Abstract
Ribonucleoprotein complexes consisting of Argonaute-like proteins and small regulatory RNAs function in a wide range of biological processes. Many of these small regulatory RNAs are predicted to act, at least in part, within the nucleus. We conducted a genetic screen to identify factors essential for RNAi in nuclei of C. elegans and identified the Argonaute protein NRDE-3. In the absence of siRNAs NRDE-3 resides in the cytoplasm. NRDE-3 binds siRNAs generated by RNA-dependent RNA Polymerases acting upon mRNA templates in the cytoplasm and redistributes to the nucleus. Nuclear redistribution of NRDE-3 requires a functional nuclear localization signal, is required for nuclear RNAi, and results in NRDE-3 association with nuclear-localized nascent transcripts. Thus, specific Argonaute proteins can transport specific classes of small regulatory RNAs to distinct cellular compartments to regulate gene expression.
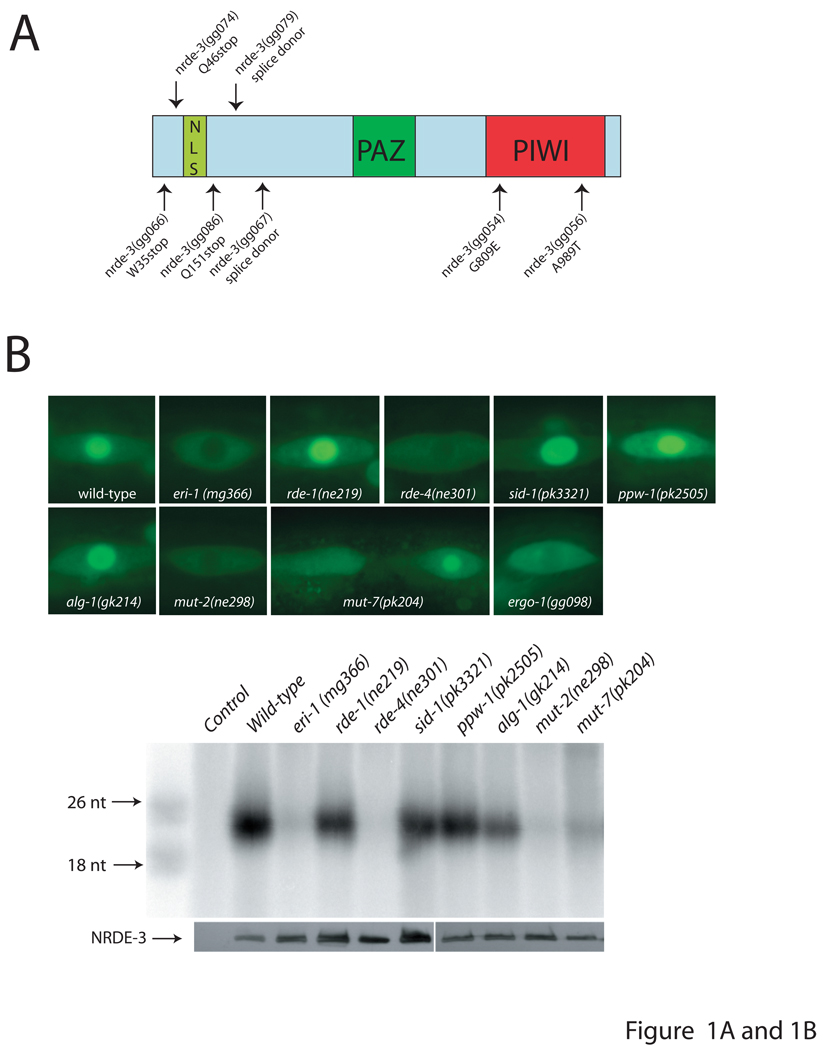
To identify factors specifically required for RNAi in C. elegans nuclei, we chemically mutagenized worms and screened for mutant animals that failed to silence nuclear-localized RNAs in response to RNAi, but retained the ability to silence RNAs not localized exclusively in the nucleus (for details see Fig. S1). We identified 55 mutant alleles that were categorized into three complementation groups. Forty-six of these alleles defined a gene that we termed nuclear RNAi defective-3 (nrde-3). We mapped nrde-3 to a < 1 cM interval. Open reading frame (ORF) R04A9.2 lies within this genetic interval. R04A9.2 is predicted to encode an Argonaute-like protein containing a bipartite nuclear localization signal (NLS), a PAZ, and a PIWI domain. The PIWI domain of R04A9.2 lacks the DDH catalytic triad of amino acids considered necessary for Argonaute-based Slicer activity: the synonymous residues are EVQ in R04A9.2 (1). Sequencing of R04A9.2 from eight independent nrde-3 alleles identified seven mutations in R04A9.2 coding sequences (Fig. 1A). Three alleles are predicted to stop translation upstream of the PAZ/PIWI domains of R04A9.2 and thus are likely to reveal the null phenotype. Transformation of wild-type R04A9.2 DNA into nrde-3 mutant animals rescued phenotypes associated with nrde-3 mutant animals (Fig. 3B). Thus, R04A9.2 corresponds to nrde-3.
Fig. 1.
siRNA binding is necessary and sufficient for redistribution of NRDE-3 to the nucleus. (A) Predicted domain structure of R04A9.2. DNA lesions, identified in nrde-3 alleles, are indicated by arrows. (B) Sub-cellular localization of NRDE-3 correlates with NRDE-3-siRNA interactions. (top) Fluorescence microscopy visualizing GFP::NRDE-3 in seam cells of animals of the indicated genotypes (2). (bottom) FLAG::NRDE-3 co-immunoprecipitating RNAs were isolated, radiolabeled, and analyzed by PAGE (2). (C) siRNA binding is necessary to localize NRDE-3 to the nucleus. (top) NRDE-3 and NRDE-3(*PAZ) sub-cellular localization in seam cells and (bottom) co-precipitating endo siRNAs. (D) siRNA binding is sufficient for nuclear redistribution of NRDE-3. (top) GFP::NRDE-3 localization in seam cells and (bottom) FLAG::NRDE-3 co-precipitating exo siRNAs, in animals lacking endo siRNAs, 36 hours following exposure to control bacteria or dsRNA expressing bacteria (2).
Fig. 3.
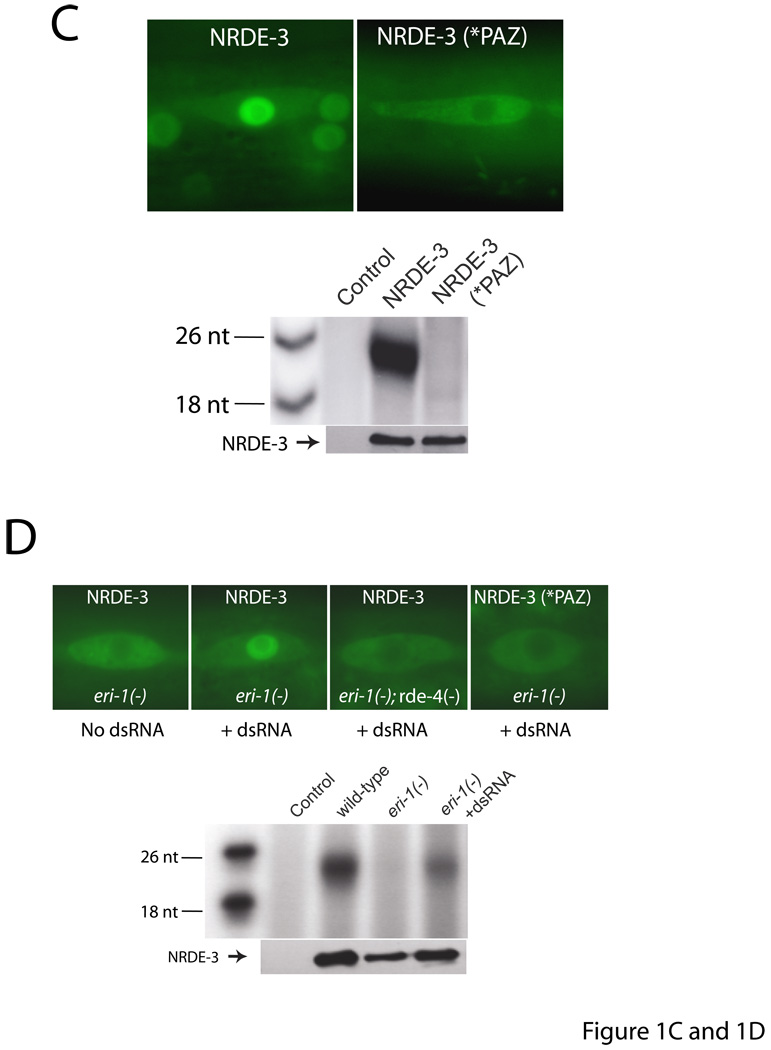
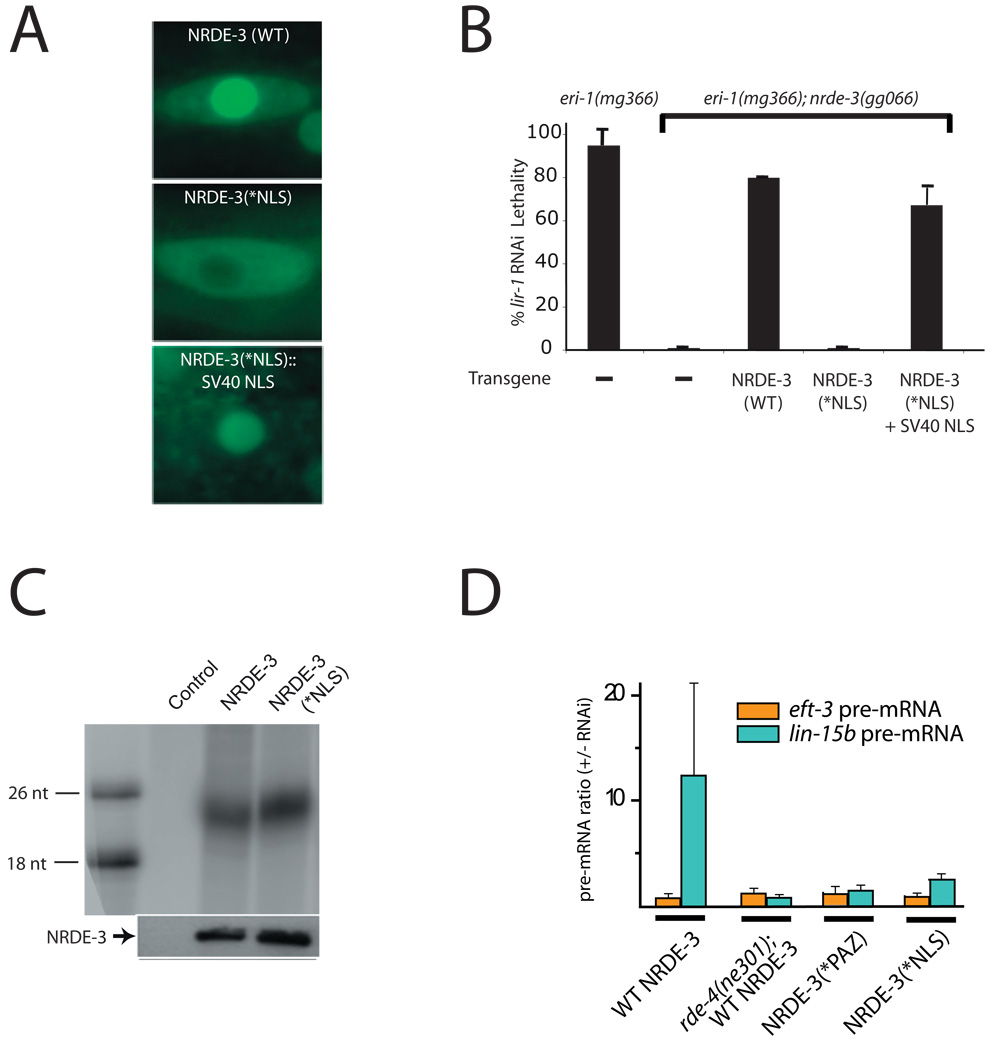
NRDE-3 transports siRNAs from the cytoplasm to the nucleus: an obligatory step for nuclear RNAi. (A) NRDE-3 contains a functional NLS. Fluorescence microscopy of seams cells of animals expressing GFP::NRDE-3 and its variants (2). (B) Nuclear localization of NRDE-3 is required for nuclear RNAi. lir-1 RNAi-mediated lethality results from silencing of the nuclear-localized lir-1/lin-26 polycistronic RNA (2, 13). L1 animals of the indicated genotypes expressing the indicated NRDE-3 variants were fed bacteria expressing lir-1 dsRNA for 60 hours and scored for viability (n=3, +/− s.d.). (C) NRDE-3 associates with siRNAs in the cytoplasm. FLAG::NRDE-3 and FLAG::NRDE-3(*NLS) associated endo siRNAs were purified and labeled as described in Fig. 1B. (D) NRDE-3 associates with pre-mRNA in an RNAi-dependent manner. FLAG::NRDE-3 was immunoprecipitated from animals expressing indicated NRDE-3 variants after exposure to (+/−) lin-15b dsRNA. cDNAs generated from associating RNAs (n=4, +/− s.d.) were analyzed by qRT-PCR (2). Similar levels of NRDE-3 were immunoprecipitated in each experiment (not shown) and similar results were obtained following unc-40 RNAi (Fig. S5A).
A fusion gene between GFP and full-length NRDE-3 under control of the endogenous nrde-3 promoter (2) rescued nrde-3 mutant phenotypes, was expressed in most somatic cells after the ≈80-cell stage of development, and localized predominantly to the nucleus (Fig. 1B, Supplemental Fig. S2). Immunoprecipitation of NRDE-3 and isolation of co-precipitating RNAs allowed us to clone 465 RNAs of approximately 20–22 nucleotides that associated with NRDE-3 in vivo. These RNAs represent endogenous (endo) siRNAs; they map to similar chromosomal loci as previously identified endo siRNAs (Table S1). Endo siRNAs are thought to mediate, or be the consequence of, ongoing negative regulation of endogenously expressed RNAs by RNAi (3, 4). In eri-1(-), ergo-1(-), mut-7(-), rde-4(-), or mut-2(-) animals, which exhibit defects in endo siRNA production (3, 4), NRDE-3 failed to associate with endo siRNAs (2) (Fig. 1B). In these mutant backgrounds, NRDE-3 localized to the cytoplasm, suggesting that siRNAs are necessary to localize NRDE-3 to the nucleus (Fig. 1B).
To examine this possibility, we mutated residues within the NRDE-3 PAZ domain (Y463A, Y464A) known to be necessary for siRNA-binding in related Argonaute proteins (termed NRDE-3(*PAZ)) (5, 6). NRDE-3(*PAZ) failed to interact with siRNAs and localized predominantly to the cytoplasm (Fig. 1C). Exposure of animals lacking endo siRNAs (due to loss of ERI-1 activity) to exogenous dsRNA caused NRDE-3 to re-localize to the nucleus and induced association of NRDE-3 with exogenous (exo) siRNAs (Fig. 1D). The nuclear redistribution of NRDE-3 required NRDE-3/siRNA interactions: NRDE-3(*PAZ) failed to localize to the nucleus in response to dsRNA treatment, and NRDE-3 re-localization was dependent on RDE-4, a dsRNA binding protein necessary for converting experimentally introduced dsRNAs to exo siRNAs (7) (Fig. 1D). We conclude that NRDE-3/siRNA binding is necessary and sufficient for NRDE-3 nuclear redistribution.
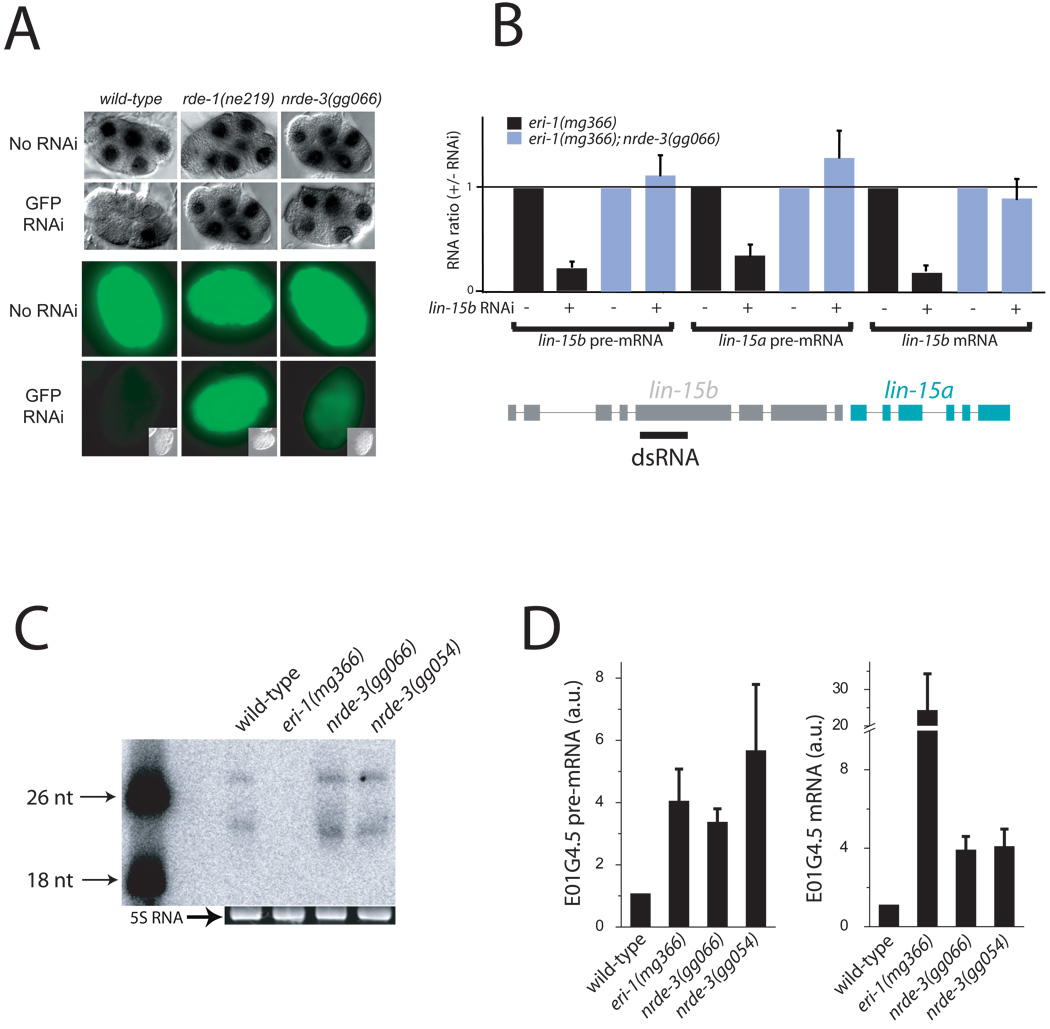
NRDE-3 is essential for silencing nuclear-localized RNAs. A pes-10::GFP transgene expresses a transcript that, for unknown reasons, accumulates in the nucleus during early (<16-cell) embryogenesis. RNAi targeting the pes-10::GFP transcript triggered a NRDE-3-dependent loss of nuclear-localized pes-10::GFP RNA (8) (Fig. 2A and Supplemental Fig. S3). NRDE-3 is also required for RNAi of endogenous nuclear-localized RNAs. The lin-15b and lin-15a genes are transcribed as a bicistronic pre-mRNA that is spliced within the nucleus into distinct lin-15b and lin-15a mRNAs (9). Animals harboring mutations in both lin-15b and lin-15a, but not either gene alone, exhibit a multi-vulva (Muv) phenotype (10, 11). RNAi targeting lin-15b results in a low percentage of animals exhibiting a Muv phenotype, consistent with nuclear silencing of the lin-15 bicistron (8). To sensitize this assay, we repeated these experiments in a strain of C. elegans (eri-1(-)) that exhibits enhanced sensitivity to dsRNAs (12). 95 +/− 4% of eri-1(-) animals exposed to dsRNA targeting lin-15b exhibited a Muv phenotype, supporting the hypothesis that nuclear-localized lin-15 bicistronic RNA can be targeted by RNAi (Table 1). Consistent with these results, RNAi targeting lin-15b in eri-1(-) animals triggered a decrease in both lin-15b and lin-15a pre-mRNA abundance (Fig. 2B). These effects were also observed in wild-type animals but were less pronounced than in eri-1(-) animals. NRDE-3 is required for these phenomena; in response to lin-15b RNAi, eri-1(-); nrde-3(-) animals did not exhibit a Muv phenotype (Table 1) and failed to silence the lin-15b and lin-15a pre-mRNAs (Fig. 2B). NRDE-3-dependent silencing of lin-15b pre-mRNA accounted for ≈80% of total lin-15b silencing elicited by lin-15b RNAi (Fig. 2B). Finally, we, and others, have detected RNAi-triggered silencing of nuclear-localized lir-1/lin-26 polycistronic RNA (2, 13). NRDE-3 is required for this silencing (Table 1). We conclude that NRDE-3 is required for silencing of many, if not all, nuclear-localized RNAs.
Fig. 2.
NRDE-3 is required for nuclear RNAi. (A) NRDE-3 is essential for silencing of the nuclear-localized pes-10::GFP RNA. (top) Light microscopy of ≈6 cell embryos subjected to in situ hybridization targeting the pes-10::gfp RNA +/− GFP dsRNA (2). (bottom) Fluorescence microscopy of ≈ 300 cell embryos +/− GFP RNAi. (B) NRDE-3 is required for RNAi-driven silencing of nuclear-localized lin-15 bicistronic RNA. qRT-PCR analysis of lin-15a/b pre-mRNA and lin-15b mRNA levels in animals +/− lin-15b dsRNA (n=4–8, +/− s.d.) (2). (C) NRDE-3 is not required for production of an endo siRNA. Total RNA isolated from animals of the indicated genotypes were subjected to Northern blot analysis detecting E01G4.5 endo siRNAs (2). (D) NRDE-3 is required for silencing of an endogenous pre-mRNA. qRT-PCR of E01G4.5 mRNA and E01G4.5 pre-mRNA levels from animals of the indicated genotypes (n=4, +/− s.d.) (2).
Table 1.
NRDE-3 preferentially targets nuclear-localized RNAs. Animals of the indicated genotypes were fed bacteria expressing indicated dsRNAs (e.g. unc-22). Phenotypes (e.g. twitcher) of eri-1(mg366) animals exposed to control or dsRNA were defined as zero and four, respectively (n=3, +/− s.d.). NS= not scored.
| dsRNA (phenotype scored) |
|||||||
|---|---|---|---|---|---|---|---|
| Genotype |
unc-22 (twitcher) |
unc-15 (paralysis) |
pos-1 (lethality) |
unc-73 (paralysis) |
dpy-13 (dumpy) |
lir-1 (*lethality) |
lin-15b (*multi-vulva) |
| eri-1(mg366) | 3.9 +/− 0.2 | 4 | 3.8 +/− 0.4 | 3.9 +/− 0.2 | 3.9 +/− 0.1 | 4 | 4 |
| eri-1(mg366); nrde-3(gg066) | 3.7 +/− 0.4 | 3 | 4 | 1.6 +/− 0.8 | 1.8 +/− 0.4 | 0 | 0 |
| eri-1(mg366); rde-1(ne219) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| eri-1(mg366); rde-4(ne301) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| eri-1(mg366); rrf-1(pk1417) | 0 | NS | 4 | 0 | NS | 0 | 0 (n=1) |
indicates phenotype predicted to be elicited by silencing of nuclear localized RNA
Thirty-three of the 465 NRDE-3 associated endo siRNAs exhibited sequence complementarity to the E01G4.5 ORF (Table S1). Northern blot analysis confirmed that NRDE-3 interacts, in vivo, with E01G4.5 endo siRNAs (Fig. S4). nrde-3(-) animals expressed E01G4.5 endo siRNAs at approximately wild-type levels (Fig. 2C). The E01G4.5 pre-mRNA and mRNA, however, were up-regulated 4–5x in nrde-3(-) animals (Fig. 2D). eri-1(-) animals (which failed to express E01G4.5 siRNAs (Fig. 2C)) exhibited a similar misregulation of the E01G4.5 pre-mRNA, but a more dramatic (24x) up-regulation of the E01G4.5 mRNA (Fig. 2D). These data suggest that NRDE-3 represents the primary, if not the sole, means for endogenous silencing of the E01G4.5 pre-mRNA, but other, NRDE-3-independent, mechanisms exist for silencing E01G4.5 mRNA. Thus, NRDE-3 is required for silencing of an endogenous, nuclear-localized RNA and likely functions downstream of endo siRNA production in these silencing events.
We investigated whether silencing in the nucleus is dependent upon nuclear localization of NRDE-3. NRDE-3 harboring a three amino acid substitution (K80A, R81A, K82A) within the predicted NRDE-3 NLS (termed NRDE-3(*NLS)) localized to the cytoplasm (Fig. 3A). Wild-type NRDE-3, but not NRDE-3(*NLS), rescued a Nrde phenotype associated with nrde-3(-) animals (Fig. 3B). Introduction of a heterologous SV40 NLS to NRDE-3(*NLS) restored NRDE-3(+) function and nuclear localization to NRDE-3(*NLS) (Figs. 3A and 3B). Thus, NRDE-3 contains a functional NLS and NRDE-3 must localize to the nucleus to trigger nuclear RNAi. NRDE-3(*NLS), which localized to the cytoplasm, associated with exo and endo siRNAs to a similar extent as wild-type NRDE-3, indicating that NRDE-3 interacts with siRNAs in the cytoplasm (Fig. 3C and not shown). Finally, RNAi targeting lin-15b and unc-40 induced an association between NRDE-3 and unspliced lin-15b and unc-40 RNAs, respectively (Fig. 3D and Supplemental Fig. S5A). NRDE-3/pre-mRNA interactions were siRNA-dependent and occurred in the nucleus: NRDE-3(*PAZ) and NRDE-3(*NLS) failed to associate with pre-mRNA to a similar extent as wild-type NRDE-3. RNAi-driven association of NRDE-3 with pre-mRNA is consistent with a role for NRDE-3 in nuclear silencing events and may provide insights into the mechanism of nuclear silencing in C. elegans. Taken together, these data indicate that NRDE-3 can escort endo and exo siRNAs from the cytoplasm to the nucleus in an NLS-dependent manner, and that this translocation step is essential for silencing nuclear-localized RNAs.
NRDE-3 is required for silencing E01G4.5 pre-mRNA, but only partially required for endogenous RNAi-mediated silencing of E01G4.5 mRNA, suggesting that NRDE-3 may not be required for cytoplasmic silencing events (Fig. 2D). Three lines of evidence support this hypothesis. First, expression of NRDE-3(*NLS) did not enhance RNAi responses: nrde-3(-), and nrde-3(-) animals expressing NRDE-3(*NLS), responded similarly to RNAi (Fig. S5B and S5C). Second, nrde-3(-) animals retained the ability to respond (albeit at somewhat attenuated levels) to RNAi targeting RNAs (such as pos-1, unc-22, unc-15, unc-73, and dpy-13) not localized exclusively to the nucleus (Table 1). Incidentally, the near wild-type response of nrde-3(-) animals to these dsRNAs indicates that (for unknown reasons) NRDE-3-dependent silencing is not always a significant component of RNAi responses following typical feeding RNAi experiments in C. elegans. Finally, at the >16-cell stage of embryonic development, the pes-10::GFP RNA is transported to the cytoplasm (8). Late stage (≈300-cell) nrde-3(-) embryos, exposed to GFP dsRNA, retained the ability to partially silence PES-10::GFP (Fig. 2A, bottom panel). Thus, while NRDE-3 is required for RNAi of nuclear-localized RNAs, it is not required for all RNAi-related silencing events, and likely does not play a significant role in silencing of cytoplasmic mRNAs.
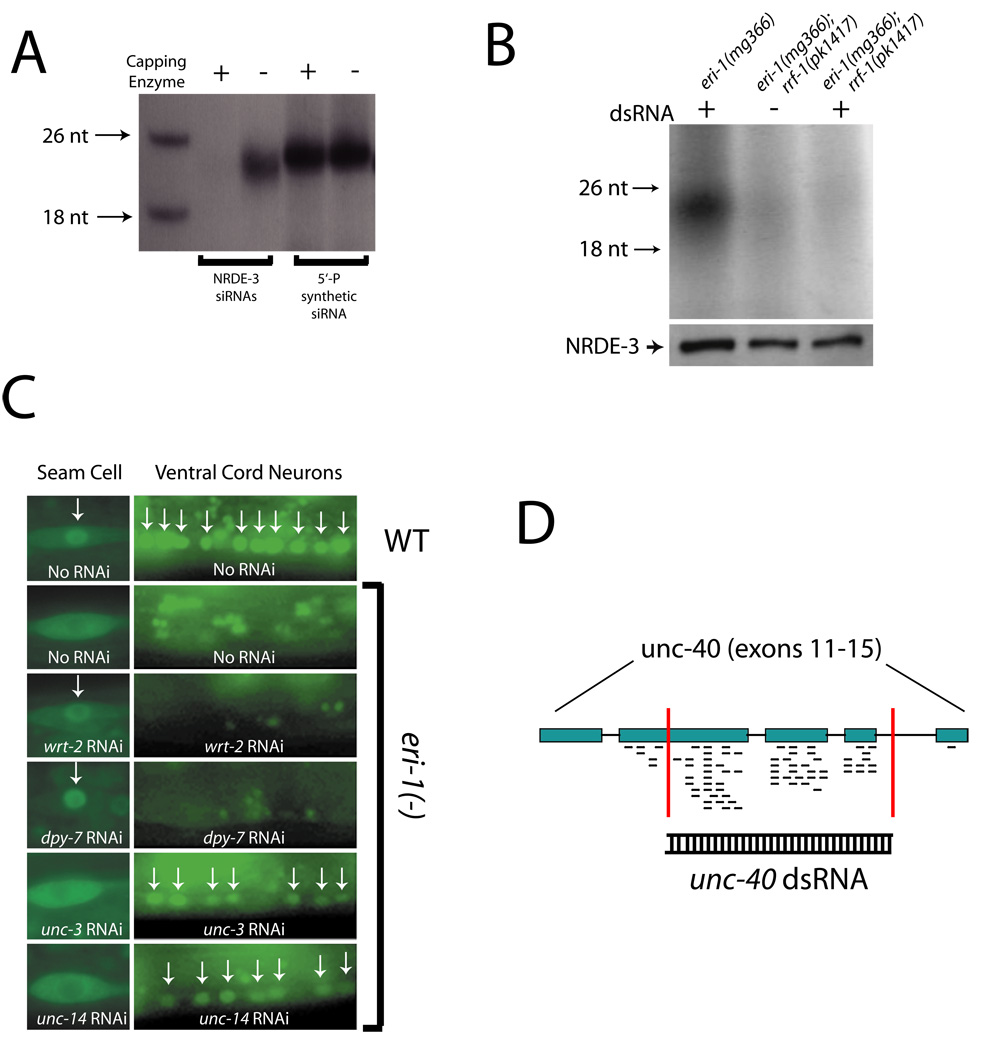
We next examined NRDE-3 associated siRNAs in more detail. >85% of NRDE-3 associated siRNAs are substrates for in vitro RNA capping reactions catalyzed by guanylyl transferase, indicating that NRDE-3 associated siRNAs carry 5’ di- or tri-phosphates (Fig. 4A). RNA-dependent RNA Polymerases (RdRPs) are thought to amplify RNAi signals by utilizing RNA molecules, which have been targeted by RNAi, as templates for transcription of additional siRNAs. The presence of 5’ di- or tri-phosphates on NRDE-3 associated siRNAs suggested that these siRNAs might be generated by RdRP action (14, 15). Three lines of evidence support this hypothesis. First, the RdRP, RRF-1 was required for silencing of the lir-1/lin-26 polycistronic RNA in response to lir-1 RNAi (Table 1). In addition, in rrf-1(-) animals NRDE-3 failed to associate with exo siRNAs following RNAi (Fig. 4B). Second, acute exposure (16 hrs) of animals lacking endo siRNAs to dsRNAs targeting wrt-2 and dpy-7, two genes expressed in hypodermal cells, triggered a nuclear redistribution of NRDE-3 in hypodermal cells, but failed to initiate NRDE-3 translocation in non-hypodermal cells (Fig. 4C). RNAi targeting unc-14 and unc-3, two genes expressed in ventral cord neurons, triggered NRDE-3 redistribution predominantly in ventral cord neurons (Fig. 4C). Thus, nuclear redistribution of NRDE-3 appears to require expression of RNA molecules exhibiting sequence similarity to the trigger dsRNA (1). Third, exposure of animals lacking endo siRNAs to dsRNA targeting the unc-40 gene induced association of NRDE-3 with siRNAs that are exclusively anti-sense to the unc-40 mRNA. 10% of these siRNAs were derived from unc-40 sequences not covered by the unc-40 dsRNA to which the animals were exposed (Fig. 4D). NRDE-3 associated with unc-40 siRNAs that exhibited complementarity exclusively to unc-40 exonic sequences despite the fact that both exonic (82%) and intronic (18%) unc-40 sequences were targeted by RNAi in these experiments (Fig. 4D). A similar bias towards exonic sequences was observed in NRDE-3 associated endo siRNAs (Table S1). These data, in conjunction with our data demonstrating that NRDE-3 associates with siRNAs in the cytoplasm, strongly suggest that NRDE-3 associates predominantly with siRNAs generated by RdRPs acting upon mRNA templates in the cytoplasm and that this association is essential for nuclear RNAi.
Fig. 4.
NRDE-3 associated siRNAs are generated by RdRPs acting upon cytoplasmic mRNA targets. (A) NRDE-3 associated siRNAs carry 5’ di- or tri-phosphates. FLAG::NRDE-3 associated endo siRNAs were treated (+/−) with guanylyl transferase and GTP and then detected as described in Fig. 1B. (B) Animals of indicated genotypes were fed (+/−) bacteria expressing unc-22 dsRNA for 24 hours and FLAG::NRDE-3-associated RNAs were detected as described in Fig. 1B. (C) eri-1(mg366) animals were fed bacteria expressing indicated dsRNAs. After 16 hours, GFP::NRDE-3 localization was assessed. Arrows indicate nuclear localization. (D) eri-1(mg366) animals were exposed to unc-40 dsRNA (targeted sequences indicated by vertical red lines) and FLAG::NRDE-3 associated siRNAs were cloned (2). Short black lines represent anti-sense unc-40 sequences identified.
Our data indicate that nuclear silencing events in C. elegans are the result of transport of siRNAs, and not long dsRNAs, to the nucleus via an active transport system involving Argonaute proteins. nrde-3(-) animals are defective for all nuclear silencing phenomena that we have assayed, indicating that other mechanisms of siRNA nuclear localization, such as passive diffusion, and/or nuclear envelope breakdown during mitosis are unlikely to be major contributors to this processes. Many small regulatory RNAs such as scanRNAs in ciliated protozoa, heterochromatin related siRNAs in plants and fungi, and piRNAs in mammals are thought to function, at least in part, within the nucleus (16–19). It will be interesting to determine whether these small regulatory RNAs are transported to the nucleus by mechanisms similar to the one described here.
Supplementary Material
References
- 1.Yigit E, et al. Cell. 2006;127:747. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Materials and methods are available as supporting material on Science Online.
- 3.Lee RC, Hammell CM, Ambros V. RNA. 2006;12:589. doi: 10.1261/rna.2231506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duchaine TF, et al. Cell. 2006;124:343. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Science. 2004;305:1434. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 6.Ma JB, Ye K, Patel DJ. Nature. 2004;429:318. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabara H, Yigit E, Siomi H, Mello CC. Cell. 2002;109:861. doi: 10.1016/s0092-8674(02)00793-6. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery MK, Xu S, Fire A. Proc. Natl. Acad. Sci. U S A. 1998;95:15502. doi: 10.1073/pnas.95.26.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenthal T, et al. Nature. 2002;417:851. doi: 10.1038/nature00831. [DOI] [PubMed] [Google Scholar]
- 10.Clark SG, Lu X, Horvitz HR. Genetics. 1994;137:987. doi: 10.1093/genetics/137.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang LS, Tzou P, Sternberg PW. Mol. Biol. Cell. 1994;5:395. doi: 10.1091/mbc.5.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy S, Wang D, Ruvkun G. Nature. 2004;427:645. doi: 10.1038/nature02302. [DOI] [PubMed] [Google Scholar]
- 13.Bosher JM, Dufourcq P, Sookhareea S, Labouesse M. Genetics. 1999;153:1245. doi: 10.1093/genetics/153.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pak J, Fire A. Science. 2007;315:241. doi: 10.1126/science.1132839. [DOI] [PubMed] [Google Scholar]
- 15.Sijen T, Steiner FA, Thijssen KL, Plasterk RH. Science. 2007;315:244. doi: 10.1126/science.1136699. [DOI] [PubMed] [Google Scholar]
- 16.Hall IM, Shankaranarayana GD, Noma K, Ayoub N, Cohen A, Grewal SIS. Science. 2002;297:2232. doi: 10.1126/science.1076466. [DOI] [PubMed] [Google Scholar]
- 17.Volpe TA, Kidner C, Hall IM, Teng G, Grewal SIS, Martienssen RA. Science. 2002;297:1833. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 18.Mochizuki K, Fine NA, Fujisawa T, Gorovsky MA. Cell. 2002;110:689. doi: 10.1016/s0092-8674(02)00909-1. [DOI] [PubMed] [Google Scholar]
- 19.Carmell MA, et al. Dev. Cell. 2007;12:503. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 20.We thank Craig Hunter for suggesting utilizing the pes-10::GFP strain, Judith Kimble for use of confocal microscope, Phil Anderson, David Wassarman, Jim Dahlberg, and Elsebet Lund for comments, and the Caenorhabditis Genetics Center for strains. This work was supported by grants from the Pew and Shaw scholars programs, the NIH, and the AHA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.