptors. Varying neurosteroid levels during the ovarian cycle, pregnancy and stress4,27 are expected to modulate the action of neurogliaform cells on network hyperpolarization and on suppressing communication in the local circuit acting on axons of resident neurons or terminals of long range projections at their arrival.
Methods summary
All procedures were performed with the approval of the University of Szeged and in accordance with the National Institutes of Health Guide to the Care and Use of Laboratory Animals. Electrophysiology was obtained at ~35 °C from up to four concomitantly recorded cells visualized in layer 2/3 of the somatosensory cortex of Wistar rats (P22–35) as described14. Presynaptic neurogliaform cells and other cell types were stimulated to elicit action potentials with brief (2 ms) suprathreshold pulses at > 120 s intervals to avoid exhaustion of transmission. Amplitudes of postsynaptic potentials riding on top of the decay phase of preceding IPSPs were corrected with the input resistance and driving force (2±1 % for IPSPs and 0.5±0.2 % for EPSPs) related changes. Data are given as mean ± s.d. Wilcoxon-test and Mann-Whitney U-test was used to compare datasets, differences were accepted as significant if p<0.05.
Visualization of biocytin and correlated light- and electron microscopy was performed as described earlier14,18. Three-dimensional light and electron microscopic reconstructions were carried out using Neurolucida (MicroBrightfield, Colchester, VT) and Reconstruct (Synapse Web) software. Using three dimensional reconstructions and/or serial ultrathin sections, synaptic junctions were defined as 20–25 nm wide rigid appositions between pre-and postsynaptic profiles with an accumulation of presynaptic vesicles in axon terminals. The absence of either of these criteria around presynaptic boutons was used to classify a particular axon terminal without synapses.
Immunocytochemistry was performed on adult Wistar rats (n=5) and GABAA receptor δ subunit −/− mice (n=2, donated by I. Mody, University of California, Los Angeles) with standard methods (see detailed methods online). For immunoreactions concerning δ subunits of GABAA receptors, a primary rabbit polyclonal antibody was used (gift from W. Sieghart, Center for Brain Research, Vienna, Austria, 1:500). Colocalizations were performed afterwards with primary antibodies given in the detailed methods section.
Methods
Electrophysiology
All procedures were performed with the approval of the University of Szeged and in accordance with the National Institutes of Health Guide to the Care and Use of Laboratory Animals. Wistar rats (P22–35) were anaesthetized by the intraperitoneal injection of ketamine (30 mg/kg) and xylazine (10 mg/kg), and following decapitation, coronal slices (350 μm thick) were prepared from the somatosensory cortex. Slices were incubated at room temperature for 1 hour in a solution composed of (in mM) 130 NaCl, 3.5 KCl, 1 NaH2PO4, 24 NaHCO3, 1 CaCl, 3 MgSO4, 10 D(+)-glucose, saturated with 95% O2 and 5% CO2. The solution used during recordings differed only in that it contained 3 mM CaCl2 and 1.5 mM MgSO4. Recordings were obtained at ~35 °C from up to four concomitantly recorded cells visualized in layer 2/3 by infrared differential interference contrast videomicroscopy (Olympus BX60WI microscope, Hamamatsu CCD camera, Luigs & Neumann Infrapatch set-up and two HEKA EPC 10/double patch-clamp amplifiers). Micropipettes (5–7 MΩ) were filled with (in mM) 126 K-gluconate, 4 KCl, 4 ATP-Mg, 0.3 GTP-NA2, 10 HEPES, 10 creatine phosphate and 8 biocytin (pH 7.25; 300 mOsm). Signals were filtered at 5 kHz, digitized at 10 kHz and analyzed with PULSE software (HEKA, Lambrech/Pfalz, Germany). Presynaptic neurogliaform cells and other cell types were stimulated to elicit action potentials with brief (2 ms) suprathreshold pulses at > 120 s intervals to avoid exhaustion of transmission. Postsynaptic cells were held at − 51 ± 4 mV membrane potential. Unless specified, traces shown are averages of 30–200 episodes. The amplitude of postsynaptic responses was defined as the difference between the peak amplitude and the baseline value measured prior to the PSP onset in control experiments (i.e. when additional preceding spikes in the neurogliaform or fast spiking basket cells were not elicited) and were derived using the same principle following subtraction of the control traces from the postsynaptic averages recorded with the preceding presynaptic spikes elicited in the neurogliaform or fast spiking basket cells. Amplitudes of postsynaptic potentials riding on top of the decay phase of preceding IPSPs were corrected with the input resistance changes measured with brief hyperpolarizing pulses timed on top of the IPSPs at 60 and 120 ms after the spike in the neurogliaform or fast spiking basket cells. Data were also corrected for driving force related changes which were 2±1 % for IPSPs and 0.5±0.2 % for EPSPs. Experiments with THDOC were performed in the presence of NBQX (10 μM) and APV (20 μM). Effects between neurogliafrom cells could not be adequately fitted with single exponentials, thus half-widths were compared. Data are given as mean ± s.d. Wilcoxon-test and Mann-Whitney U-test was used to compare datasets, differences were accepted as significant if p<0.05.
Histology
Visualization of biocytin and correlated light- and electron microscopy was performed as described earlier14,18. Three-dimensional light microscopic reconstructions were carried out using Neurolucida (MicroBrightfield, Colchester, VT) with 100× objective; measurements of the overlap between axonal and dendritic fields were aided by Neuroexplorer (MicroBrightfield) software. Three-dimensional electron microscopic reconstructions were performed with Reconstruct (Synapse Web) software axons and were aided by the especially large range (±80 degrees) of the goniometer fitted to our electron microscope (Tecnai BioTwin 120, Rotterdam, The Netherlands). Using three dimensional reconstructions and/or serial ultrathin sections, synaptic junctions were defined as 20–25 nm wide rigid appositions between pre-and postsynaptic profiles with an accumulation of presynaptic vesicles in axon terminals. The absence of either of these criteria around presynaptic boutons was used to classify a particular axon terminal without synapses.
Immunocytochemistry
Adult Wistar rats (n=5) and GABAA receptor δ subunit −/− mice (n=2, donated by I. Mody, University of California, Los Angeles) were perfused with fixative containing 4% paraformaldehyde made up in 0.1 M phosphate buffer (PB, pH=7.3) for 10 minutes in deep anaesthesia. For immunoreactions concerning δ subunits of GABAA receptors, 60 μm thick coronal sections were incubated in citrate buffer containing 10mM citric acid and 0.05% Tween 20 (Sigma) made up in distilled water (pH=6.0) at 95–100°C for 10 minutes. After cooling to room temperature, sections were blocked with normal horse serum (NHS, 10%) made up in Tris-buffered saline (TBS, pH=7.4) for 1 hour and incubated with the primary rabbit polyclonal antibody (gift from W. Sieghart, Center for Brain Research, Vienna, Austria, 1:500) diluted in TBS containing 2% NHS and 0.1% TritonX-100 for 72 hours at 4°C. Washes were done between steps with TBS containing 0.05% Tween 20 until incubation in a cocktail containing biotinylated donkey anti-rabbit secondary antibody (Jackson Immunoresearch, 1:250), then, with TBS only. Sections were treated with ABC complex dissolved in TBS (Vector Laboratories, 1:100) and GABAAδ receptor immunoreactions were visualized by Alexa488 conjugated Tyramide signal amplification kits (Molecular Probes). Colocalizations were performed afterwards with the following primary antibodies: mouse anti-α-actinin (Sigma, Saint Louis, Missouri, A7811, 1:40000), goat anti-parvalbumin (Swant, PGV-214, 1:5000), mouse anti-calbindin (Sigma, C8666, 1:100) and guinea pig anti- vasoactive intestinal peptide (Peninsula Laboratories, T-5030, 1:200), mouse anti-calretinin (Swant, 6B3, 1:1000), and rat anti-somatostatin (Chemicon, MAB354, 1:50), mouse anti-chicken ovalbumin upstream promoter transcription factor II (PPMX, Tokyo, Japan, 2ZH7147H, 1:500) and mouse anti-reelin (Chemicon, MAB5366, 1:50000). Primaries were diluted in cocktail mentioned above and were visualized by the following secondaries: Cy3 conjugated donkey anti-mouse (Jackson, 1:500), Cy3 conjugated donkey anti-rabbit (Jackson, 1:500), Cy3 conjugated donkey anti-rat (Jackson, 1:500), Cy5 conjugated donkey anti-guinea pig (Jackson, 1:500) and Alexa350 conjugated donkey anti-goat (Molecular Probes, 1:500). Finally, sections were mounted on slides in Vectashield (Vector). Images were taken by light microscope (BX60, Olympus) using 5× objective or a confocal laser scanning microscope (IX81, Olympus) using a 20× (NA=0.75) or a 40× (NA=1.30) objective. Automated sequential acquisition of multiple channels were used. Z-stack images were made up from 3–9 images in 5 – 45 μm depth of tissue.
Supplementary Material
Acknowledgments
The authors thank W. Sieghart for donating, A. L rincz and Z. Nusser for initial testing of the GABAAδ antibody, I. Mody for the GABAAδ −/− animals, and A. Simon and E. Toth for reconstructions. This work was supported by the EURYI, NKTH Polanyi Award, HHMI, NIH NS535915, Boehringer Ingelheim Fonds and the Hungarian Academy of Sciences.
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Author contributions. S.O. performed experiments, analysed data and wrote the paper, M.F., G. K., C. V. and R. B. performed experiments and analysed data, P. B. performed experiments and G.T designed and performed experiments, analysed data and wrote the paper.
References
- 1.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–7. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 4.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–70. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 7.Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–50. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 8.Vizi ES. Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol Rev. 2000;52:63–89. [PubMed] [Google Scholar]
- 9.Barbour B, Hausser M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–84. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- 10.Guastella J, et al. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–6. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- 11.Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23:2618–26. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–81. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci. 2000;20:8651–8. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Sience. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 15.Szabadics J, Tamas G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA, slow and GABAA, fast. Proc Natl Acad Sci U S A. 2007;104:14831–6. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price CJ, Scott R, Rusakov DA, Capogna M. GABA(B) receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J Neurosci. 2008;28:6974–82. doi: 10.1523/JNEUROSCI.4673-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karube F, Kubota Y, Kawaguchi Y. Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J Neurosci. 2004;24:2853–65. doi: 10.1523/JNEUROSCI.4814-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 19.Simon A, Olah S, Molnar G, Szabadics J, Tamas G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci. 2005;25:6278–85. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters APSL, Webster H. The Fine Structure of the Nervous System. Oxford University Press; New York: 1991. [Google Scholar]
- 21.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–75. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 22.Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–8. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 23.Guetg N, et al. The GABAB1a Isoform Mediates Heterosynaptic Depression at Hippocampal Mossy Fiber Synapses. J Neurosci. 2009;29:1414–1423. doi: 10.1523/JNEUROSCI.3697-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- 25.Kulik A, et al. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–35. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vardya I, Drasbek KR, Dosa Z, Jensen K. Cell type-specific GABA A receptor-mediated tonic inhibition in mouse neocortex. J Neurophysiol. 2008;100:526–32. doi: 10.1152/jn.01224.2007. [DOI] [PubMed] [Google Scholar]
- 27.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–44. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 29.Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–60. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- 30.Kullmann DM, et al. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.