Abstract
APRIL (a proliferation-inducing Ligand) and BLyS/BAFF (B-lymphocyte stimulator/B-cell-activating factor of the TNF (tumor necrosis factor) family have been shown to be the survival factors for certain myeloma cells in vitro. BAFF binds to the TNF-related receptors such as B-cell maturation antigen (BCMA), transmembrane activator and CAML interactor (TACI) and BAFFR, whereas APRIL binds to TACI and BCMA and to heparan sulfate proteoglycans (HSPG) such as syndecan-1. TACI gene expression in myeloma reportedly can distinguish tumors with a signature of microenvironment dependence (TACIhigh) versus a plasmablastic signature (TACIlow). We tested the effect of atacicept (formerly TACI-Ig, which blocks APRIL and BAFF) and BAFFR-Ig (which blocks BAFF only) on primary myeloma growth in the SCID-hu model and in coculture with osteoclasts. With only few exceptions, atacicept and to a lesser extent BAFFR-Ig, inhibited growth of TACIhigh but not TACIlow myeloma samples in vivo and ex vivo, and the response rate was inversely correlated with TACI expression. Most TACIhigh myeloma cells were molecularly classified as being low risk with our recently described 70-gene model. APRIL and BAFF were highly expressed by osteoclasts and were upregulated in myeloma cells after coculture with osteoclasts. Our findings suggest that APRIL plays an essential role in the survival of TACIhigh bone marrow-dependent myeloma cells and TACI gene expression may be a useful predictive marker for patients who could benefit from atacicept treatment.
Keywords: myeloma, APRIL, BAFF, TACI, gene expression, microenvironment
Introduction
In multiple myeloma, a plasma cell malignancy, a majority of the tumor cells reside in the hematopoietic bone marrow (BM). As a consequence of interaction with certain microenvironmental components, myeloma cells are protected from spontaneous and drug-induced apoptosis.1,2 In late-stage disease, myeloma cells become independent of BM microenvironmental signals and extramedullary disease is not uncommon. Recent clinical studies indicate that using drugs that simultaneously target myeloma cells and their supportive BM microenvironment (for example, thalidomide, bortezomib) provides a promising approach to control disease progression and overcome drug resistance.3,4 Interaction of myeloma cells with cellular and extracellular components results in upregulation of growth factors such as interleukin (IL)-65–7 and vascular endothelial growth factor (VEGF).8 However, inhibiting their activity in patients with myeloma results in partial or no response,9,10 suggesting that additional growth factors and/or cell-to-cell interactions may be involved in the growth and survival of myeloma cells within the BM.
Two TNF (tumor necrosis factor) family members known to play key roles in normal B-cell biology, BLyS/BAFF (B-lymphocyte stimulator/B cell activating factor of the TNF family) and APRIL (A PRoliferation-Inducing Ligand), also promote the survival of various malignant B-cell types, including myeloma.11–13 BAFF binds to three TNF-R-related receptors, BCMA (B-cell maturation antigen), TACI (transmembrane activator and CAML interactor), and BAFFR (BAFF-receptor), whereas APRIL binds to TACI and BCMA and to heparan sulfate proteoglycans (HSPG) such as syndecan-1 (CD138).11–13 All myeloma cells express high levels of syndecan-1 and one or more of these three TNF-R-related receptors. Culture of myeloma cells with BAFF and/or APRIL leads to enhanced survival of these malignant cells in vitro14,15 and promotes their adhesion to stromal cells.16 APRIL and BAFF induce myeloma cell adhesion and survival through activation of nuclear factor-κB (NF-κB), phosphatidylinositol-3 (PI-3) kinase/Akt and mitogen-activated protein kinase (MAPK) pathways and upregulation of the antiapoptotic factors MCL-1 and BCL-2 in myeloma cells.14,16 Although myeloma cells can express very low levels of BAFF and APRIL, circulating levels of these cytokines in myeloma patients is higher than that in normal individuals, suggesting that BM microenvironmental cells are a major source of these factors in myeloma patients.14,16 It has been shown that differences in TACI gene expression can distinguish tumors with a BM microenvironment-dependent signature (TACIhigh) from those with a plasmablastic signature (TACIlow),17 suggesting that TACIhigh myeloma cells may be more sensitive to growth factor withdrawal.
Inhibition of BAFF and APRIL using a soluble receptor, TACI-Ig, in culture of L363 and RPMI8226 cell lines causes cultured myeloma cells to die rapidly.14 In coculture of certain myeloma cell lines with the supporting osteoclasts or dendritic cells, TACI-Ig addition resulted in reduced myeloma cell growth and diminished clonogenic potential.18,19 In the current study, we further tested the involvement of APRIL and BAFF in myeloma pathogenesis using two inhibitors—atacicept (TACI-Ig) and BAFFR-Ig—in our in vivo20,21 and ex vivo22,23 experimental systems for primary myeloma. Whereas atacicept binds and neutralizes both BAFF and APRIL, BAFFR-Ig blocks only BAFF y. For our in vivo model, SCID- (severe combined immunodeficiency) hu mice are constructed by implanting a human fetal bone into which primary human myeloma cells are directly injected. Myeloma cells from the majority of patients interact with the human BM, grow exclusively in or on the implanted bone and produce myeloma manifestations, including stimulation of osteoclastogenesis and angiogenesis of human origin, suppression of osteoblastogenesis and induction of severe osteolytic bone disease. This SCID-hu system has been successfully used to study the role of BM microenvironment in myeloma.24–26 We also recently established a coculture system for the growth of primary myeloma cells ex vivo using authentic bone-resorbing osteoclasts, which are typically activated in myelomatous BM. Cultured osteoclasts consistently support long-term survival and proliferation and protect myeloma cells from drug-induced apoptosis.22,23 Osteoclasts have been shown to express high levels of APRIL and BAFF and thus may represent an important paracrine source of these survival factors.17 Here, for the first time we demonstrate the association between TACI gene expression by myeloma cells and their response to inhibitors of APRIL and BAFF in vivo and ex vivo.
Materials and methods
Myeloma cells
Myeloma cells were obtained from heparinized BM aspirates from 17 patients with active myeloma during scheduled clinic visits. Signed Institutional Review Board-approved informed consent forms are kept on record. Pertinent patient information used in animal experiments is provided in Table 1. The BM samples were separated by density centrifugation using Ficoll-Paque (specific gravity, 1.077 g ml−1; Amersham Biosciences Corp., Piscataway, NJ, USA), and the proportion of myeloma plasma cells in the light-density cell fractions was determined by CD38/CD45 flow cytometry. Aliquots of BM cells were taken for in vivo studies. The rest of the BM cells were used for isolation of plasma cells by CD138 immunomagnetic bead selection (Miltenyi-Biotec, Auburn, CA, USA).27 The isolated plasma cells were subjected to global gene expression profiling as described previously.27–29
Table 1.
Patients' characteristics, changes in hlg levels in control-, atacicept- and BAFFR-lg-treated SCID-hu mice, and myeloma cell response to atacicept in coculture with osteoclastsa
| In vivo studies | hlg (% of pretreatment)g | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Stageb | Prior therapy |
Isotype | 70-gene modelc |
7-group modeld |
TACI signalb |
BAFFR signale |
BCMA signalf |
PBS | Atacicept | BAFFR-lg | ||
| 1 | llla | NO | IgGκ | Low risk | Low bone | 2882 | 254 | 17 034 | 471 | 36 | 240 | ||
| 2 | llla | NO | IgAκ | High risk | MAF | 2015 | 36 | 10 773 | 150 | 34 | 85 | ||
| 3 | llla | NO | IgGλ | High risk | Proliferation | 1669 | 22 | 15 759 | 244 | 54 | 114 | ||
| 4 | llla | NO | IgGκ | Low risk | Myeloidy | 1491 | 101 | 10 635 | 1448 | 120 | 244 | ||
| 5 | llla | NO | IgGκ | Low risk | Hyperdiploid | 1370 | 37 | 17 630 | 120 | 51 | 120 | ||
| 6 | llla | NO | IgGκ | High risk | CCND1-2 | 855 | 71 | 20 346 | 798 | 68 | 150 | ||
| 7 | llla | NO | IqAλ | Low risk | CCND1-2 | 805 | 819 | 13 643 | 137 | 47 | 346 | ||
| 8 | llla | NO | IgGκ | High risk | Proliferation | 655 | 42 | 14 247 | 1699 | 1541 | 1710 | ||
| 9 | lllb | NO | IgGλ | Low risk | Low bone | 599 | 104 | 11 589 | 1465 | 148 | 315 | ||
| 10 | llla | NO | IgGλ | High risk | Proliferation | 559 | 55 | 3985 | 1466 | 1354 | ND | ||
| 11 | llla | YES | IgAλ | High risk | Proliferation | 303 | 30 | 22 122 | 1747 | 439 | ND | ||
| Ex vivo studies | MTT (% of control)i | ||||||||||||
| Patient | Staged | Prior therapy | Isotype | 70-gene modelc | 7-group modeld | TACI signalh | BAFFR signale | BCMA signalf | Atacicept | BAFFR-Ig | |||
| 12 | llla | NO | IgGκ | Low risk | CCND1-2 | 1720 | 485 | 14 658 | 44 | ND | |||
| 13 | lllb | NO | IgGλ | High risk | Proliferation | 1444 | 59 | 17 575 | 64 | 105 | |||
| 14 | llla | NO | IgGλ | Low risk | Hyperdiploid | 1113 | 56 | 16 244 | 54 | ND | |||
| 15 | llla | NO | IgGκ | Low risk | MMSET | 574 | 820 | 15 418 | 96 | 93 | |||
| 10 | llla | NO | IgGλ | High risk | Proliferation | 559 | 55 | 3985 | 89 | ND | |||
| 16 | lllb | YES | IgGκ | High risk | Proliferation | 362 | 108 | 13740 | 100 | 102 | |||
| 17 | lllb | YES | Freeλ | High risk | Proliferation | 175 | 28 | 3388 | 88 | ND | |||
Abbreviations: BAFFR, B-cell activating factor of the TNF family receptor; BCMA, B-cell maturation antigen; hIg, human Ig; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SCID, severe combined immunodeficiency.
Patients were numbered in each study according to TACI signal expression level.
Stage at diagnosis, according to the Durie-Salmon staging system.
Patients were segregated into low- and high-risk groups according to Shaughnessy et al.29
Patients were segregated into low- and high-risk groups according to Zhan et al.28
BAFFR expression was analyzed by microarray. Signal level >107 is considered high.
BCMA expression was analyzed by microarray. Signal level > 15 000 is considered high.
Circulating hlg was determined by ELISA and calculated as percent of pretreatment level (pre-Rx).
TACI expression was analyzed by microarray. Signal level > 800 is considered TACIhigh.
Response to atacicept and BAFFR-lg (10 μg ml−1) was determined using MTT assay following 5–7 days of treatment. Results are expressed as percentage of control.
Construction of primary myelomatous SCID-hu mice
SCID-hu mice were constructed as described previously.20,21,24 Briefly, 6- to 8-week-old CB.17/Icr-SCID mice were obtained from Harlan Sprague Dawley (Indianapolis, IN, USA) and were housed and monitored in our animal facility. The University of Arkansas for Medical Sciences (UAMS) Institutional Animal Care and Use Committee approved all experimental procedures and protocols. The human fetal bones were obtained from Advanced Bioscience Resources (ABR, Alameda, CA, USA). The bone (femur or tibia) was inserted subcutaneously through a small (5 mm) incision. The incision was then closed with sterile surgical staples, and engraftment of the bones was allowed to take place for 6–8 weeks. For each experiment, 5–10 × 106 mononuclear cells containing >15% plasma cells in 100 μl of phosphate-buffered saline (PBS) were injected directly into the implanted bone. Mice were periodically bled from the tail vein and changes in the levels of circulating human Ig (hIg) of the tumor M-protein isotype were used as an indicator of myeloma growth. When hIg levels reached 15 μg ml−1 or higher, two to three mice injected with cells from the same patient were used for each experiment. Usually, the hosts with the higher hIg levels, indicative of higher tumor burden, were selected for treatment, while the others served as controls. At the end of each experiment, the myelomatous human bone was histologically processed and bone sections were stained with hematoxylin and eosin (H&E).
In vivo treatment with soluble receptors
In 9 of 11 experiments, myeloma-bearing SCID-hu mice were injected three times a week intraperitoneally with approximately 10 mg kg−1 atacicept (200 μg per mouse; n = 11), 10 mg kg−1 BAFFR-Ig (n = 9) or with vehicle (PBS; n = 11) for 4–6 weeks. Hosts engrafted with cells from patients 10 and 11 were similarly treated with 5 mg kg−1 atacicept. TACI–Fc5 (atacicept) and BAFFR–Fc5 Fc fusion proteins were provided by ZymoGenetics Inc., Seattle, WA, USA. These soluble receptors were constructed using the extracellular domain of TACI or BAFFR fused to a form of the Fc portion of human IgG1 that contains mutations preventing complement binding and FcγR engagement.
Determination of human Ig levels
Levels of human κ and λ light chains were determined by ELISA as described previously.20,21 All samples from each experiment were analyzed in the same assay at the end of each experiment to avoid potential complications from interassay variability.
Myeloma plasma cells and osteoclasts cocultures
Highly purified bone-resorbing osteoclasts were prepared as described previously.22,23 Briefly, human peripheral blood mononuclear cells were cultured at 2.5 × 106 cells per ml in α-minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), antibiotics, RANKL (50 ng ml−1) and macrophage colony-stimulating factor (25 ng ml−1) (osteoclast medium) for 10–14 days, at which time the cultures contained large number of multinucleate osteoclasts exhibiting bone-resorbing activity.22,23
For coculture experiments, osteoclasts were washed three times with phosphate-buffered saline (PBS) to detach and remove nonadherent cells. To test the effects of atacicept and BAFFR-Ig inhibition on osteoclast-induced tumor growth/survival, myeloma plasma cells (0.5–1 × 106 cells per ml in osteoclast medium) were cultured with osteoclasts in 24-well plates (1 ml per well) in the presence or absence of 1–10 μg ml−1 atacicept or BAFFR-Ig for 5–7 days. At the end of each experiment, myeloma plasma cells were collected and subjected to an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.22
Immunohistochemistry
Cytospin slides of myeloma cells (40 000 per slide) were fixed with HistoChoice (Amersco, Solon, Ohio) for 20 min, thoroughly washed and incubated in citrate buffer in a water bath (80 °C, 30 min) for antigen retrieval. Following peroxidase quenching with 3% hydrogen peroxide for 10 min, the slides were incubated with monoclonal antibodies against human APRIL (Axxora, San Diego, CA, USA; 10 μg ml−1) or BAFF (ZymoGenetics Inc. 15 μg ml−1) for 60 min. The assays were completed using immunoperoxidase kit from DAKO (Carpinteria, CA, USA) and counterstaining with hematoxylin.
Statistical analysis
All values are expressed as mean ± s.e.m. Student's paired t-test was used to test the effect of treatment on myeloma burden. The Spearman's rank correlation coefficient analysis was used to determine the association between levels of TACI gene expression and changes in myeloma burden in vivo. The Fisher's exact test was used to determine the between levels of TACI gene expression and global gene expression-based prognosis systems.
Results
Antimyeloma response of atacicept and BAFFR-Ig in SCID-hu mice
Myeloma cells from 11 patients were successfully engrafted in SCID-hu mice and used for this study. The growth pattern of myeloma cells in this model has been well characterized.20,21,24 In nine experiments, myeloma growth was restricted to the implanted BM (medullary myeloma), while myeloma cells from patients 8 and 10 grew also on the outer surface of the implanted bone (extramedullary myeloma). When SCID-hu mice had established myeloma as indicated by increased hIg,24 hosts engrafted with myeloma cells from different patients were treated with atacicept (n = 11), BAFFR-Ig (n = 9) or PBS (n = 11) for 4–6 weeks. Atacicept and BAFFR-Ig did not cause apparent toxicities in any of the experiments.
For assessing antitumor response, reduced tumor burden defined as reduction of hIg levels from pretreatment levels and growth rate delay defined as reduced myeloma burden by more than 25% compared with PBS-treated hosts.30 Compared with PBS-treated hosts, in which tumor burden (hIg) increased from pretreatment levels in all experiments, atacicept treatment markedly reduced tumor burden from pretreatment level in six experiments (patients 1, 2, 3, 5, 6 and 7), inhibited myeloma growth rate in three experiments (patients 4, 9 and 11) and had no antitumor effect in two experiments (patients 8 and 10) (Table 1). In contrast, BAFFR-Ig treatment resulted in tumor reduction in one experiment (patient 2), growth rate inhibition in five experiments (patients 1, 3, 4, 6 and 9) and no effect in three experiments (patients 5, 7 and 8) (Table 1). Overall changes in hIg levels relative to pretreatment values in atacicept-treated hosts were significantly lower than that in PBS-treated hosts (P<0.05, Figure 1). BAFFR-Ig treatment also resulted in reduced hIg from pretreatment levels but the change was insignificantly different than in control PBS-treated hosts (P<0.08, Figure 1).
Figure 1.
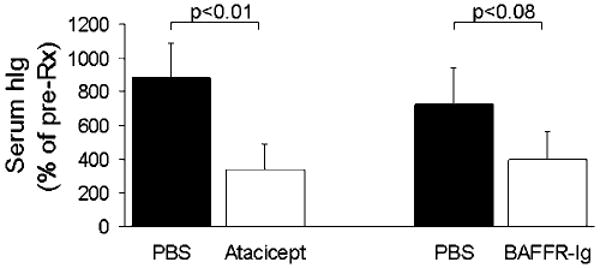
Significant antimyeloma efficacy of atacicept, but not BAFFR-Ig, in SCID-hu mice. Myelomatous SCID-hu mice engrafted with myeloma cells from different patients were treated with atacicept (n = 11), BAFFR-Ig (n = 9) or PBS (n = 11) for 4–6 weeks. Serum hIg levels, reflecting tumor burden, were assessed by ELISA and expressed as percentage of pre-Rx level. BAFFR, B-cell activating factor of the TNF family receptor; PBS, phosphate-buffered saline.
The in vivo effect of atacicept and BAFFR-Ig on hIg levels and tumor burden in three representative experiments is shown in Figure 2. Histological examinations of myelomatous bone sections from these experiments further confirmed changes in myeloma burden as assessed by measurement of hIg levels and demonstrated parallel changes in myeloma cell infiltration following treatment (Figure 2). Since atacicept significantly inhibited myeloma growth in primary myelomatous SCID-hu mice more consistently than BAFFR-Ig, we focused our further analyses and in vitro work on atacicept.
Figure 2.
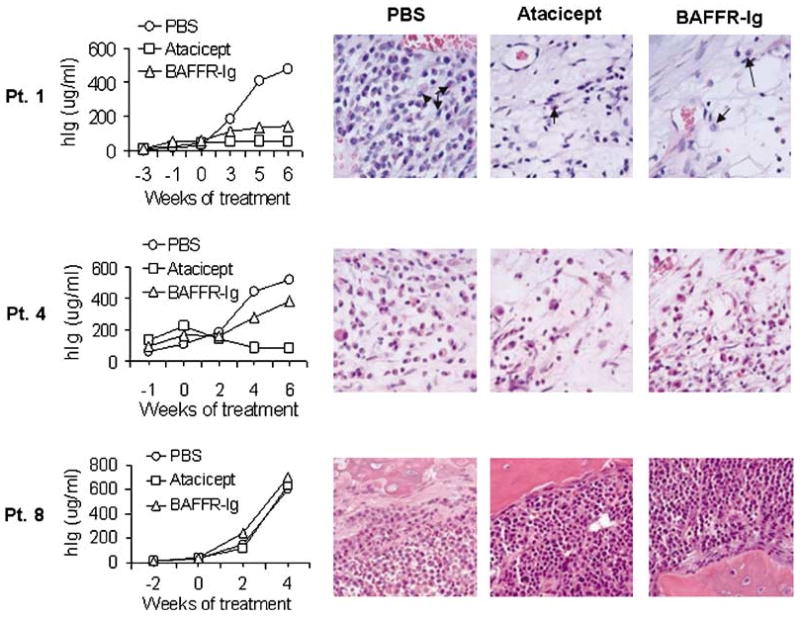
Heterogeneous in vivo antimyeloma effect of atacicept and BAFFR-Ig. Three representative experiments (patients 1, 4 and 8) are shown, demonstrating time course effect on serum hIg (left panel) and histological examinations of myelomatous bone sections from the engrafted mice (right panel). Note parallel changes in hIg levels and myeloma cell infiltration of the implanted human bone in SCID-hu mice following treatment. Both atacicept and BAFFR-Ig inhibited growth of myeloma cells in patient 1 and had no effect on the growth of myeloma cells in patient 8. The myeloma cells in patient 4 responded to atacicept but not to BAFFR-Ig. Arrows indicate myeloma plasma cells. BAFFR, B-cell activating factor of the TNF family receptor.
Association between response to atacicept in SCID-hu mice and myeloma cell TACI expression and molecular classification
Next, we looked for an association between TACI gene expression in myeloma plasma cells and changes in myeloma burden in PBS- and atacicept-treated hosts (Figure 3a). It has been shown previously that global gene expression profiling can be successfully used to assess TACI expression accurately by myeloma plasma cells and the differences in TACI gene expression can distinguish tumors with a BM microenvironment-dependent signature (TACIhigh) from those with a plasmablastic signature (TACIlow).17 Using global gene expression profiling on CD138-selected myeloma plasma cells from newly diagnosed myeloma patients enrolled in total therapy 2 clinical trial in our institute,28 we measured a TACI signal level of <800 in 25% of all subjects and designated those samples as TACIlow17. Myeloma cells expressing TACI mRNA at a signal level >800 were designated TACIhigh. In our in vivo experiments, myeloma cells from 4 of the 11 patients were TACIlow (patients 8–11; Table 1). Atacicept treatment in six of seven TACIhigh cases led to reduced serum hIg compared with pretreatment levels, and two of the four TACIlow cases exhibited delayed growth (patients 9 and 11), whereas none of the TACIlow myeloma had reduced hIg level from pretreatment following treatment with atacicept (P<0.01, Fisher's exact test, Table 1).
Figure 3.
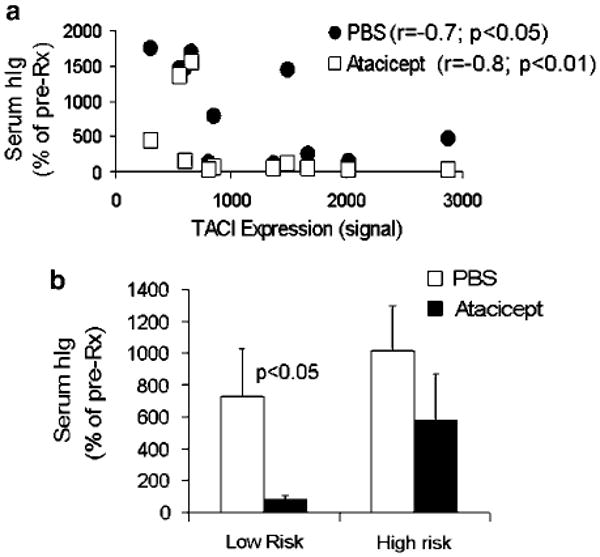
Response to atacicept is associated with TACI gene expression in myeloma cells and is higher in low-risk myeloma. (a) Correlation coefficients (Spearman's rank test) between myeloma growth and TACI expression by myeloma cells in PBS- and atacicept-treated SCID-hu mice were −0.7 (P<0.05) and −0.8 (P<0.01), respectively. (b) Response to atacicept was determined in myeloma samples that were classified into low-risk (n = 5) and high-risk (n = 6) groups according to the 70-gene model.29 Note that the low-risk myeloma were highly sensitive to the antimyeloma effect of atacicept.
In the control SCID-hu mice treated with PBS, we observed an inverse correlation between the levels of TACI gene expression and increased hIg compared with pretreatment levels (r= −0.7, P<0.05, Spearman's rank correlation coefficient), suggesting that TACIlow myeloma cells, which are typically associated with a proliferative phenotype,17 also have higher growth rate in our in vivo model system (Figure 3a). An even higher inverse correlation rate was observed between TACI gene expression level and changes in tumor burden following treatment with atacicept (rSpearman's = −0.8, P<0.01), reflecting the ability of atacicept treatment to reduce tumor burden efficiently in the TACIhigh myeloma-bearing mice. In contrast, most myeloma cases expressed very low levels of BAFFR (as expected, since BAFFR is downregulated as B cells differentiate into plasma cells) and a high levels of BCMA (Table 1). Levels of BAFFR or BCMA expression were not associated with response to atacicept or BAFFR-Ig (data not shown).
To further analyze the association within TACI expression level, response to atacicept in SCID-hu mice and patients' outcome, we utilized two of our myeloma plasma cell molecular classification models: the 70-gene model, which accurately defines low- and high-risk patients29 and the 7-group model, which segregates all cases into seven disease subtypes based on known genetic lesions.28 Based on the 70-gene model, five cases from our in vivo study (patients 1, 4, 5, 7 and 9) were classified as the low-risk group and six were classified as the high-risk group (patients; 2, 3, 6, 8, 10 and 11). While four of the five low-risk cases (80%) were TACIhigh, three of six high-risk cases (50%) were TACIlow (Table 1). We then sought to analyze the response to atacicept of low- and high-risk myeloma cases. The analysis revealed that atacicept reduced tumor burden from pretreatment level in low-risk myeloma, and that atacicept-treated hosts had lower hIg levels than that in matching control hosts by the end of the experiment (P<0.03). However in atacicept-treated hosts harboring high-risk myeloma, hIg levels were increased from pretreatment and were not statistically significantly different from hIg levels in matching PBS-treated control hosts (Figure 3b).
We also looked for the association between TACI expression level and the gene expression profile-based prognostic system using the 7-group model.28 To increase statistical validity, we tested patients whose samples used in the in vivo and in vitro study (n = 17, Table 1). Based on the 7-group model, 7 of 10 (70%) TACIhigh cases were categorized in the subgroups associated with superior clinical outcome (low bone, hyperdiploid, CCND1-2) groups, while 6 of 7 TACIlow cases (85%) belonged to the poor outcome (proliferation, MAF, MMSET) groups (P<0.036, Fisher's exact test, Table 1).28
Role of APRIL and BAFF in the osteoclast-induced myeloma growth
To further shed light on the role of APRIL and BAFF in myeloma and validate our in vivo findings of an antimyeloma effect of atacicept, we employed our primary myeloma–osteoclast coculture system. We previously demonstrated the stimulatory effect of highly purified bone-resorbing osteoclasts on long-term in vitro survival of primary myeloma plasma cells.22 To shed light on the molecular consequences of this interaction, myeloma cells from eight patients were cocultured with osteoclasts, generated from mobilized blood from eight different patients for 4 days. We then performed global gene expression profiling and looked for changes in expression levels of APRIL and BAFF in pre and cocultured myeloma plasma cells, and in osteoclasts cultured alone or cocultured with myeloma cells.31,32 As reported previously,17 osteoclasts express high levels of both APRIL and BAFF compared with myeloma cells, which had low but detectable levels of these cytokines. After coculture, expression level of APRIL and BAFF remained high in the osteoclasts and was also significantly upregulated in the myeloma plasma cells themselves (Figure 4a). Using immunohistochemistry for APRIL and BAFF, we further demonstrated increased production of these cytokines in cocultured myeloma cells, thus excluding the possibility of fault microarray results due to contamination with osteoclasts (Figures 4b and c). These data support previous reports on autocrine production of these APRIL and BAFF by myeloma cells.14,15
Figure 4.
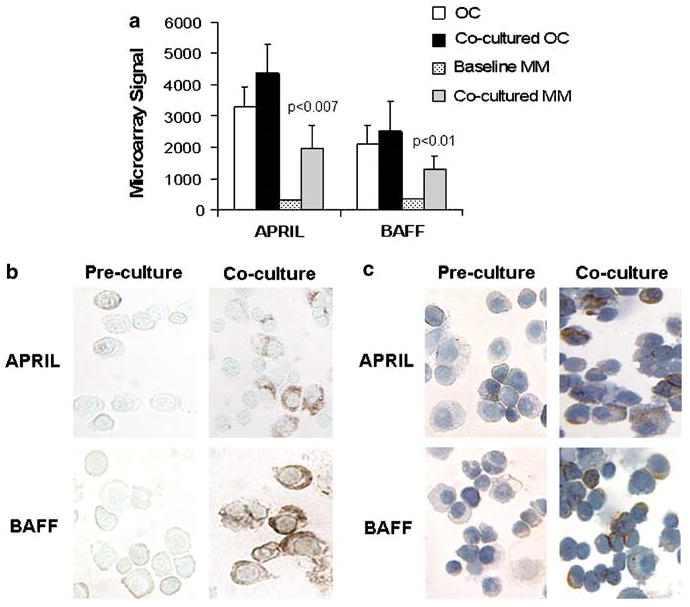
APRIL and BAFF expression and production in myeloma-osteoclast cocultures. (a) APRIL and BAFF gene expression in MM and osteoclasts (OC, n = 8) at baseline and after coculture as determined by microarray. Note the high expression of these cytokines by osteoclasts and their upregulation in myeloma cells after coculture. (b, c) Immunohistochemical staining for APRIL and BAFF in precultured and cocultured myeloma cells isolated from two patients. Note the increased production of these cytokines in cocultured myeloma cells. APRIL, a proliferation-inducing Ligand; BAFF, B-cell activating factor of the TNF family; MM, myeloma cells.
To test the effect of atacicept on myeloma cell growth ex vivo, myeloma plasma cells from seven patients were cocultured with osteoclasts in the absence or presence of atacicept for 5–7 days. Atacicept-inhibited survival of myeloma cells by >35% in three of six experiments (Table 1; P<0.03).18 As in the SCID-hu mice, the antimyeloma effect of atacicept was evident on TACIhigh but not TACIlow cases and, as in the in vivo experiments, was inversely correlated with TACI expression level (r= −0.9, P<0.01; Table 1; Figure 5a). For the three TACIhigh myeloma samples, atacicept-inhibited myeloma cell survival in a dose-related manner (Figure 5b). In three experiments (patients 13 (TACIhigh), 15 and 16 (TACIlow); Table 1), we also tested the ex vivo effect of BAFFR-Ig on myeloma growth. BAFFR-Ig had no antitumor effect in coculture of myeloma cells with osteoclasts, regardless of the level of TACI expression by the tumor cells, suggesting that APRIL plays a key role in myeloma survival in this coculture system.
Figure 5.
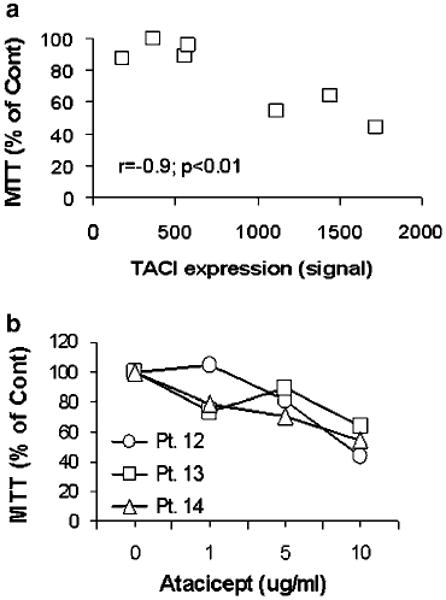
Antimyeloma response of atacicept in myeloma-osteoclast cocultures. (a) Primary myeloma cells (n = 7) were cocultured with osteoclasts in the absence and presence of atacicept (10 μg ml−1) for 5–7 days and then subjected to MTT assay. Percentage inhibition of myeloma cell growth is plotted against TACI gene expression determined by microarray analysis. Response to atacicept was highly correlated with TACI expression in myeloma cells (r= −0.9, P<0.01). (b) Atacicept-inhibited growth of TACIhigh myelomas (patients 12, 13 and 14; see Table 1) in a dose-related manner. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Discussion
APRIL and BAFF have been shown to be upregulated in the myeloma microenvironment, stimulate survival and proliferation of myeloma cells in vitro through autocrine and paracrine mechanisms and protect tumor cells from drug-induced apoptosis.15–18,33 In this study, we demonstrated that osteoclasts express high levels of APRIL and BAFF and that these cytokines are upregulated by myeloma cells after coculture with osteoclasts. Atacicept effectively reduced the growth of TACIhigh primary myeloma cells in SCID-hu mice and in long-term coculture with the supportive osteoclasts. For the first time, we also demonstrated using global gene expression profiling that antimyeloma efficacy of atacicept in vivo and ex vivo was significantly correlated with expression of TACI by myeloma cells. These results are in accordance with the previous reports demonstrating the ability of TACI-Ig to induce apoptosis of TACI-expressing myeloma cell lines in vitro14,18 and to inhibit the stimulatory effects of osteoclasts18 and dendritic cells19 on myeloma cells. Our findings that atacicept more consistently demonstrated antimyeloma effects in our experimental systems than BAFFR-Ig suggest that APRIL, either alone or together with BAFF, plays a role in the growth and/or survival of myeloma cells from patients of specific subtypes.
Our data strongly support a recent report associating TACIhigh myeloma with a BM microenvironment-dependence signature, suggesting that these myeloma cells are likely to be more sensitive to growth factor withdrawal than TACIlow myeloma cases harboring plasmablastic and proliferative gene signatures.17 The significant inverse correlation that we found between TACI expression and the growth rate of myeloma cells in SCID-hu mice (Figure 3) emphasizes the clinical relevance of this experimental system, as TACIlow patients were reportedly characterized by an increased percentage of stage III myeloma, reduced hemoglobin level and increased bone lesions.17 Studies by Moreaux et al.,17 and our current data indicate that global gene expression profiling for assessing TACI expression and molecular classifications may prove to be a useful approach for tailoring therapeutic intervention with APRIL and BAFF inhibitors in myeloma. These findings are supported by the data demonstrating high correlation in expression of TACI at the RNA and protein levels14,17 as well as with other genes critically involved in myeloma pathogenesis.27,28,32
The majority of TACIhigh cases were associated with low-risk myeloma according to our recently reported 70-gene model29 or molecularly classified subgroups with superior survival based on the 7-group model (Table 1).28 Furthermore, the majority of TACIhigh samples tested in vivo and all three TACIhigh cases tested ex vivo responded to atacicept. Our in vivo study also identified exceptional TACIhigh cases that responded to atacicept but were classified as high-risk myeloma (in vivo studies patients 2, 3 and to a lesser extent patient 6; ex vivo studies patient 13), while two TACIlow cases (in vivo studies patient 9; ex vivo studies patient 15) were molecularly classified in the low-risk group (Table 1). This suggests that some myeloma cells from cases with high-risk classifications may also still maintain a certain degree of dependency on the BM microenvironment compared with myeloma cells from most high-risk cases. If true, this refinement suggests that even some high-risk subtype patients may also benefit from microenvironment-targeted therapy. Intriguingly, two in vivo experiments with TACIlow myeloma (patients 9 and 11) showed a partial response to atacicept. The heterogeneous effect of atacicept may be related to yet unknown functions of APRIL and/or BAFF in myelomatous BM or confounding factors associated with the model systems.
In contrast to atacicept, BAFFR-Ig (which binds to BAFF but not APRIL) partially inhibited growth of myeloma cells in vivo and had no antitumor effect in cocultures. It has been demonstrated previously that TACI-expressing myeloma cells had a higher response to APRIL than to BAFF, and that certain myeloma cells responded to APRIL but not to BAFF.14 Our study suggests that blocking BAFF by itself may not be sufficient to impact myeloma progression. However, since BAFF has been demonstrated to protect myeloma cells from drug-induced apoptosis, combination therapy of anti-BAFF compounds with antimyeloma agents such as dexamethasone and lenalidomide may help overcome de novo drug resistance.16 Previous reports indicated that heparan sulfate proteoglycans (HSPGs) are important for APRIL-induced tumor growth by mediating the binding of APRIL to tumor cells and activating TACI signaling.34,35 Syndecan-1 is produced and shed from myeloma plasma cells and is involved in myeloma cell metastasis and growth.36–38 A recent study also demonstrated that syndecans, including syndecan-1, directly bind to tumor cells and activate TACI signaling.39 These studies may suggest that in contrast to BAFF, APRIL plays a nonredundant role in the survival of myeloma due to its interaction with syndecan-1 and possibly other HSPGs.
Myeloma is typically associated with increased osteoclast activity and severe osteolytic lesions in more than 80% of patients.40 Recent experimental studies suggest that myeloma bone disease drives tumor progression.24,30,41 Cultured osteoclasts alone support long-term survival of myeloma cells in vitro22,42 and protect them from drug-induced apoptosis.23,32 The molecular mechanisms by which osteoclasts affect myeloma cells are progressively being revealed. IL-6 and osteopontin, two myeloma growth factors produced by osteoclasts, play certain roles in osteoclast-induced myeloma growth.22,42 We have reported that fibroblast activation protein (FAP), a cell-surface serine protease with dual dipeptidyl peptidase (DPP) and collagenase proteolytic activity, was consistently upregulated in cocultured osteoclasts and supported myeloma cell survival.31 In the current study, we further confirmed that osteoclasts are a major source of APRIL and BAFF in myelomatous bone17 and that the direct interaction between osteoclasts and myeloma cells results in upregulation of these survival factors by myeloma cells. The coculture experiments revealed that, as in our animal studies, growth of TACIhigh myeloma cells was severely attenuated in the majority of cases by atacicept but not by BAFFR-Ig, suggesting that APRIL is a critical survival component in myeloma-osteoclast cocultures.
In summary, our studies in SCID-hu mice and myeloma-osteoclast cocultures have revealed that atacicept, and to a lesser extent BAFFR-Ig, effectively attenuates growth of TACIhigh myelomas, the majority of which were molecularly classified into the low-risk, superior survival subgroups and are associated with a BM-dependence gene signature. Thus, myeloma patients whose tumors can be selectively classified according to high TACI gene expression may benefit from atacicept treatment.
Acknowledgments
This work was supported by Grants CA-93897 (SY), CA55819 (BB and JS), and CA97513 (JS) from the National Cancer Institute, by Senior and Translational Research Awards from the Multiple Myeloma Research Foundation (SY) and by a grant from ZymoGenetics Inc. and Merck-Serono International, SA (JS and SY). SY performed research, analyzed and interpreted data, and wrote the paper. AP and XL performed research experiments. SRD provided materials, analyzed and interpreted data. FZ analyzed all microarray data. BB contributed resources including patient material and clinical data. JS conceptualized the work, analyzed and interpreted data. We recognize the efforts of the members of Dr Yaccoby's laboratory; Rinku Saha, Wen Ling and Paul Perkins, and Derek Janszen (ZymoGenetics Inc.) for his assistance with the statistical analysis. We also thank the faculty, staff and patients of the Myeloma Institute for Research and Therapy for their support.
References
- 1.Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. Eur J Cancer. 2006;42:1564–1573. doi: 10.1016/j.ejca.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Hazlehurst LA, Landowski TH, Dalton WS. Role of the tumor microenvironment in mediating de novo resistance to drugs and physiological mediators of cell death. Oncogene. 2003;22:7396–7402. doi: 10.1038/sj.onc.1206943. [DOI] [PubMed] [Google Scholar]
- 3.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 5.Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989;84:2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 7.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between Myeloma cells and marrow stromal cells stimulates production of DKK1 and IL-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 8.Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105:1383–1395. doi: 10.1182/blood-2004-07-2909. [DOI] [PubMed] [Google Scholar]
- 9.Moreau P, Harousseau JL, Wijdenes J, Morineau N, Milpied N, Bataille R. A combination of anti-interleukin 6 murine monoclonal antibody with dexamethasone and high-dose melphalan induces high complete response rates in advanced multiple myeloma. Br J Haematol. 2000;109:661–664. doi: 10.1046/j.1365-2141.2000.02093.x. [DOI] [PubMed] [Google Scholar]
- 10.Kovacs MJ, Reece DE, Marcellus D, Meyer RM, Mathews S, Dong RP, et al. A phase II study of ZD6474 (Zactima, a selective inhibitor of VEGFR and EGFR tyrosine kinase in patients with relapsed multiple myeloma—NCIC CTG IND.145. Invest New Drugs. 2006;24:529–535. doi: 10.1007/s10637-006-9022-7. [DOI] [PubMed] [Google Scholar]
- 11.Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5:235–246. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- 12.Jelinek DF, Darce JR. Human B lymphocyte malignancies: exploitation of BLyS and APRIL and their receptors. Curr Dir Autoimmun. 2005;8:266–288. doi: 10.1159/000082107. [DOI] [PubMed] [Google Scholar]
- 13.Shivakumar L, Ansell S. Targeting B-lymphocyte stimulator/B-cell activating factor and a proliferation-inducing ligand in hematologic malignancies. Clin Lymphoma Myeloma. 2006;7:106–108. doi: 10.3816/CLM.2006.n.046. [DOI] [PubMed] [Google Scholar]
- 14.Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103:689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 16.Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66:6675–6682. doi: 10.1158/0008-5472.CAN-06-0190. [DOI] [PubMed] [Google Scholar]
- 17.Moreaux J, Cremer FW, Reme T, Raab M, Mahtouk K, Kaukel P, et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood. 2005;106:1021–1030. doi: 10.1182/blood-2004-11-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe M, Kido S, Hiasa M, Nakano A, Oda A, Amou H, et al. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia. 2006;20:1313–1315. doi: 10.1038/sj.leu.2404228. [DOI] [PubMed] [Google Scholar]
- 19.Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaccoby S, Barlogie B, Epstein J. Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood. 1998;92:2908–2913. [PubMed] [Google Scholar]
- 21.Yaccoby S, Epstein J. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood. 1999;94:3576–3582. [PubMed] [Google Scholar]
- 22.Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, et al. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 2004;64:2016–2023. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 23.Yaccoby S. The phenotypic plasticity of myeloma plasma cells as expressed by dedifferentiation into an immature, resilient, and apoptosis-resistant phenotype. Clin Cancer Res. 2005;11:7599–7606. doi: 10.1158/1078-0432.CCR-05-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaccoby S, Pearse RN, Johnson CL, Barlogie B, Choi Y, Epstein J. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br J Haematol. 2002;116:278–290. doi: 10.1046/j.1365-2141.2002.03257.x. [DOI] [PubMed] [Google Scholar]
- 25.Yaccoby S, Johnson CL, Mahaffey SC, Wezeman MJ, Barlogie B, Epstein J. Antimyeloma efficacy of thalidomide in the SCID-hu model. Blood. 2002;100:4162–4168. doi: 10.1182/blood-2002-03-0939. [DOI] [PubMed] [Google Scholar]
- 26.Yaccoby S, Wezeman MJ, Zangari M, Walker R, Cottler-Fox M, Gaddy D, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–1757. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]
- 28.Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 30.Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge Y, Zhan F, Barlogie B, Epstein J, Shaughnessy J, Yaccoby Y. Fibroblast activation protein (FAP) is upregulated in myelomatous bone and supports myeloma cell survival. Br J Haematol. 2006;133:83–92. doi: 10.1111/j.1365-2141.2006.05976.x. [DOI] [PubMed] [Google Scholar]
- 32.Colla S, Zhan F, Xiong W, Wu X, Xu H, Stephens O, et al. The oxidative stress response regulates DKK1 expression through the JNK signaling cascade in multiple myeloma plasma cells. Blood. 2007;109:4470–4477. doi: 10.1182/blood-2006-11-056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–1383. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendriks J, Planelles L, de Jong-Odding J, Hardenberg G, Pals ST, Hahne M, et al. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–648. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- 36.Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Yaccoby S, Liu W, Langford JK, Pumphrey CY, Theus A, et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002;100:610–617. doi: 10.1182/blood.v100.2.610. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, MacLeod V, Dai Y, Khotskaya-Sample Y, Shriver Z, Venkataraman G, et al. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood. 2007;110:2041–2048. doi: 10.1182/blood-2007-04-082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bischof D, Elsawa SF, Mantchev G, Yoon J, Michels GE, Nilson A, et al. Selective activation of TACI by syndecan-2. Blood. 2006;107:3235–3242. doi: 10.1182/blood-2005-01-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 41.Croucher PI, De Hendrik R, Perry MJ, Hijzen A, Shipman CM, Lippitt J, et al. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18:482–492. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- 42.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]


