Abstract
Background
Young infants are susceptible to developmental factors influencing the pharmacokinetics of drugs. Fluconazole is increasingly used to prevent and treat invasive candidiasis in infants. Dosing guidance remains empiric and variable because limited pharmacokinetic data exist.
Methods
Our population PK model derived from 357 fluconazole plasma concentrations from 55 infants (23–40 week gestation) illustrates expected changes in fluconazole clearance based upon gestational age, postnatal age, weight, and creatinine. We used a Monte Carlo simulation approach based on parametric description of a patient population’s pharmacokinetic response to fluconazole to predict fluconazole exposure (median, 10th and 90th percentile population variability range) after 3, 6 and 12 mg/kg dosing.
Results
For the treatment of invasive candidiasis, a dose of at least 12 mg/kg/day in the 1st 90 days after birth is needed to achieve an AUC of >400 mg*h/L and an AUC/MIC>50 for Candida species with MIC<8 µg/ml in ≥ 90% of <30 week gestation infants and 80% of 30–40 week gestation infants. The more preterm infants achieve a higher median AUC (682 mg*hr/L) compared with more mature infants (520 mg*hr/L). For early prevention of candidiasis in 23–29 week infants, a dose of 3 or 6 mg/kg twice weekly during the first 42 days of life is equivalent to an AUC of 50 and 100 mg*hr/L, respectively, and maintains fluconazole concentrations ≥ 2 or 4 µg/ml, respectively, for half of the dosing interval. For late prevention, the 6 mg/kg dose every 72 hours provides similar exposure to 3 mg/kg daily dose. Infants with serum creatinine ≥ 1.3 mg/dl have delayed drug clearance and dose adjustment is indicated if creatinine does not improve within 96 hours.
Conclusions
A therapeutic concentration of fluconazole in premature infants with invasive candidiasis requires dosing substantially greater than commonly recommended in most reference texts. To prevent invasive candidiasis, twice weekly prophylaxis regimens can provide adequate exposure when unit specific MICs are taken into account.
Keywords: neonate, pharmacology, antifungal treatment
MATERIALS AND METHODS
Population pharmacokinetic model
The population PK model of fluconazole in young infants developed from the Open Label PK study of fluconazole in the Pediatric Pharmacology Research Unit Network has been described previously13. PK data were analyzed with a non-linear mixed effect modeling approach using NONMEM (version 5)16. This model was created from 357 fluconazole plasma concentrations collected from 55 infants who were 23 – 40 weeks gestation and 1 – 90 days of age. All infants were receiving fluconazole as routine care for the prevention or treatment of invasive Candida infection in doses of 3 –12 mg/kg. Although post menstrual age was associated with clearance, it was less informative in the model than postnatal age and gestational age at birth combined. The final PK model explains the expected changes in fluconazole clearance based upon the infant’s postnatal age, gestational age at birth, and present body weight. Serum creatinine factors into the model only when the creatinine is greater than 1.0 mg/dl at least 3 days after birth. Model performance was successfully qualified using an external predictive check method with a PK dataset from a different study22. The model construction, evaluation and initial simulations were previously published13.
Assessment of dose-exposure relationship
Monte Carlo simulations using the final population PK model (including population typical value, inter-individual covariance, and residual variance parameters) were used to explore the impact of postnatal age, gestational age at birth, and serum creatinine on dose-exposure relationships. For each dose evaluation, we performed 100 simulated trials within NONMEM (version VI; ICON Development Solutions, Ellicott City, MD) and R (version 2.5.1; R Core Development Team; www.r-project.org). We were interested in the central tendency of concentration time profile and the 80th percentile of variation around this central tendency. Additional simulations, up to 1000, did not change the predicted central tendency or surrounding 80th percentile. We used five simulation infant cohorts (Table 1, online only). The majority of infants in these simulated cohorts had the demographic characteristics of the infants enrolled in the previous PK study of infants receiving fluconazole for clinical care. Additional hypothetical infants, such as 30–33 weeks gestation infants or infants with serum creatinine >1.0 mg/dl, made up the remainder of the simulated cohorts. After simulation, we summarized the concentration time profiles of the approximately 3000 simulated infants.
Table 1.
Description of simulation cohorts.
| Therapeutic Goal |
BGA (wk)* |
PNA on day 1* |
Serum creatinine mg/dl |
Dose (mg/kg) |
Interval (hours) |
Duration (days) |
PK/PD Target # |
|---|---|---|---|---|---|---|---|
| Treatment IC very preterm |
25 (23–29) |
21 (1–50) |
<1.3 | 6–12 | 24–48 | 14 | 90 % with AUC>400 Median AUC 600–800 AUC/MIC>50 for MIC≤8 |
| Treatment IC late preterm-term |
35 (30–40) |
21 (1–50) |
<1.3 | 6–12 | 24 | 14 | 90 % with AUC>400 Median AUC 600–800 AUC/MIC>50 for MIC≤8 |
| Early Prevention from birth |
25 (23–29) |
2 (1–5) |
<1.3 | 3–6 | 24–96 | 42 | None available, summarize exposure with concentration time profile, AUC, and T>MIC |
| Late Prevention during high risk |
25 (23–29) |
21 (7–50) |
<1.3 | 3–6 | 24–72 | 21 |
IC, invasive candidiasis
median (range), wk (week), BGA (gestational age at birth in weeks), PNA (post natal age on 1st day of fluconazole treatment)
AUC, area under the concentration curve at steady state per 24 hour dosing interval (mg*hour/L). T>MIC is the percent of dosing interval time that the fluconazole concentration is maintained above the MIC of the Candida species.
We explored simulated exposure after a range of fluconazole dosage from 3–12 mg/kg given intravenously at intervals of 24–96 hours as suggested in neonatal reference texts14, 15. Infants in the PK trial used to derive the model received fluconazole in dosages of between 3 and 12 mg/kg. Table 1 summarizes the dosage, dosing interval, and duration of treatment evaluated for the treatment of systemic candidiasis, and for both early and late prevention of invasive candidiasis. Simulation cohorts included infants with serum creatinine of <1.3 mg/dl as long as the creatinine returned to ≤1.0 mg/dl within 96 hours since this clinical scenario was observed in our prior PK study.
A separate simulation population was generated to evaluate fluconazole exposure in infants with renal insufficiency as defined as serum creatinine >1.0 beyond 3 days of life. For treatment strategies, we evaluated simulated exposure in infants who had creatinine values of 1.1 – 2.0 mg/dl that normalized to ≤ 1.0 mg/dl by day 5 of treatment. For prevention strategies, we evaluated exposure in infants with creatinine values of 1.1 – 2.0 mg/dl that normalized to ≤1.0 mg/dl by two weeks of therapy.
We evaluated the median and population variability range (10th and 90th percentile) of simulated fluconazole concentration-time profile for each observation time after dose input from each 100 simulated trial scenario of groups of approximately 30 infants (3000 infants total). If an infant in our previous PK trial received the dosing regimen under evaluation then we plotted the observed fluconazole concentrations within the simulation graphs. Because the prior PK trial relied on sparse sampling, the observed fluconazole concentrations did not span the simulated concentration time curve.
We determined the interval median, 10th, and 90th percentile AUC to be the AUC for each 24 hours interval after a given dose. The AUC for each 24 hours of exposure for each simulated subject was generated using NONMEM based on the final population PK model cited in our previous publication13 with the median and percentiles derived from the individual predictions (≈3000 simulated subjects) for each scenario presented in table 1 (on-line only). For calculation of AUC/MIC we used the Clinical and Laboratory Standards Institute (CLSI) sensitivity breakpoint MIC of 8 µg/ml for C. albicans and C. parapsilosis. We calculated the percent of the dosing interval that the fluconazole concentration was above the typical MIC for C. albicans and parapsilosis species in neonates (MIC90 ≤4 µg/ml)10, 11, 17, 18. Both the AUC24 and percent T>MIC were calculated by numerical integration using NONMEM’s differential equation solver (PREDPP ADVAN6).
Therapeutic Exposure Targets
For the treatment of invasive candidiasis we chose to target exposure to a minimum AUC24 of 400 mg*hr/L in at least 90% of infants and a median AUC24 of 600–800 mg*hr/L. The minimum AUC of 400 mg*hr/L ensures that the PK/PD index of AUC/MIC is ≥50 for Candida species sensitive to fluconazole based on the CLSI sensitivity breakpoint of a MIC ≤ 8 µg/ml for all Candida species3, 6, 7. The median AUC range was chosen to reflect exposure in critically adults receiving 800 mg/day. Since no PK/PD index has been described for preventative regimens, we evaluated the concentration time profile, the AUC24, and the T>MIC in which the fluconazole concentration was above the MIC of the Candida species found in the NICU, MIC90 ≤ 4 µg/ml. An AUC24 of 100 mg*hr/L was considered to be equivalent to exposure obtained in adults receiving 100 mg/day 8. The predicted steady state AUC was used to compare infant daily dose with equivalent adult daily dose.
RESULTS
Our infant population PK model of fluconazole describes the PK of fluconazole in preterm and term infants who were all receiving fluconazole for the prevention or treatment of invasive candidiasis in dosages of 3–12 mg/kg13. Monte Carlo simulation of 5 cohorts (Table 1, online only) was used to predict fluconazole exposure and thus guide dosing for the use of fluconazole in the care of neonates and young infants.
Treatment of Invasive Candidiasis
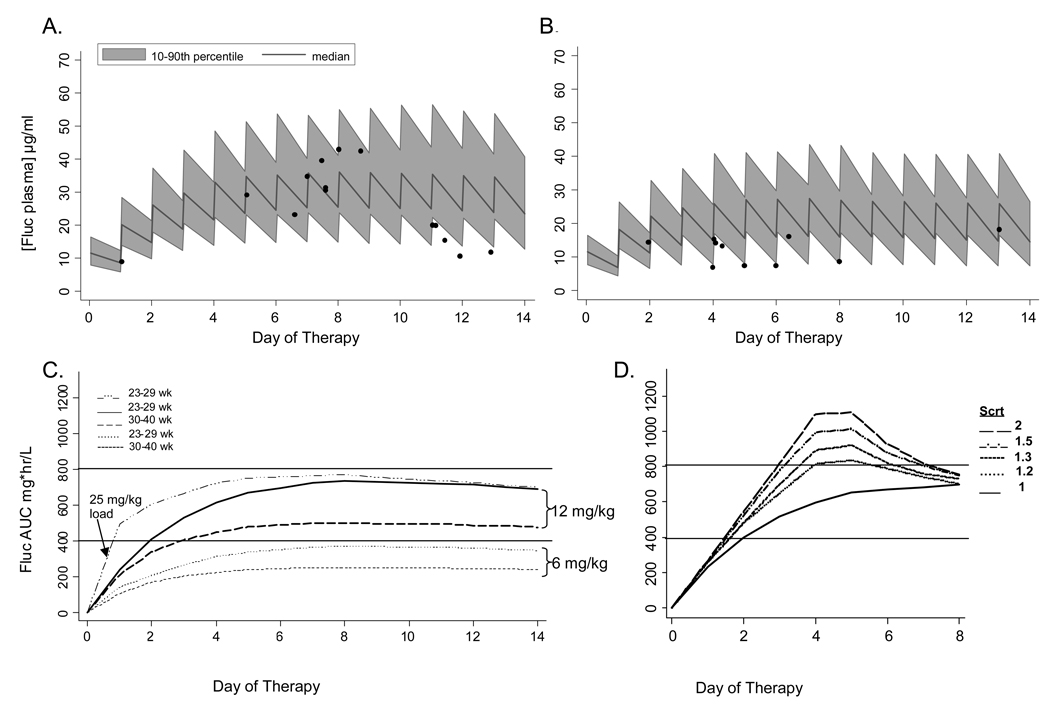
We predicted fluconazole exposure achieved after a 12 mg/kg daily dose regimen in 2 groups of infants with serum creatinine values <1.3 mg/dl: group 1 (BGA 23–29 weeks) and group 2 infants (GA 30–40 weeks) (Table 2, Figure 1). In our previous PK trial, six infants received 10–12 mg/kg dosing and the sparse samples collected from such infants yielded fluconazole concentrations that were within the predicted variability range of simulated fluconazole concentrations (closed circles, Figure 1A, B). Given the sparse sampling, the observed infant values were not dispersed evenly across the concentration-time profile. The two infants with concentrations just below the predicted 10th percentile were receiving 10–11 mg/kg fluconazole if actual weight was used to calculate dose rather than the empiric dosing weight estimated by clinicians because of fluid overload. Among infants 23–29 weeks gestation, the simulated steady state median AUC24 was 680 mg*hr/L, and 90% achieved an AUC24 of ≥400 mg*hr/L. Among infants 30–40 weeks gestation, the simulated steady state median AUC24 was lower at 520 mg*hr/L and 80% achieved an AUC24 of ≥400 mg*hr/L. Traditional 6 mg/kg/day dosing did not reach the therapeutic AUC target (Figure 1C). Since the half life of fluconazole in young infants is 30–50 hours, these steady state values would not be achieved until after 5 days of therapy. A loading dose would be needed to achieve the AUC target by day 2 (Figure 1C).
Table 2.
Simulated Steady State Dose Exposure Summary
| Indication | Dose mg/kg |
Interval (hours) |
BGA A (wks) |
PNA A (days) |
[Fluc] B (µg/ml) |
AUC B (mg*hr/L) |
AUC C 10th % |
AUC C 90th % |
%Time>MIC E (median) |
|---|---|---|---|---|---|---|---|---|---|
| Treatment IC |
12 | Q24 | 23–29 | 7–80 | 23 (11) 22 | 720 (247) 680 | 430 | 1030 | NA |
| 12 | Q24 | 30–40 | 1–80 | 15 (7.4) 14 | 540 (190) 520 | 340 | 790 | NA | |
| Early Prevention |
3 | Q Tue/Fri | 23–29 | 1–42 | 2.3 (1.3) 2.1 | 53 (28) 50 | 20 | 90 | 58% T>MIC2 3 % T>MIC4 |
| 6 | Q Tue/Fri | 23–29 | 1–42 | 4.2 (2.6) 4.0 | 107 (55) 101 | 40 | 180 | 93% T>MIC2 47% T>MIC4 |
|
| Late Prevention |
3 | Q24 | 23–29 | 7–42 | 6.9 (3.1) 6.5 | 163 (60) 156 | 93 | 243 | 85% T>MIC4 |
| 6 | Q72 | 23–29 | 7–42 | 5.0 (2.7) 4.4 | 112 (59) 108 | 43 | 190 | 57% T>MIC4 | |
| 6 | Q48 | 23–29 | 42–80 | 6.0 (3.2) 5.5 | 135 (64) 131 | 59 | 218 | 67% T>MIC4 | |
| 6 | Q48 | 30–40 | 1–42 | 5.5 (3.0) 5.0 | 128 (57) 122 | 60 | 205 | 63% T>MIC4 |
IC, invasive candidiasis; wk, weeks; BGA, gestational age at birth; PNA postnatal age; [Fluc] plasma concentration of fluconazole;
range of infant characteristics
mean (standard deviation) median
percentile of simulation
Typical MIC for Candida species in NICU, MIC90 ≤ 4 µg/ml.
Figure 1.
Treatment of empiric or proven invasive candidiasis. Predicted median and population variability range (10th–90th percentile) of fluconazole plasma concentrations from 100 simulated trials of preterm 23–29 week gestation infants (A) and 30–40 week gestation infants (B) treated with 12 mg/kg/day for 14 days. Closed circles in (A) and (B) represent the observed fluconazole plasma concentrations in six infants who were receiving 10–12 mg/kg/day in a previous PK trial. Predicted median fluconazole AUC is shown in (C) for 23–29 week and 30–40 week infants receiving 6 or 12 mg/kg/day. A loading dose of 25 mg/kg followed by 12 mg/kg/day is predicted to achieve target AUC by day 2 (C). Predicted median fluconazole AUC is shown for 23–29 week gestation infants receiving 12 mg/kg/day with renal insufficiency if creatinine improves to ≤ 1 mg/dl after 4 days (D).
We predicted the concentration of fluconazole achieved in infants with renal insufficiency receiving 12 mg/kg/ daily. This renal insufficiency simulation cohort contained infants 23–29 weeks gestation who had serum creatinine values of 1.1 – 2.0 mg/dl for to the first 4 days of treatment and then had a normalized creatinine value of ≤ 1.0 mg/dl by day 5 of treatment. Figure 1D shows accumulation of fluconazole and an increase in AUC24 with increasing creatinine up to 2.0 mg/dL. The median AUC remains in the therapeutic window as long as the creatinine normalizes to ≤1 mg/dl by day 5 of treatment.
Early Prevention of Invasive Candidiasis
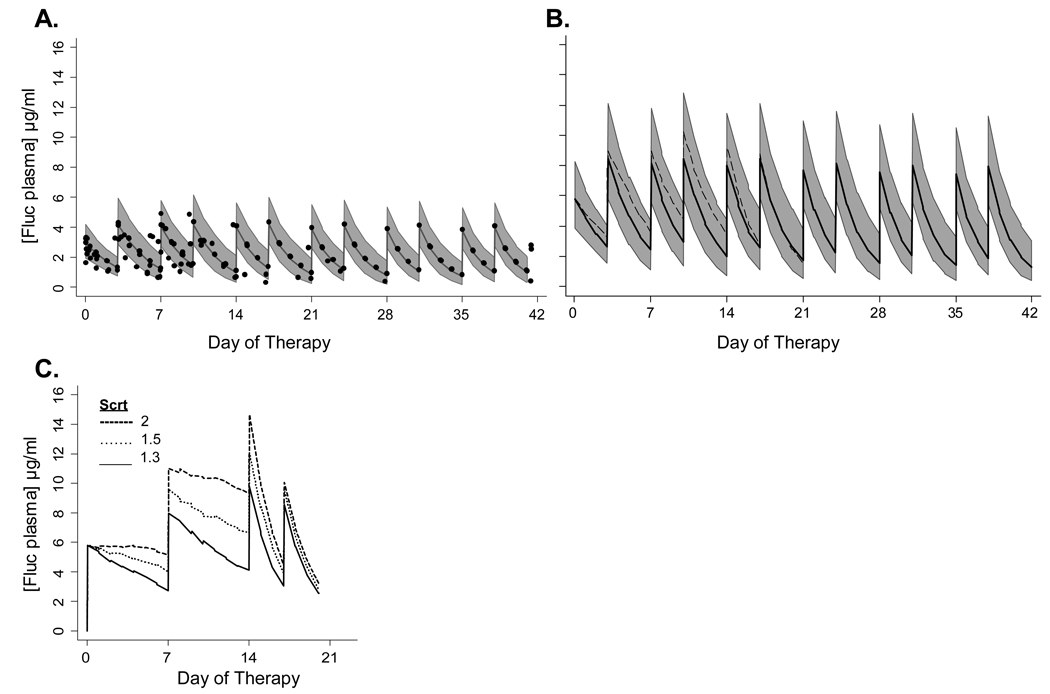
We evaluated simulated exposure in 23–29 week gestation infants who began fluconazole in the first 5 days of life by comparing fluconazole exposure in infants receiving 3 or 6 mg/kg twice weekly, for example every Tuesday and Friday (Table 2, Figure 2). All infants used in this simulation had creatinine values that remained <1.3 mg/dl. Infants receiving 6 mg/kg twice weekly had a simulated median AUC24 of 101 mg*hr/L with fluconazole concentrations above 4 µg/ml for 47% of the dosing interval. Infants receiving 3 mg/kg twice weekly had a simulated median AUC24 of 50 mg*hr/L with fluconazole concentrations above 2 µg/ml for 58% of the dosing interval.
Figure 2.
Early prevention of invasive candidiasis in 23–29 week gestation infants. Predicted median and population variability range (10th–90th percentile) of fluconazole plasma concentrations from 100 simulated trials of preterm 23–29 week gestation infants treated with 3 mg/kg (A) or 6 mg/kg (B) twice weekly for 42 days starting in the first 5 days of life. Closed circles in (A) represent the observed fluconazole plasma concentrations in infants participating in a previous PK trial who received 3 mg/kgl. Dash line in B represents the median fluconazole concentration predicted in infants with serum creatinine of 1.2 mg/dl for first two weeks of therapy. (C) shows the predicted median fluconazole plasma concentrations in 23–29 week infants with renal insufficiency treated with 6 mg/kg weekly for first two weeks and then twice weekly beginning on day 14 when creatinine decreases to ≤1.0 mg/dl in simulation.
We performed a separate simulation analysis for infants born at 23–29 weeks gestation who also had significant renal insufficiency. All infants in this simulation cohort had creatinine values of 1.1 – 2.0 mg/dl for two weeks and then normalization of creatinine to ≤ 1.0 mg/dl by day 14 of therapy. Infants with creatinine values of 1.1 and 1.2 mg/dl who received 6 mg/kg twice a week had predicted median fluconazole concentrations < 12 µg/ml (Figure 2B) and a median AUC24 of <200 mg*hr/L. Infants with creatinine values of 1.3 – 2.0 mg/dl achieved similar exposure when they were treated with 6 mg/kg once a week. The simulated median fluconazole concentrations were <12 µg/ml (Figure 2C) and a median AUC24 of 130, 159, and 179 mg*hr/L for infants with creatinine of 1.3, 1.5, and 2.0 mg/dl, respectively.
Late prevention of invasive candidiasis
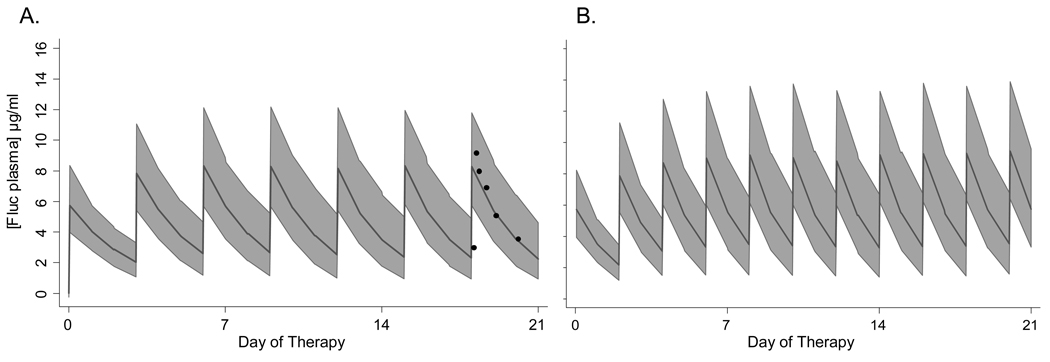
Infants are at risk for invasive candidiasis during broad spectrum antibiotic exposure, particularly in the setting of gastrointestinal disease2, 19–21. We explored a fluconazole dose of 6 mg/kg for the prevention of invasive candidiasis in infants between 8–80 days of age. We evaluated infants in two groups based upon gestational age at birth, 23 to 29 weeks and 30–40 weeks (Table 2). Infants used in this simulation had creatinine values that remained < 1.3 mg/dl. For extremely preterm infants born at 23 to 29 week gestation who are < 42 days old, doses of 3 mg/kg daily or 6 mg/kg every 72 hours (Figure 3A, online only) can achieve a simulated median AUC24 near 100 mg*hr/L and maintain concentrations above 4 µg/ml for at least 40 % of the dosing interval (Table 2). Once these preterm infants are >42 days old, a dose of 6 mg/kg every 48 hours is needed to maintain these concentrations (Figure 3B, online only). For more mature infants born at 30–40 week gestation who are < 42 days old, a dose of 6 mg/kg twice every 48 hours is needed to maintain an AUC near 100 mg*hr/L and maintain concentrations above 4 µg/ml for 40% of the dosing interval. Infants with renal insufficiency with creatinine ≥ 1.3 mg/dl have similar exposure using a weekly dose of 6 mg/kg fluconazole as previously shown for early prophylaxis. Once the creatinine has normalized to ≤ 1.0 mg/dl then standard dosing can be resumed.
Figure 3.
Fluconazole has been used for the late prevention of invasive candidiasis during broad spectrum antibiotic exposure or necrotizing enterocolitis in infants born at 23–29 weeks gestation. Predicted fluconazole plasma concentrations in 23–29 week gestation infants treated with 6 mg/kg every 72 hours in infants who are 7–42 days old (A) and every 48 hours if in infants who are 43–80 days old (B). Closed circles in (A) represent observed fluconazole plasma concentrations in a preterm infant who participated in a previous PK trial and received 6 mg/kg every 72 hours.
DISCUSSION
Young infants with invasive candidiasis ideally should receive antifungal medications at exposures designed to meet PK/PD indices. Current fluconazole dosing guidance has been primarily empiric and based upon limited PK information22. We used Monte Carlo simulation to predict fluconazole exposure in infants using a population PK model of fluconazole disposition derived from preterm and term infants <90 days of age. This model has been thoroughly qualified and hence its use for dosing considerations in this population is acceptable13. Simulated exposures revealed the predicted variation and were consistent with observed fluconazole concentrations from the prior PK study. We have developed fluconazole dosing guidance for young infants based on PK/PD indices.
For the treatment of invasive candidiasis, the pharmacologic properties of fluconazole offer many advantages including its minimal metabolism, bioavailability, renal elimination of active drug, penetration of tissues and cerebral spinal fluid, and excellent safety profile23–27. However, fluconazole exhibits time dependent fungistatic activity. Optimal treatment of invasive candidiasis requires fluconazole concentrations and the AUC to be maintained above the MIC at the site of infection4, 7, 12. Adult dosing regimens of 400–800 mg/day target an AUC24 of 400–800 mg*hr/L3 to achieve the PK/PD index, AUC/MIC ≥ 50, for Candida species up to the CLSI susceptibility breakpoint, MIC ≤ 8 µg/ml 6–8. The 800 mg dose is intended for individuals who are immunocompromised, are critically ill, or have deep seeded fungal infections.
These simulations suggest that a 12 mg/kg/day dose of fluconazole provides therapeutic exposure for the treatment of invasive candidiasis in young infants. Plasma concentrations were 2–4 times above the typical MIC (≤ 4 µg/ml) for Candida albicans or parapsilosis species in infants10, 11, 17, 18, 28. At least 90% of the most preterm infants achieved the minimum AUC24 of 400 mg*hr/L. The most immature infants have delayed clearance and achieve higher median exposures than late preterm and term infants. This higher exposure is likely to be advantageous because infants < 30 weeks gestation have the most immature immune systems and are at high risk of invasive disease, including meningo-encephalitis20, 29, 30. The higher median AUC is consistent with higher dose (800mg/day) therapy in critically ill adults with severe fungal infections 3, 31–35. In the rare clinical situations of treating an extremely preterm infant in the first few days of life, delay in clearance is expected in those with limited urine output and elevated creatinine. At least 2 daily doses are needed to achieve therapeutic concentrations. Subsequent dosing at 24–48 hour intervals in the first week of life would depend of the clinical scenario and predicted renal function. Prospective trials evaluating PK and safety of this 12 mg/kg/day dosing regimen and potentially higher doses in more mature infants are needed.
The high mortality and high rates of neurodevelopmental impairment among surviving infants suggest that improved treatment strategies are needed20. Loading doses, as used in adults, would be needed to reach therapeutic exposure in the first 2 days of therapy. Doses of up to 1600 mg/day (approximately 25 mg/kg) have been well tolerated in adults31–39. Clinical studies to evaluate loading doses in infants are needed.
Clinicians should balance the possibility of subtherapeutic exposure with the unclear risk of toxicity and monitor liver function tests closely. In adults, doses up to 1600 mg/day, which typically yield an AUC24 1600 mg*hr/L and fluconazole concentrations < 80 µg/ml, have been well tolerated 31–39. The simulated 90th percentile of fluconazole exposure in infants receiving 12 mg/kg/day was maintained below these limits. Although rare but serious hepatic toxicity has occurred in patients taking fluconazole, this toxicity does not appear to be related to total drug exposure or duration of therapy36, 40, 41.
Fluconazole has excellent properties for preventative therapies however the PD exposure targets are not well described for the prevention of candidiasis. Preventative strategies must consider plasma concentrations, predicted tissue concentrations at sites of potential colonization, and the MIC of colonizing Candida species. Dosing for preventative strategies, primarily designed for severely immunocompromised adults, range from 50–200 mg/day with predicted AUC24 of 50–200 mg*hr/L. Clinical trials of early fluconazole prophylaxis in preterm infants from birth to 6 weeks of age have found that both 3 mg/kg and 6 mg/kg dosing are effective at preventing invasive candidiasis in centers with a high burden of Candida infections10, 11. No change in azole resistance patterns were observed during either study although the power to detect such a change was low.
Lower exposures achieved in clinical trials with early 3 mg/kg twice weekly regimens may be sufficient to prevent colonization and infection when initiated shortly after birth because higher fluconazole concentrations are anticipated at sites of early colonization including the skin, gastrointestinal mucosa, and urine19. The typical MICs of Candida species in neonates range from 0.25–4 µg/ml 10, 11, 17, 18. Dosing regimens that maintain T>MIC of at least 40% may prevent the emergence of more resistant Candida isolates in vitro12. The low dose 3 mg/kg twice weekly regimen would be expected to prevent the growth of Candida species with an MIC of ≤ 2 µg/ml; the 6 mg/kg twice weekly regimen would be needed if the targeted MIC range of Candida species is ≤ 4 µg/ml. Neonatal units may want to consider their unit specific Candida MICs from surveillance and susceptibility testing of fungal isolates when considering dosing for prevention. Further studies need to identify the PK-PD target for prevention of candidiasis and prevention of emerging resistance.
High risk infants who receive fluconazole for prevention of invasive candidiasis after the first two weeks of age may already be heavily colonized42, 43. Higher fluconazole exposures may be required to both eradicate colonization and prevent late onset invasive candidiasis. Randomized controlled trials to evaluate later prevention strategies are needed.
Prophylactic fluconazole exposure has been associated with the development or selection of drug-resistant microorganisms44, 45. Mechanisms of azole resistance include alteration in fungal cell drug influx, drug efflux, or the target enzyme lanosterol demethylase. Factors considered to impact upon emerging resistance include intermittent dosing intervals, the amount of drug, the length of treatment, and the immune status of the patients. Total NICU exposure of high doses has been associated with the emergence of some resistance46. It is not clear what exposure strategies minimize emerging resistance in the NICU environment. Surveillance and susceptibility testing of fungal isolates is warranted.
This study has important limitations. The fidelity and generalizability of the population PK model is key to the simulation interpretation and dosing guidance discussed herein. The strength of the model is derived from the magnitude of the PK dataset and the inclusion of infants receiving fluconazole for treatment or prevention of candidiasis at doses used for these simulations. However, longitudinal prediction of clearance changes with postnatal age over the first two months of life is difficult because infants beyond two weeks of age typically began fluconazole during a period of clinical deterioration. These dosing guidelines rely on PK/PD indices derived in adult, animal and in vitro studies3, 4, 7. The safety of fluconazole, potential drug interactions, and factors contributing to fungal resistance patterns needs further study. These limitations reflect a lack of knowledge specific to young infants. The modeling and simulation framework utilized in this exercise can easily accommodate new knowledge as the data become available. The extension of the PK/PD index to incorporate safety as well as efficacy targets would likewise add value to our dosing guidance.
CONCLUSIONS
Infants with invasive candidiasis required a minimum of 12 mg/kg/day of fluconazole to achieve therapeutic exposure, an AUC of 400 mg*hr/L to meet the PK/PD index of AUC/MIC ≥50 for Candida isolates at the susceptibility breakpoint, MIC 8 µg/ml. For the early prevention of candidiasis, dosages of 3 or 6 mg/kg twice weekly have demonstrated efficacy in randomized controlled trials and can maintain the fluconazole concentrations above 2 or 4 µg/ml, respectively, for >40% of the dosing interval. For the late prevention of candidiasis, dosages of 6 mg/kg, every 48 to 72 hours based upon gestational age at birth and postnatal age, are reasonable based upon adult exposures achieved after 100 mg/day dose and the ability to maintain fluconazole concentration above an MIC of 4 for at least 40% of the dosing interval. Late prevention strategies have not been subjected to randomized controlled trials. These results are based on simulated clinical trials. Confirmatory, prospective trials of fluconazole exposure, safety, and efficacy are needed.
ACKNOWLEDGEMENTS
This project described was supported by grant # 1U10-HD037255-06 (Drs. Wade, Barrett), 1U10-HD045986-05 (RM Ward), 1U10-HD045962-05 (DK Benjamin Jr.) from the National Institute of Child Health and Development Pediatric Pharmacology Research Unit. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Development, or the National Institutes of Health.
Footnotes
No potential conflict of interests relevant to this article was reported.
Portions of this work were presented at the Society for Pediatric Research Annual Meeting, Honolulu, HI May 2008.
References
- 1.Kaufman D. Fungal infection in the very low birthweight infant. Curr Opin Infect Dis. 2004;17:253–259. doi: 10.1097/00001432-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Smith PB, Steinbach WJ, Benjamin DK., Jr Neonatal candidiasis. Infect Dis Clin North Am. 2005;19:603–615. doi: 10.1016/j.idc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 4.Andes D, van Ogtrop M. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother. 1999;43:2116–2120. doi: 10.1128/aac.43.9.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klepser ME, Wolfe EJ, Jones RN, Nightingale CH, Pfaller MA. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother. 1997;41:1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clancy CJ, Staley B, Nguyen MH. In vitro susceptibility of breakthrough Candida bloodstream isolates correlates with daily and cumulative doses of fluconazole. Antimicrob Agents Chemother. 2006;50:3496–3498. doi: 10.1128/AAC.00741-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy CJ, Yu VL, Morris AJ, Snydman DR, Nguyen MH. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob Agents Chemother. 2005;49:3171–3177. doi: 10.1128/AAC.49.8.3171-3177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai MP, Turpin RS, Garey KW. Association of Fluconazole Area under the Concentration-Time Curve/MIC and Dose/MIC Ratios with Mortality in Nonneutropenic Patients with Candidemia. Antimicrob Agents Chemother. 2007;51:35–39. doi: 10.1128/AAC.00474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:662–678. doi: 10.1086/313749. [see comment] [DOI] [PubMed] [Google Scholar]
- 10.Kaufman D, Boyle R, Hazen KC, et al. Fluconazole Prophylaxis against Fungal Colonization and Infection in Preterm Infants. N Engl J Med. 2001;345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 11.Manzoni P, Stolfi I, Pugni L, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356:2483–2495. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 12.Andes D, Forrest A, Lepak A, et al. Impact of Antimicrobial Dosing Regimen on Evolution of Drug Resistance In Vivo: Fluconazole and Candida albicans. Antimicrob Agents Chemother. 2006;50:2374–2383. doi: 10.1128/AAC.01053-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wade KC, Wu D, Kaufman D, et al. Population Pharmacokinetics of Fluconazole in Young Infants. Antimicrob Agents Chemother. 2008;52:4043. doi: 10.1128/AAC.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taketomo C, Hodding J, Kraus D. Pediatric Dosage Handbook. Lexi-Comp; 2005. [Google Scholar]
- 15.Young T, Magnum B. NeoFax 2008. Physician's Desk Reference; 2008. [Google Scholar]
- 16.Beal S, Boeckmann A, Sheiner L. NONMEM Users Guides Parts I–VIII, NONMEM Project Group. San Francisco, CA: University of California; [Google Scholar]
- 17.Kaufman D, Boyle R, Hazen KC, et al. Twice weekly fluconazole prophylaxis for prevention of invasive Candida infection in high-risk infants of <1000 grams birth weight. J Pediatr. 2005;147:172–179. doi: 10.1016/j.jpeds.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics. 2001;107:293–298. doi: 10.1542/peds.107.2.293. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman DA. Fungal infections in neonates: update on prevention and treatment. Minerva Ginecol. 2007;59:311–329. [PubMed] [Google Scholar]
- 20.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal Candidiasis Among Extremely Low Birth Weight Infants: Risk Factors, Mortality Rates, and Neurodevelopmental Outcomes at 18 to 22 Months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 21.Roilides E, Farmaki E, Evdoridou J, et al. Neonatal candidiasis: analysis of epidemiology, drug susceptibility, and molecular typing of causative isolates. Eur J Clin Microbiol Infect Dis. 2004;23:745–750. doi: 10.1007/s10096-004-1210-9. [DOI] [PubMed] [Google Scholar]
- 22.Saxen H, Hoppu K, Pohjavuori M. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin Pharmacol Ther. 1993;54:269–277. doi: 10.1038/clpt.1993.147. [DOI] [PubMed] [Google Scholar]
- 23.Bodey GP. Azole antifungal agents. Clin Infect Dis. 1992;14 Suppl 1:S161–S169. doi: 10.1093/clinids/14.supplement_1.s161. [DOI] [PubMed] [Google Scholar]
- 24.Brammer KW, Coates PE. Pharmacokinetics of fluconazole in pediatric patients. Eur J Clin Microbiol Infect Dis. 1994;13:325–329. doi: 10.1007/BF01974613. [DOI] [PubMed] [Google Scholar]
- 25.Brammer KW, Farrow PR, Faulkner JK. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis. 1990;12 Suppl 3:S318–S326. doi: 10.1093/clinids/12.supplement_3.s318. [DOI] [PubMed] [Google Scholar]
- 26.Fischman AJ, Alpert NM, Livni E, et al. Pharmacokinetics of 18F-labeled fluconazole in healthy human subjects by positron emission tomography. Antimicrob Agents Chemother. 1993;37:1270–1277. doi: 10.1128/aac.37.6.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frattarelli DAC, Reed MD, Giacoia GP, Aranda JV. Antifungals in systemic neonatal candidiasis. Drugs. 2004;64:949–968. doi: 10.2165/00003495-200464090-00003. [DOI] [PubMed] [Google Scholar]
- 28.Pfaller MA, Hazen KC, Messer SA, et al. Comparison of results of fluconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS global antifungal surveillance program. J Clin Microbiol. 2004;42:3607–3612. doi: 10.1128/JCM.42.8.3607-3612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. 2004;17:638–680. doi: 10.1128/CMR.17.3.638-680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin DK, Jr, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634–640. doi: 10.1542/peds.112.3.634. [DOI] [PubMed] [Google Scholar]
- 31.Torres HA, Kontoyiannis DP, Rolston KVI. High-dose fluconazole therapy for cancer patients with solid tumors and candidemia: an observational, noncomparative retrospective study. Support Care Cancer. 2004;12:511–516. doi: 10.1007/s00520-004-0601-x. [DOI] [PubMed] [Google Scholar]
- 32.De Bellis P, Bonfiglio M, Gerbi G, et al. High-dose fluconazole therapy in Intensive Care Unit. Minerva Anestesiol. 69:145–152. [PubMed] [Google Scholar]
- 33.Rex JH, Pappas PG, Karchmer AW, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis. 2003;36:1221–1228. doi: 10.1086/374850. [DOI] [PubMed] [Google Scholar]
- 34.Voss A, de Pauw BE. High-Dose Fluconazole Therapy in Patients with Severe Fungal Infections. Eur J Clin Microbiol Infect Dis. 1999;18:165–174. doi: 10.1007/s100960050252. [DOI] [PubMed] [Google Scholar]
- 35.Louie A, Liu W, Miller DA, et al. Efficacies of high-dose fluconazole plus amphotericin B and high-dose fluconazole plus 5-fluorocytosine versus amphotericin B, fluconazole, and 5-fluorocytosine monotherapies in treatment of experimental endocarditis, endophthalmitis, and pyelonephritis due to Candida albicans. Antimicrob Agents Chemother. 1999;43:2831–2840. doi: 10.1128/aac.43.12.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anaissie EJ, Kontoyiannis DP, Huls C, et al. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis. 1995;172:599–602. doi: 10.1093/infdis/172.2.599. [DOI] [PubMed] [Google Scholar]
- 37.Menichetti F, Fiorio M, Tosti A, et al. High-dose fluconazole therapy for cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22:838–840. doi: 10.1093/clinids/22.5.838. [DOI] [PubMed] [Google Scholar]
- 38.Haubrich RH, Haghighat D, Bozzette SA, Tilles J, McCutchan JA The California Collaborative Treatment Group. High-dose fluconazole for treatment of cryptococcal disease in patients with human immunodeficiency virus infection. J Infect Dis. 1994;170:238–242. doi: 10.1093/infdis/170.1.238. [DOI] [PubMed] [Google Scholar]
- 39.Berry AJ, Rinaldi MG, Graybill JR. Use of high-dose fluconazole as salvage therapy for cryptococcal meningitis in patients with AIDS. Antimicrob Agents Chemother. 1992;36:690–692. doi: 10.1128/aac.36.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novelli V, Holzel H. Safety and tolerability of fluconazole in children. Antimicrob Agents Chemother. 1999;43:1955–1960. doi: 10.1128/aac.43.8.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JW, Seibel NL, Amantea M, et al. Safety and pharmacokinetics of fluconazole in children with neoplastic diseases. J Pediatr. 1992;120:987–993. doi: 10.1016/s0022-3476(05)81975-4. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman DA, Gurka MJ, Hazen KC, et al. Patterns of fungal colonization in preterm infants weighing less than 1000 grams at birth. Pediatr Infect Dis J. 2006;25:733–737. doi: 10.1097/01.inf.0000226978.96218.e6. [DOI] [PubMed] [Google Scholar]
- 43.Huang YC, Kao HT, Lin TY, Kuo AJ. Antifungal susceptibility testing and the correlation with clinical outcome in neonatal candidemia. Am J Perinatol. 2001;18:141–146. doi: 10.1055/s-2001-14524. [DOI] [PubMed] [Google Scholar]
- 44.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loeffler J, Stevens DA. Antifungal drug resistance. Clin Infect Dis. 2003;36:S31–S41. doi: 10.1086/344658. [DOI] [PubMed] [Google Scholar]
- 46.Sarvikivi E, Lyytikainen O, Soll DR, et al. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. J Clin Microbiol. 2005;43:2729–2735. doi: 10.1128/JCM.43.6.2729-2735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]