Abstract
Objective
Plasminogen activator inhibitor-1 (PAI-1) over-expression is implicated in vascular disease. However, the effects of a primary increase in PAI-1 expression on arterial remodeling are poorly defined. We tested the hypothesis that recombinant PAI-1 inhibits intimal hyperplasia after vascular injury.
Methods and Results
Rats underwent carotid artery injury and received intraperitoneal injections of saline or mutant forms of PAI-1 for 14 days, including an active, stable mutant (PAI-1-14-1b), a mutant lacking anti-PA activity (PAI-1-R), or a mutant defective in vitronectin (VN) binding (PAI-1-K). All forms of PAI-1 significantly inhibited neointima formation, while elastase-cleaved PAI-1, which lacks both anti-PA and VN-binding functions, did not. Similar effects were observed in a murine model. However, the anti-proliferative effect of PAI-1-R was lost in Vn−/− mice, suggesting that PAI-1 can inhibit intimal hyperplasia in vivo by a VN-dependent pathway not involving direct inhibition of proteases. In vitro, recombinant PAI-1 inhibited wild-type vascular smooth muscle cell (VSMC) proliferation, promoted apoptosis, and inhibited migration. These effects were lost in VN-deficient VSMC.
Conclusion
Recombinant PAI-1 inhibits intimal hyperplasia by inhibiting proteases and binding VN. VN is a key determinant of the anti-proliferative effect of PAI-1 over-expression. PAI-1-R has therapeutic potential to inhibit vascular restenosis without promoting thrombosis.
Keywords: plasminogen activator inhibitor-1, vitronectin, neointima, vascular smooth muscle cell
Plasminogen activator inhibitor-1 (PAI-1) is the primary inhibitor of urinary-type and tissue-type plasminogen activators and a key regulator of fibrinolysis.1, 2 PAI-1 also regulates the function of vascular cells, including vascular smooth muscle cells (VSMC). PAI-1 inhibits VSMC migration by inhibiting plasmin formation and preventing degradation of extracellular matrix (ECM) and elastic laminae.3 PAI-1 binds vitronectin (VN), an ECM protein whose PAI-1 binding site overlaps with those on VN for integrin αVβ3 and u-PA receptor (uPAR), cell surface proteins that control VSMC migration.4 Therefore, PAI-1 can competitively block VSMC-VN interactions and inhibit migration. However, PAI-1 can also promote VSMC migration by binding to uPAR-bound u-PA, leading to conformational changes in PAI-1 and exposure of its high-affinity binding site for LDL receptor-related protein (LRP).5, 6 Binding of PAI-1 to LRP triggers internalization of PAI-1, along with associated u-PA, uPAR, and integrin.7 This internalization process, which appear to occur predominantly at the trailing edge of cells, allows VSMC to detach from the ECM, a process necessary for migration.7–9 In addition to modulating VSMC migration, PAI-1 controls VSMC proliferation and apoptosis.10–12
Several diseases, including diabetes mellitus and atherosclerosis, are characterized by increased PAI-1 expression, both within blood and the vascular wall.13, 14 PAI-1 over-expression may promote intimal hyperplasia and adverse vascular remodeling.15 However, published animal studies have predominantly used knock-out mice to compare the effect of normal vs. absent PAI-1 expression on arterial remodeling.3, 16–20 Relatively little is known about the impact of enhanced PAI-1 expression on arterial remodeling.21 In addition, the role of PAI-1’s cofactor, VN, in controlling the vascular response to PAI-1 over-expression is poorly defined. To examine the impact of a primary increase in PAI-1 expression on vascular remodeling, we administered recombinant PAI-1 to rodents after mechanical arterial injury, using not only active PAI-1, but also PAI-1 mutants lacking inhibitory or vitronectin binding functions. To examine the role of VN in determining the vascular response to PAI-1 over-expression, we studied vascular remodeling after arterial injury in VN-deficient (Vn−/−) mice and performed in vitro experiments with wild-type- and VN-deficient VSMC.
Methods
Proteins
The following recombinant human proteins were expressed and purified as described.22 1) PAI-1-14-1b (PAI-1 N150H, K154T, Q319L, M354I), an active, stable mutant that binds VN normally;23 2) PAI-1-R (T333R, A335R), a reactive center mutant that binds VN normally, but has no detectable anti-proteolytic activity and cannot assume a latent conformation;24 3) PAI-1-K (PAI-1 N150H, K154T, Q319L, M354I, Q123K), which exhibits markedly reduced VN binding, but stable anti-protease activity;25, 26 and 4) PAI-1-AK (PAI-1 N150H, K154T, Q319L, M354I, R101A, Q123K), an active, stable mutant with no detectable VN binding.27, 28 Endotoxin level of proteins was below the FDA limit (5 U/kg) for parenteral drugs. Latent and elastase-cleaved human PAI-1 and mouse multimeric VN were from Molecular Innovations.
Animals
Male Sprague-Dawley rats (300–350 g) were from Harlan Laboratories. C57BL/6J mice were from Jackson Labs. C57BL/6J-congenic VN-deficient (Vn−/−) mice were from Dr. David Ginsburg, University of Michigan.29 Animals received normal chow (Mice: Diet 5001; Rats: Diet 5012, LabDiet). All animal care and experiments were approved by appropriate institutional committees.
Rodent arterial injury models
Injury of rat carotid arteries was performed with a balloon-tipped catheter.21 Animals were injected with bromodeoxyuridine (BrdU) prior to euthanasia.30 Wire injury of femoral arteries of adult male mice (approximately 25 g) was performed as described.31 Operator was blinded to genotype and treatment group during all procedures and analyses.
Statistical analyses
Data are presented as mean ± standard error of the mean. Experimental groups were compared by two-tailed Student’s t test, Mann-Whitney rank sum test, or one-way analysis of variance (ANOVA).
On-line methods supplement
A detailed description of the methods briefly described below is available as a supplement online at http://atvb.ahajournals.org.
Animal experiments
Recombinant PAI-1 was administered by daily intraperitoneal injection. Plasma PAI-1 antigen and activity were measured by ELISA and functional assays. Cross-sections of injured arterial segments were stained with hematoxylin-eosin and intima and media areas were measured. Cell proliferation was assessed by anti-BrdU staining.
Cell culture experiments
Murine aortic VSMC were grown in culture. Effects of recombinant PAI-1 on VSMC proliferation, apoptosis, and migration were measured.
Results
Recombinant PAI-1 inhibits intimal hyperplasia after arterial injury
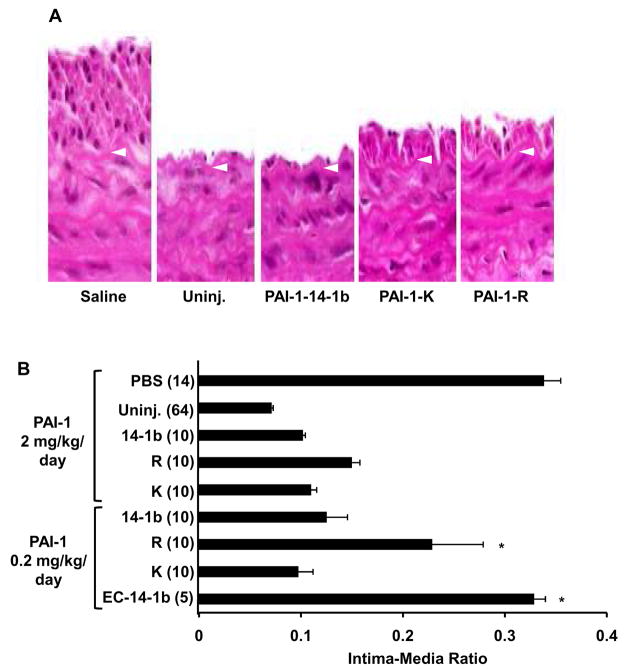
Rat carotid arteries were subjected to balloon injury, after which animals received twice-daily injections of PAI-1-14-1b, PAI-1-R, PAI-1-K (2 mg/kg/day), or saline. Fourteen days later the injured and uninjured carotid arteries were retrieved. Injured arteries in all groups were patent with no detectable thrombus. Injured arteries from saline-treated animals exhibited a robust neointima with significantly increased intima/media ratio (Fig. 1A). Non-injured carotid arteries from all groups appeared normal. Injured arterial segments of rats treated with PAI-1-14-1b demonstrated a marked reduction in intima/media ratio as compared to saline-treated controls. Treatment with PAI-1-R and PAI-1-K also markedly inhibited neointima formation, producing an effect that did not differ significantly from that of PAI-1-14-1b (Fig. 1B). Additional rats underwent balloon carotid injury and received 10-fold lower doses of PAI-1 (i.e. 0.2 mg/kg/day for 14 days). Inhibition of neointima formation was preserved at the lower dose, though the reduced intima-media ratio in PAI-1-R-treated rats did not differ statistically from that of saline-treated controls (Fig. 1B). As another control, 5 rats received elastase-cleaved PAI-1-14-1b (0.2 mg/kg/day for 14 days), which completely lacks protease inhibitory activity and has markedly reduced VN binding affinity. Elastase-cleaved PAI-1-14-1b had no apparent effect on neointima formation (Fig. 1B). To determine if the inhibition of neointima formation by recombinant PAI-1 was accompanied by an inhibition of vascular cell proliferation, we administered BrdU to subsets of the rats described above and detected proliferating cells in the intima by anti-BrdU antibody staining. The % BrdU positive cells at 14 days after injury was significantly higher (P<0.01) in injured carotid segments of rats treated with saline (38.3±2.8%, n=3) than in corresponding segments of rats treated with PAI-1-14-1b (9.7±2.8%, n=3) or PAI-1-R (11.3±3.3%, n=3). As shown in Table 1, peak plasma PAI-1 antigen levels were >1.5 μg/mL in rats treated with recombinant PAI-1 (2 mg/kg/day). Trough plasma PAI-1 antigen levels were approximately 0.5 μg/mL. Rats treated with PAI-1-R showed an increase in plasma PAI-1 antigen, but no increase in plasma PAI-1 activity.
Figure 1.
Inhibition of neointima formation in rats by PAI-1. (A) Representative images of injured carotid segments treated with saline, PAI-1-14-1b, PAI-1-K, and PAI-1-R, (2 mg/kg/day). An uninjured (Uninj.) segment of a saline-treated rat is shown. Arrow heads denote internal elastic lamina. Magnification: ×400. (B) Composite morphometric data, including 2 mg/kg/day and 0.2 mg/kg/day PAI-1 treatment groups. EC-14-1b, elastase-cleaved PAI-1-14-1b. PBS, phosphate-buffered-saline. Intima/media ratio of each group was statistically significantly less (P<0.05) than that of PBS group, except as denoted by asterisk.
Table 1.
Plasma levels of recombinant PAI-1 achieved in rats by intraperitoneal administration (2 mg/kg/day).
| PAI-1 Mutant | Plasma PAI-1 Antigen (μg/mL) | Plasma PAI-1 Activity (μg/mL) | ||
|---|---|---|---|---|
| Peak | Trough | Peak | Trough | |
| Vehicle control | <0.01 | <0.01 | <0.01 | <0.01 |
| 14-1b | 1.61±0.08 | 0.41±0.04 | 1.64±0.08 | 0.56±0.06 |
| R | 1.87±0.14 | 0.54±0.05 | 0.00047±0.00031 | 0.0012±0.00066 |
| K | 1.85±0.16 | 0.42±0.05 | ||
Peak and trough levels were measured in 8 rats/group.
VN deficiency inhibits intimal hyperplasia and blocks suppression of neointima formation by PAI-1
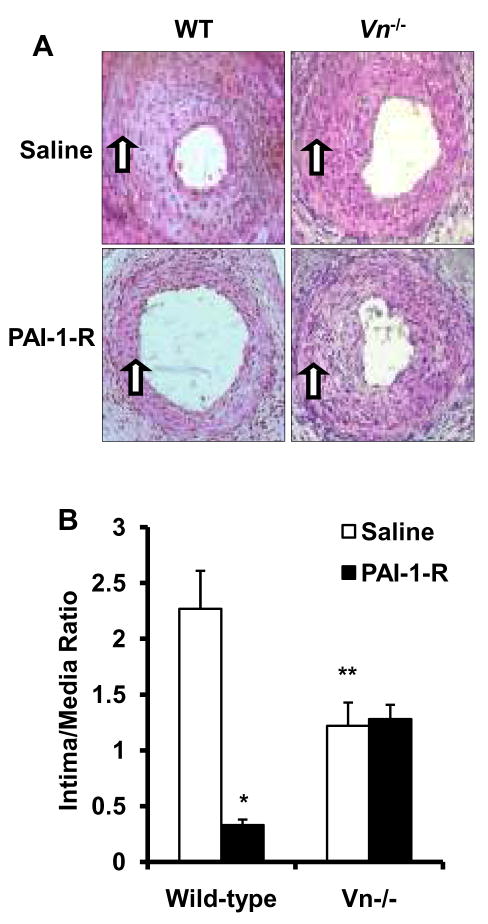
Wild-type mice were subjected to femoral artery injury, after which PAI-1 (2 mg/kg/day) or saline were administered for 21 days. Mean intima-media ratio of mice administered PAI-1-14-1b (0.97±0.19, n=10) was significantly less than that of saline-treated mice (1.73±0.18, n=15, P<0.01). PAI-1-AK, a mutant with no detectable VN binding, also significantly inhibited neointima formation (mean intima-media ratio 0.72±0.12, n=10, P<0.005 vs. saline treated mice). To examine the role of VN in mediating the anti-proliferative effect of PAI-1, wild-type- and Vn−/− mice (n=5/group) were given PAI-1-R (2 mg/kg/day) or saline for 21 days after femoral artery injury. Saline-treated Vn−/− mice exhibited less neointima formation than saline-treated wild-type mice did (Fig. 2). PAI-1-R markedly suppressed neointima formation in wild-type mice, but did not inhibit neointima formation in Vn−/− mice. Peak (Pk) and trough (Tr) plasma PAI-1-R antigen did not differ significantly (P>0.5) between wild-type mice (Pk: 1.76±0.24; Tr: 0.48±0.11 μg/mL) and Vn−/− mice (Pk: 1.66±0.07; Tr: 0.51±0.01 μg/mL). These results suggested that VN supports intimal hyperplasia after arterial injury and that the inhibition of intimal hyperplasia by recombinant PAI-1-R is VN-dependent.
Figure 2.
PAI-1-R inhibits neointima formation in wild-type (WT)-, but not in Vn−/− mice. (A) Representative cross sections. Arrows denote internal elastic lamina. (B) Mean intima/media ratios (n=5/group). *P <0.01, **P <0.03 vs. wild-type, saline-treated group, respectively.
Inhibition of VSMC proliferation by PAI-1 is VN-dependent
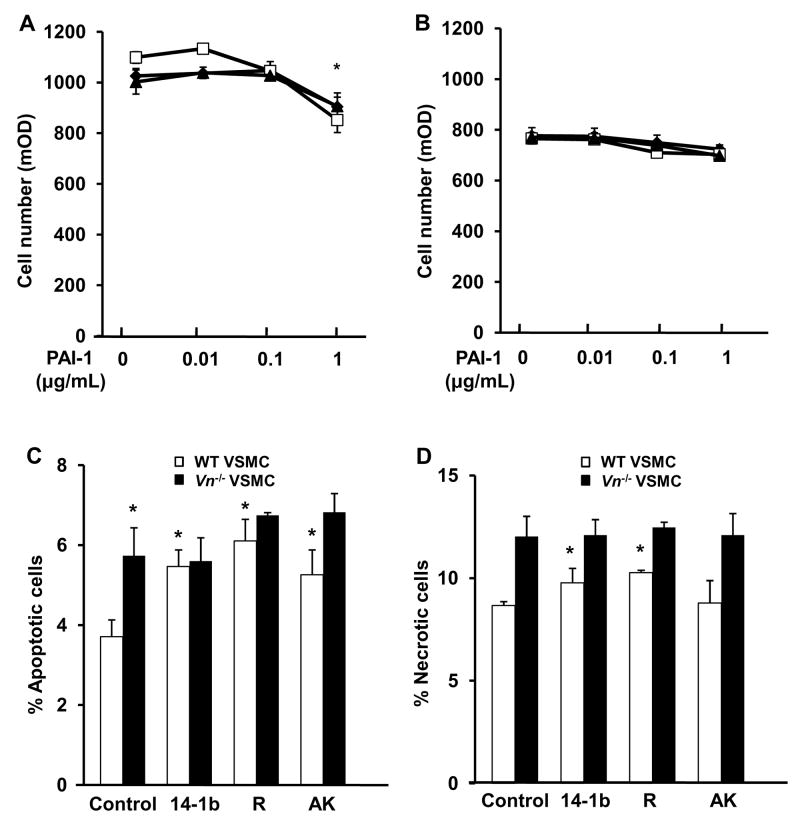
We conducted in vitro experiments to examine potential mechanisms underlying the effects of recombinant PAI-1 and VN-deficiency observed in vivo. Recombinant PAI-1 (1 μg/mL) inhibited wild-type VSMC proliferation, with similar effects observed with PAI-1-14-1b, PAI-1-R, and PAI-1-AK, though lower concentrations of PAI-1 did not produce a significant effect (Fig. 3A), nor did latent PAI-1 (1 μg/mL, data not shown). VSMC isolated from Vn−/− mice grew more slowly in culture than wild-type VSMC did. However, recombinant PAI-1 did not inhibit proliferation of VN-deficient VSMC (Fig. 3B).
Figure 3.
Effects of recombinant PAI-1 on VSMC proliferation and apoptosis are VN dependent. (A, B) VSMC were cultured for 48 hr in the presence of PAI-1-14-1b (diamonds), PAI-1-R (squares), PAI-1-AK (triangles), or vehicle lacking PAI-1 (i.e. PAI-1 0 μg/mL) and cell number was measured. PAI-1 inhibits proliferation of wild-type- (A), but not VN-deficient VSMC (B). *P<0.05 vs. vehicle control. Exposure of VSMC to PAI-1 for 24 hr did not inhibit proliferation relative to vehicle-treated VSMC (data not shown). (C, D) VSMC were cultured 24 hours and analyzed by flow cytometry. Note significant increase in apoptosis (C) and necrosis (D) in response to recombinant PAI-1 in wild-type- (white bars), but not in VN-deficient VSMC (black bars). *P<0.05 vs. vehicle-treated (Control) VSMC. After 48 hr of exposure to PAI-1 rates of apoptosis and necrosis were not significantly in PAI-1- vs. vehicle-treated VSMC (data not shown).
Effect of PAI-1 and VN on VSMC apoptosis
VN-deficient VSMC exhibited a higher incidence of apoptosis than wild-type VSMC did (Fig. 3C). Addition of PAI-1 mutants (1 μg/mL) to growth media promoted apoptosis of wild-type VSMC, but did not promote apoptosis of VN-deficient VSMC (Fig. 3C). The pro-apoptotic effects of PAI-1-14-1b and PAI-1-R were accompanied by increases in the incidence of necrotic cells (Fig. 3D).
PAI-1 and VN co-regulate VSMC migration
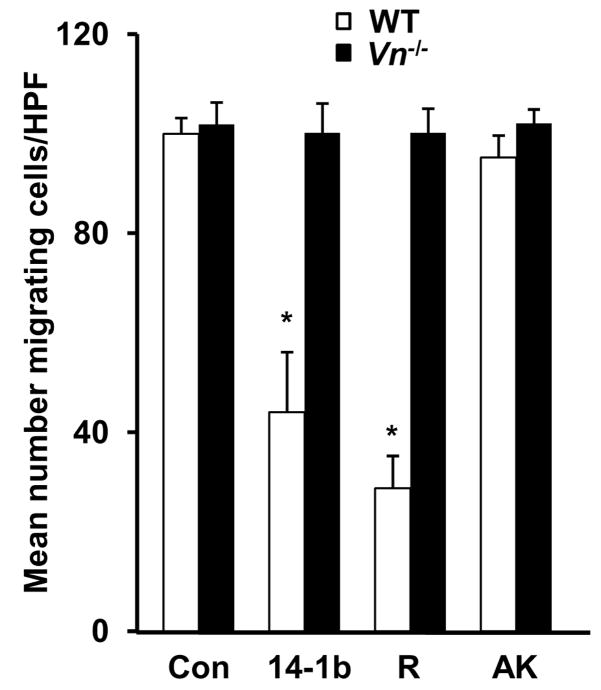
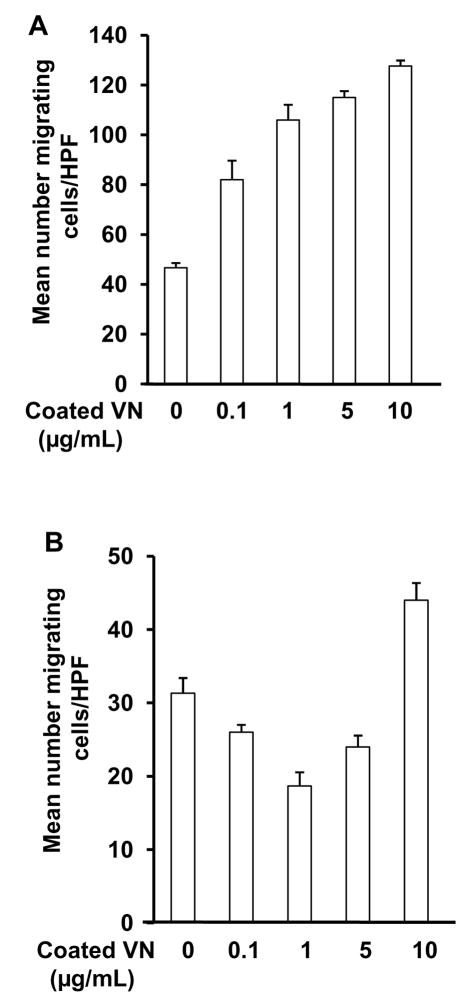
PAI-1-14-1b and PAI-1-R (1 μg/mL) inhibited migration (assessed in all experiments 24 hrs after initial cell seeding) of wild-type VSMC on murine VN (10 μg/mL), while PAI-1 AK had no effect (Fig. 4A). Lower concentrations of PAI-1-14-1b and PAI-1-R (i.e. 0.01-0.1 μg/mL) inhibited wild-type VSMC migration to a proportionately lesser extent (data not shown). However, none of the forms of PAI-1 inhibited migration of VN-deficient VSMC on VN (Fig. 4D), indicating that even when a VN ECM is provided, VN expression by VSMC is required for PAI-1 to inhibit migration. ECM VN promoted migration of wild-type VSMC in a concentration-dependent manner (Fig. 5A). However, in the presence of recombinant PAI-1-14-1b (1 μg/mL), the shape of the dose-response curve of ECM VN concentration vs. migration was distinctly altered, with increasing concentrations of VN initially inhibiting and subsequently increasing VSMC migration (Fig 5B).
Figure 4.
Inhibition of VSMC migration by PAI-1 is VN-dependent. PAI-1-14-1b and PAI-1-R (1 μg/mL) inhibit wild-type VSMC (white bars) migration on VN, but PAI-1-AK does not. PAI-1 does not inhibit migration of VN-deficient VSMC (black bars) on VN. *P<0.05 vs. control (Con).
Figure 5.
Effect of ECM VN on VSMC migration is PAI-1-dependent. (A) When PAI-1 concentration is low (i.e. in absence of added PAI-1), ECM VN vs. VSMC migration dose-response is linear. (B) When PAI-1 concentration is high (i.e. PAI-1-14-1b present at 1 μg/mL) ECM VN vs. VSMC migration dose-response is J-shaped.
Discussion
We administered recombinant PAI-1 to rodents to examine the impact of a primary increase in PAI-1 expression on arterial remodeling. We focused our investigations on: 1) the functional roles of PAI-1’s anti-proteolytic and VN-binding domains in arterial remodeling, and 2) the impact of PAI-1’s cofactor, VN, on PAI-1’s effects in vivo and on cultured VSMC. Our in vivo experiments demonstrate that systemically administered PAI-1 inhibits intimal hyperplasia after arterial injury. Inhibition of neointima formation by PAI-1-R indicates that the capacity of recombinant PAI-1 to directly inhibit proteases is not required to regulate vascular remodeling. The capacity of PAI-1-K and PAI-1-AK to inhibit intimal hyperplasia suggests that direct binding of PAI-1 to VN is also not required to inhibit intimal hyperplasia. However, elastase-cleaved PAI-1, which lacks both anti-protease and VN-binding functions, did not inhibit neointima formation. Therefore, while the capacity of recombinant PAI-1 to either bind VN or directly inhibit proteases each appears sufficient to inhibit neointima formation, neither function is absolutely required, suggesting that PAI-1 can suppress intimal hyperplasia by multiple mechanisms.
Our murine experiments suggest that VN is a key determinant of the anti-proliferative effects of PAI-1, as inhibition of neointima formation by PAI-1-R required VN expression. Multiple mechanisms could account for this effect. Migration of VSMC from the tunica media into the intima is a critical step in neointima formation. By binding VN, PAI-1 blocks VSMC-VN interactions, thereby inhibiting VSMC migration. However, a proteolysis-independent, anti-migratory effect of PAI-1 has only been shown in vitro on purified VN matrices.4 We show that PAI-1-R inhibits intimal hyperplasia in wild-type mice, but not in VN-deficient mice, suggesting that blockade of VN-VSMC interactions--independent of protease inhibition--constitutes a mechanism by which PAI-1 functions in vivo to control vascular remodeling. Nevertheless, other mechanisms could account for the anti-proliferative effect of PAI-1-R. By binding VN, PAI-1-R could displace and destabilize endogenous PAI-1, thereby decreasing PAI-1 activity in the peri-cellular environment, which is proposed to play a key role in cell migration.7, 32–34 In addition, PAI-1 is a downstream mediator of the proliferative effects of TGF-beta, and PAI-1 suppresses TGF-beta expression.18 Therefore, PAI-1-R could block TGF-beta signaling by a dominant-negative effect on endogenous PAI-1 and/or by feedback-inhibition of TGF-beta expression. However, the anti-proliferative effects of PAI-1-K and PAI-1-AK suggest that PAI-1 can also inhibit intimal hyperplasia by inhibiting cell-associated PAs and/or other proteases, such as thrombin, which exhibits proliferative and pro-migratory vascular effects.19 We showed that Vn−/− mice have less neointima formation after arterial injury than wild-type mice do, supporting a key role for VN and its receptors in arterial remodeling after vascular injury.16, 19, 35
Our cell culture experiments offer important insights into mechanisms by which PAI-1 controls VSMC function. Recombinant PAI-1-14-1b, PAI-1-R, and PAI-1-AK, at concentrations achieved in plasma in our in vivo experiments, inhibited proliferation and promoted apoptosis of wild-type VSMC. However, these effects were lost in VN-deficient VSMC, consistent with our in vivo data and suggesting that PAI-1 regulates vascular remodeling by controlling VSMC proliferation and apoptosis. The VN-binding function of PAI-1 would be expected to inhibit proliferation and promote apoptosis by inhibiting cell adhesion to VN.12 Inhibition of plasmin formation could potentially account for the capacity of PAI-1-AK to promote apoptosis, as plasmin exerts anti-apoptotic effects.36 However, PAI-1 exerted anti-apoptotic effects in some studies.11, 37 Further studies are necessary to determine the mechanisms by which PAI-1 mutants lacking VN-binding function promote VSMC apoptosis. In addition, further experiments are needed to determine if active forms of PAI-1 inhibit intimal hyperplasia in VN-deficient mice. Loss of wild-type PAI-1’s anti-proliferative effect in VN-deficient mice would support the hypotheses that PAI-1’s anti-protease function requires VN to inhibit intimal hyperplasia (e.g. effective inhibition thrombin, a mitogen, by PAI-1 in vivo could require VN 38) and that control of vascular wall cell function by PAI-1 requires engagement of cells with ECM VN (e.g. PAI-1 can dissociate cells from VN without directly binding to VN 9). We found that only forms of PAI-1 that bind VN significantly inhibited migration of wild-type VSMC on VN, consistent with previous studies.4 However, recombinant PAI-1 did not inhibit migration of VN-deficient VSMC, even when they were seeded on VN. While VSMC express VN in vivo,39 a functional significance of VSMC VN expression has not previously been reported. Our results suggest that VN production by VSMC themselves is a critical determinant of the effect of PAI-1 on VSMC proliferation, apoptosis, and migration.
Our studies of the effect of ECM VN concentration on VSMC migration (Fig. 5) offer important insights into the inter-connected functions of VN and PAI-1. At low PAI-1 concentrations (i.e. none added), VN promoted VSMC migration in a linear, concentration-dependent fashion, consistent with a previous study.40 However, at high PAI-1 concentrations, the effect of ECM VN was complex, with increasing VN concentration initially inhibiting VSMC migration and subsequently promoting it. The “J-shaped” effect of VN under conditions of PAI-1 over-expression may be explained by the fact that free PAI-1 can promote VSMC migration, while VN-bound PAI-1 does not, as VN-bound PAI-1 does not bind LRP.41 Therefore, at high-levels of PAI-1 expression, VN could inhibit VSMC migration by binding PAI-1 and preventing its LRP-dependent motogenic effect, while higher VN concentrations (i.e. in excess of those needed to saturate PAI-1) could promote VSMC migration by providing “un-blocked” integrin attachment sites. These results have important implications regarding the impact of PAI-1 over-expression on vascular remodeling—i.e. the concentration of PAI-1 in the ECM may play a key role in determining whether VN promotes or inhibits intimal hyperplasia.
Several molecular counterparts of PAI-1 and VN are likely to mediate their effects in our experiments. Integrin αVβ3 binds VN in the ECM to promote VSMC migration and neointima formation.35 This interaction also inhibits apoptosis.12 Blockade of this interaction by binding of PAI-1 to VN would be expected to inhibit intimal hyperplasia. Inhibition of cell-surface-bound uPA and other pro-migratory/mitogenic proteases, such as thrombin, would also be expected to inhibit intimal hyperplasia. However, binding of PAI-1 to LRP can promote VSMC migration in vitro.5 Our in vivo experiments suggest that high concentrations of PAI-1 do not necessarily promote intimal hyperplasia by a LRP-dependent pathway, perhaps because inhibition of integrin-VN interactions and/or proliferative/pro-migratory proteases by PAI-1 produce a dominant effect. Alternatively, as PAI-1-LRP interactions mediate cell detachment from ECM via cross-talk with integrins,9 excessive LRP-signaling, produced in our in vivo experiments and in diseases characterized by PAI-1 over-expression, could inhibit intimal hyperplasia by over-stimulating cell detachment. Additional experiments are necessary to precisely define the molecular targets that mediate the effects of PAI-1 and VN on intimal hyperplasia.
Plasma PAI-1 concentrations in our experiments were higher than those normally achieved by endogenous mechanisms. We do not know the vascular wall concentration of PAI-1 attained in our experiments, nor are the vascular wall concentrations of PAI-1 attained in vivo by endogenous mechanisms known. However, PAI-1 expression in the arterial wall is markedly increased under pathologic conditions.13, 14 Our data suggest that enhanced PAI-1 expression can inhibit neointima formation and that the high vascular wall expression of PAI-1 found in diseased blood vessels is not necessarily a cause of the associated intimal hyperplasia. Our data also have therapeutic implications. We hypothesize that recombinant PAI-1-R could be used to inhibit neointima formation without inhibiting fibrinolysis. In fact, PAI-1-R could potentially promote fibrinolysis by competing with endogenous PAI-1 for binding to VN, fibrin, and cells. Further studies are needed to determine if transient delivery of PAI-1-R produces long-term suppression of neointima formation. DeYoung et al. demonstrated that elevated vascular PAI-1 expression, induced by infusion adenovirus into rat carotid arteries, increased neointima formation after balloon injury.21 The differences between these results and ours may be explained by the fact that recombinant PAI-1 would be expected to remain in the extracellular compartment, whereas in DeYoung’s study PAI-1 was over-expressed within vascular cells. Intracellular PAI-1 expression inhibits apoptosis and promotes proliferation of VSMC.11, 42 In addition, adenovirus may induce inflammatory mediators of intimal hyperplasia and fibrin formation.18
In summary, we demonstrate that recombinant PAI-1 inhibits intimal hyperplasia after vascular injury. This effect depends both on the anti-proteolytic and VN-binding properties of PAI-1. VN expression is a critical determinant of intimal hyperplasia and the anti-proliferative properties of PAI-1. The capacity of recombinant PAI-1 to inhibit intimal hyperplasia has important pathologic implications, as increased PAI-1 expression in diseased blood vessels may not necessarily be a cause of intimal hyperplasia observed in vascular disease, as has been proposed.15 Our study also has therapeutic implications and supports examination of the anti-proliferative effect of PAI-1-R in other preclinical models, as new strategies are needed to inhibit restenosis without increasing thrombotic risk.
Acknowledgments
Funding Sources
Supported by NIH grants HL57346 (WPF) and HL55374, HL54710, HL57346, and HL89407 (DAL).
Footnotes
Disclosures
None.
References
- 1.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–654. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- 2.Ploplis VA, Castellino FJ. Gene targeting of components of the fibrinolytic system. Thromb Haemost. 2002;87:22–31. [PubMed] [Google Scholar]
- 3.Carmeliet P, Moons L, Lijnen HR, Janssens S, Lupu F, Collen D, Gerard RD. Inhibitory role of plasminogen activator inhibitor-1 in arterial wound healing and neointima formation--A gene targeting and gene transfer study in mice. Circulation. 1997;96:3180–3191. doi: 10.1161/01.cir.96.9.3180. [DOI] [PubMed] [Google Scholar]
- 4.Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin alpha-V beta-3 binding to vitronectin. Nature. 1996;383:441–443. doi: 10.1038/383441a0. [DOI] [PubMed] [Google Scholar]
- 5.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Yi, Loskutoff DJ. The Low Density Lipoprotein Receptor-related Protein Is a Motogenic Receptor for Plasminogen Activator Inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 6.Stefansson S, Muhammad S, Cheng X-F, Battey F, Strickland DK, Lawrence DA. Plasminogen activator inhibitor-1 contains a cryptic high afinity binding site for the low density lipoprotein receptor-related protein. J Biol Chem. 1998;273:6358–6366. doi: 10.1074/jbc.273.11.6358. [DOI] [PubMed] [Google Scholar]
- 7.Czekay RP, Loskutoff DJ. Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exper Biol Med. 2004;229:1090–1096. doi: 10.1177/153537020422901102. [DOI] [PubMed] [Google Scholar]
- 8.Stefansson S, Lawrence DA. Old Dogs and New Tricks, Proteases, Inhibitors, and Cell Migration. Sci STKE. 2003;2003:e24. doi: 10.1126/stke.2003.189.pe24. [DOI] [PubMed] [Google Scholar]
- 9.Czekay R-P, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Budd RC, Kelm RJ, Jr, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Kelm JR, Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspase-3 in vascular smooth muscle cells by plasminogen activator inhibitor type-1. J Cellul Biochem. 2004;92:178–188. doi: 10.1002/jcb.20058. [DOI] [PubMed] [Google Scholar]
- 12.Al-Fakhri N, Chavakis T, Schmidt-Woll T, Huang B, Cherian SM, Bobryshev YV, Lord RSA, Katz N, Preissner KT. Induction of apoptosis in vascular cells by plasminogen activator inhibitor-1 and high molecular weight kininogen correlates with their anti-adhesive properties. Biological Chem. 2003;384:423–435. doi: 10.1515/BC.2003.048. [DOI] [PubMed] [Google Scholar]
- 13.Pandolfi A, Cetrullo D, Polishuck R, Alberta MM, Calafiore A, Pellegrini G, Vitacolonna E, Capani F, Consoli A. Plasminogen activator inhibitor type 1 is increased in the arterial wall of type II diabetic subjects. Arterioscler Thromb Vasc Biol. 2001;21:1378–1382. doi: 10.1161/hq0801.093667. [DOI] [PubMed] [Google Scholar]
- 14.Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci. 1992;89:6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauhanen P, Sirén V, Carpén O, Vaheri A, Lepäntalo M, Lassila R. Plasminogen activator inhibitor-1 in neointima of vein grafts - Its role in reduced fibrinolytic potential and graft failure. Circulation. 1997;96:1783–1789. doi: 10.1161/01.cir.96.6.1783. [DOI] [PubMed] [Google Scholar]
- 16.Peng L, Bhatia N, Parker AC, Zhu Y, Fay WP. Endogenous vitronectin and plasminogen activator inhibitor-1 promote neointima formation in murine carotid arteries. Arterioscler Thromb Vasc Biol. 2002;22:934–939. doi: 10.1161/01.atv.0000019360.14554.53. [DOI] [PubMed] [Google Scholar]
- 17.Ploplis VA, Cornelissen I, Sandoval-Cooper MJ, Weeks L, Noria FA, Castellino FJ. Remodeling of the vessel wall after copper-induced injury is highly attenuated in mice with a total deficiency of plasminogen activator inhibitor-1. Am J Pathol. 2001;158:107–117. doi: 10.1016/S0002-9440(10)63949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otsuka G, Agah R, Frutkin AD, Wight TN, Dichek DA. Transforming growth factor beta-1 induces neointima formation through plasminogen activator inhibitor-1-dependent pathways. Arterioscler Thromb Vasc Biol. 2006;26:737–743. doi: 10.1161/01.ATV.0000201087.23877.e1. [DOI] [PubMed] [Google Scholar]
- 19.de Waard V, Arkenbout EK, Carmeliet P, Lindner V, Pannekoek H. Plasminogen activator inhibitor 1 and vitronectin protect against stenosis in a murine carotid artery ligation model. Arterioscler Thromb Vasc Biol. 2002;22:1978–1983. doi: 10.1161/01.atv.0000042231.04318.e6. [DOI] [PubMed] [Google Scholar]
- 20.Lijnen HR, Van Hoef B, Umans K, Collen D. Neointima formation and thrombosis after vascular injury in transgenic mice overexpressing plasminogen activator inhibitor-1 (PAI-1) J Thromb Haemost. 2004;2:16–22. doi: 10.1111/j.1538-7836.2003.00533.x. [DOI] [PubMed] [Google Scholar]
- 21.DeYoung MB, Tom C, Dichek DA. Plasminogen activator inhibitor type 1 increases neointima formation in balloon-injured rat carotid arteries. Circulation. 2001;104:1972–1977. doi: 10.1161/hc4101.097110. [DOI] [PubMed] [Google Scholar]
- 22.Stefansson S, Petitclerc E, Wong MKK, McMahon GA, Brooks PC, Lawrence DA. Inhibition of angiogenesis in vivo by plasminogen activator inhibitor-1. J Biol Chem. 2001;276:8135–8141. doi: 10.1074/jbc.M007609200. [DOI] [PubMed] [Google Scholar]
- 23.Berkenpas MB, Lawrence DA, Ginsburg D. Molecular evolution of plasminogen activator inhibitor-1 functional stability. EMBO J. 1995;14:2969–2977. doi: 10.1002/j.1460-2075.1995.tb07299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefansson S, Petitclerc E, Wong MKK, McMahon GA, Brooks PC, Lawrence DA. Inhibition of Angiogenesis in Vivo by Plasminogen Activator Inhibitor-1. J Biol Chem. 2001;276:8135–8141. doi: 10.1074/jbc.M007609200. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Balsara RD, Gorlatova NV, Lawrence DA, Castellino FJ, Ploplis VA. Conservation of Critical Functional Domains in Murine Plasminogen Activator Inhibitor-1. J Biol Chem. 2004;279:17914–17920. doi: 10.1074/jbc.M314197200. [DOI] [PubMed] [Google Scholar]
- 26.Leik CE, Su EJ, Nambi P, Crandall DL, Lawrence DA. Effect of pharmacologic plasminogen activator inhibitor-1 inhibition on cell motility and tumor angiogenesis. J Thromb Haemost. 2006;4:2710–2715. doi: 10.1111/j.1538-7836.2006.02244.x. [DOI] [PubMed] [Google Scholar]
- 27.Stefansson S, Su EJ, Ishigami S, Cale JM, Gao Y, Gorlatova N, Lawrence DA. The contributions of integrin affinity and integrin-cytoskeletal engagement in endothelial and smooth muscle cell adhesion to vitronectin. J Biol Chem. 2007;282:15679–15689. doi: 10.1074/jbc.M702125200. [DOI] [PubMed] [Google Scholar]
- 28.Li SH, Gorlatova NV, Lawrence DA, Schwartz BS. Structural differences between active forms of plasminogen activator inhibitor type 1 revealed by conformationally-sensitive ligands. J Biol Chem. 2008;283:18147–18157. doi: 10.1074/jbc.M709455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Saunders T, Camper S, Samuelson L, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proc Natl Acad Sci. 1995;92:12426–12430. doi: 10.1073/pnas.92.26.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y, Farrehi PM, Fay WP. Plasminogen activator inhibitor type 1 enhances neointima formation after oxidative vascular injury in atherosclerosis-prone mice. Circulation. 2001;103:3105–3110. doi: 10.1161/01.cir.103.25.3105. [DOI] [PubMed] [Google Scholar]
- 31.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neoinitmal hyperplasia. J Mol Cell Cardiol. 2000;32:2097–2104. doi: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P, Moons L, Herbert JM, Crawley J, Lupu F, Lijnen R, Collen D. Urokinase but not tissue plasminogen activator mediates arterial neointima formation in mice. Circ Res. 1997;81:829–839. doi: 10.1161/01.res.81.5.829. [DOI] [PubMed] [Google Scholar]
- 33.Quax PHA, Lamfers MLM, Lardenoye JHP, Grimbergen JM, de Vries MR, Slomp J, de Ruiter MC, Kockx MM, Verheijen JH, van Hinsbergh VWM. Adenoviral expression of a urokinase receptor-targeted protease inhibitor inhibits neointima formation in murine and human blood vessels. Circulation. 2001;103:562–569. doi: 10.1161/01.cir.103.4.562. [DOI] [PubMed] [Google Scholar]
- 34.Schafer K, Konstantinides S, Riedel C, Thinnes T, Muller K, Dellas C, Hasenfuss G, Loskutoff DJ. Different mechanisms of increased luminal stenosis after arterial injury in mice deficient for urokinase- or tissue-type plasminogen activator. Circulation. 2002;106:1847–1852. doi: 10.1161/01.cir.0000031162.80988.2b. [DOI] [PubMed] [Google Scholar]
- 35.Lundgren C, Vinogradsky B, Guala A, Fujii S. A Vitronectin Receptor Antagonist Inhibits Neointimal Formation After Balloon Arterial Injury in Rabbits in Vivo. Vasc Endovasc Surg. 1998;32:47–53. [Google Scholar]
- 36.Mitchell JW, Baik N, Castellino FJ, Miles LA. Plasminogen inhibits TNF{alpha}-induced apoptosis in monocytes. Blood. 2006;107:4383–4390. doi: 10.1182/blood-2005-07-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwaan HC, Wang J, Svoboda K, Declerck PJ. Plasminogen activator inhibitor 1 may promote tumour growth through inhibition of apoptosis. Br J Cancer. 2000;82:1702–1708. doi: 10.1054/bjoc.2000.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrlich HJ, Gebbink RK, Keijer J, Linders M, Preissner KT. Alteration of serpin specificity by a protein cofactor. Vitronectin endows plasminogen activator inhibitor 1 with thrombin inhibitory properties. J Biol Chem. 1990;265:13029–13035. [PubMed] [Google Scholar]
- 39.Dufourcq P, Louis H, Moreau C, Daret D, Boisseau MR, Lamazière JMD, Bonnet J. Vitronectin expression and interaction with receptors in smooth muscle cells from human atheromatous plaque. Arterioscler Thromb Vasc Biol. 1998;18:168–176. doi: 10.1161/01.atv.18.2.168. [DOI] [PubMed] [Google Scholar]
- 40.Brown SL, Lundgren CH, Nordt T, Fujii S. Stimulation of migration of human aortic smooth muscle cells by vitronectin: Implications for atherosclerosis. Cardiovasc Res. 1994;28:1815–1820. doi: 10.1093/cvr/28.12.1815. [DOI] [PubMed] [Google Scholar]
- 41.Kamikubo Y, Neels JG, Degryse B. Vitronectin inhibits plasminogen activator inhibitor-1-induced signalling and chemotaxis by blocking plasminogen activator inhibitor-1 binding to the low-density lipoprotein receptor-related protein. Int J Biochem Cell Biol. 2009;41:578–585. doi: 10.1016/j.biocel.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Budd RC, Kelm RJ, Jr, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]