Abstract
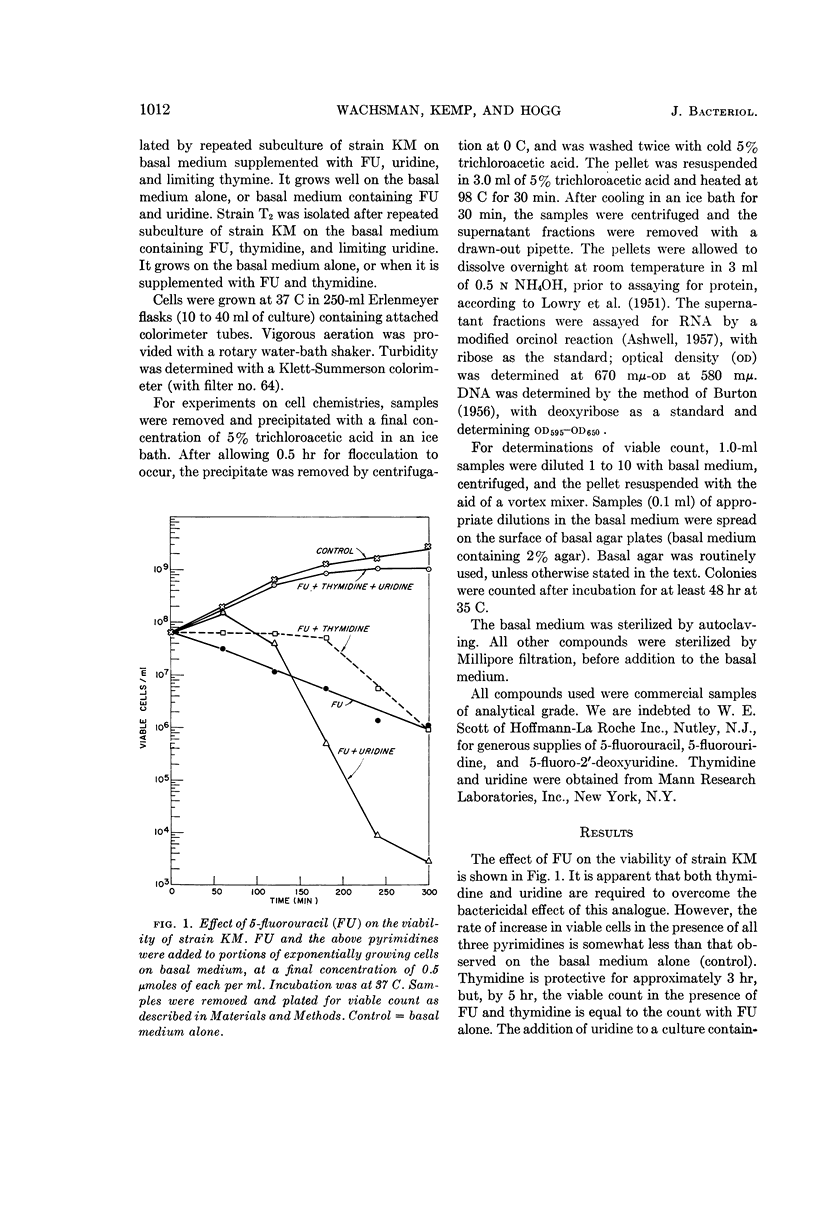
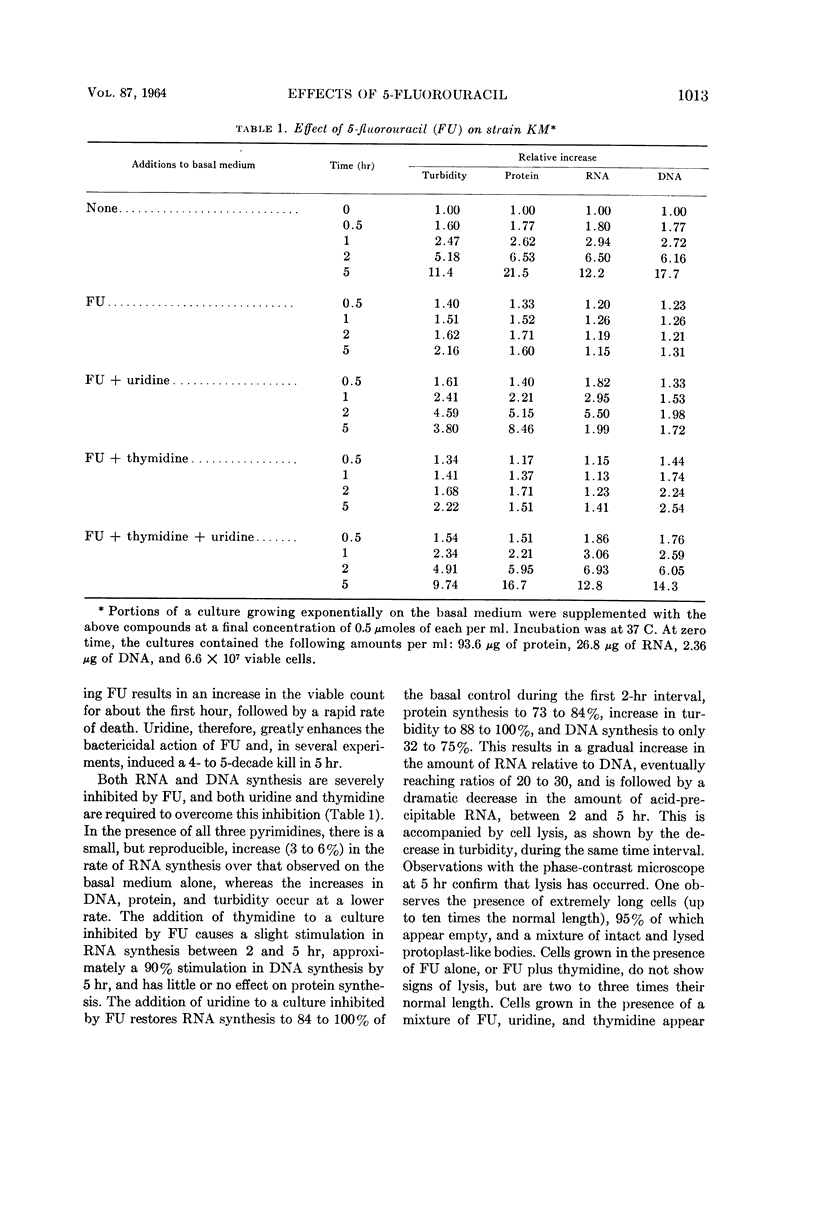
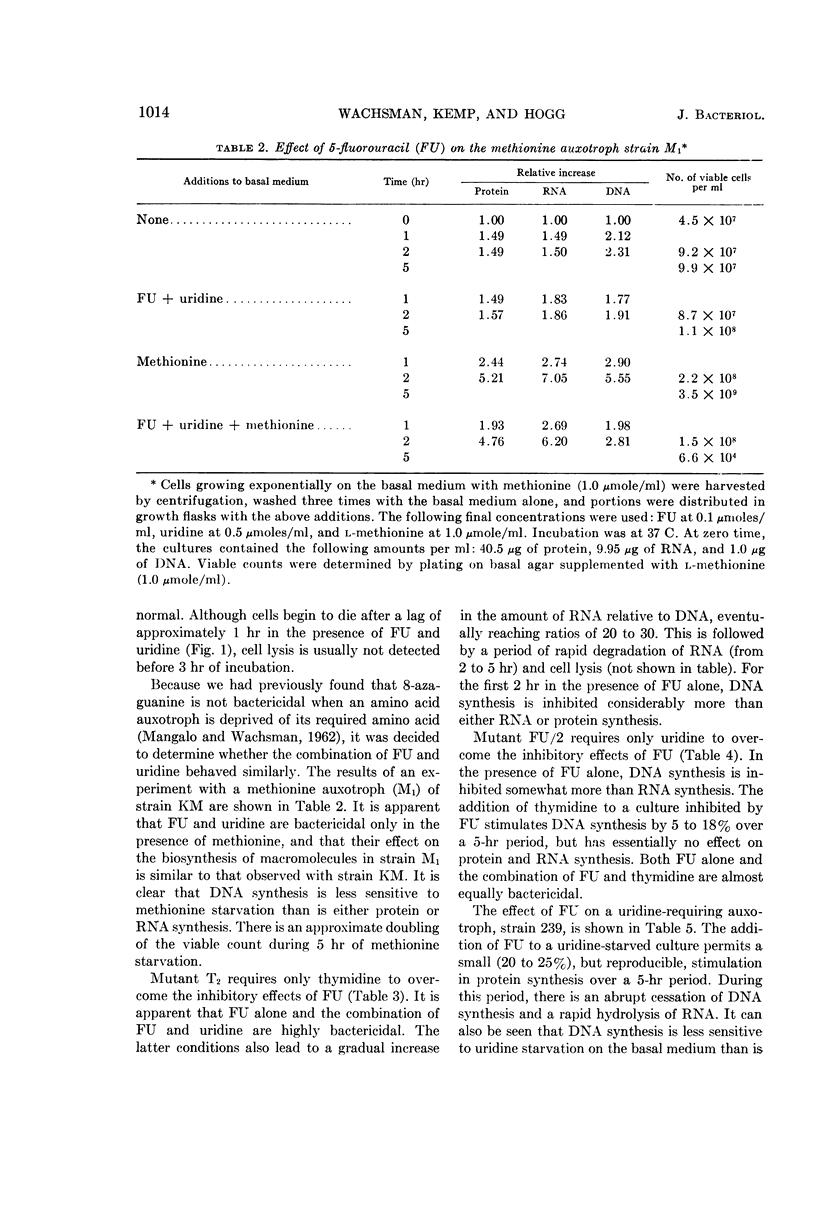
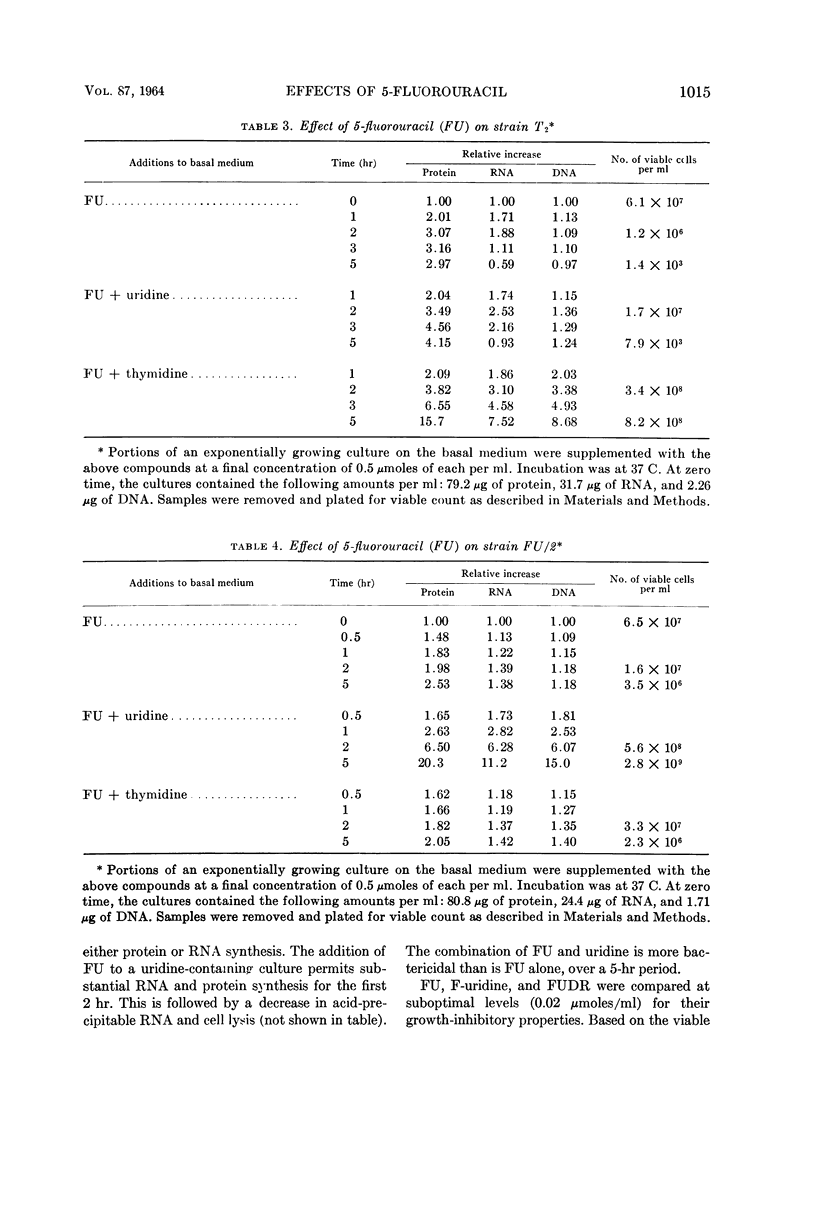
Wachsman, J. T. (University of Illinois, Urbana), S. Kemp, and L. Hogg. Comparative effects of 5-fluorouracil on strains of Bacillus megaterium. J. Bacteriol. 87:1011–1018. 1964.—Growth of Bacillus megaterium strain KM is severely inhibited by 5-fluorouracil (FU). Both thymidine and uridine are required to overcome this inhibition. The addition of uridine alone to a FU-inhibited culture permits good ribonucleic acid (RNA) and protein synthesis for the first 2 hr, but rather poor deoxyribonucleic acid synthesis. Uridine enhances the bactericidal effect of FU, promoting a decrease in the viable count of from 4 to 5 decades in 5 hr. Death begins after a 1-hr lag and is accompanied by hydrolysis of RNA and cell lysis, commencing during the 2- to 5-hr interval. The combination of FU and uridine is not bactericidal, when a methionine auxotroph is deprived of its required amino acid. Substrains of KM, partially resistant to FU, were isolated. Strain T2 requires only thymidine to overcome the inhibitory effects of FU, whereas strain FU/2 requires only uridine. With a uridine auxotroph of strain KM, FU partially replaces uridine by permitting a small, but reproducible, increase in the amount of protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNER H. D., COHEN S. S. The isolation and properties of amino acid requiring mutants of a thymineless bacterium. J Bacteriol. 1957 Sep;74(3):350–355. doi: 10.1128/jb.74.3.350-355.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAKS J. G., COHEN S. S. Virus-induced acquisition of metabolic function. III. Formation and some properties of thymidylate synthetase of bacteriophage-infected Escherichia coli. J Biol Chem. 1959 Nov;234:2981–2986. [PubMed] [Google Scholar]
- GORDON M. P., STAEHELIN M. Studies on the incorporation of 5-fluorouracil into a virus nucleic acid. Biochim Biophys Acta. 1959 Dec;36:351–361. doi: 10.1016/0006-3002(59)90177-5. [DOI] [PubMed] [Google Scholar]
- HARBERS E., CHAUDHURI N. K., HEIDELBERGER C. Studies on fluorinated pyrimidines. VIII. Further biochemical and metabolic investigations. J Biol Chem. 1959 May;234(5):1255–1262. [PubMed] [Google Scholar]
- HEIDELBERGER C., CHAUDHURI N. K., DANNEBERG P., MOOREN D., GRIESBACH L., DUSCHINSKY R., SCHNITZER R. J., PLEVEN E., SCHEINER J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957 Mar 30;179(4561):663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- HOROWITZ J., SAUKKONEN J. J., CHARGAFF E. Effects of fluoropyrimidines on the synthesis of bacterial proteins and nucleic acids. J Biol Chem. 1960 Nov;235:3266–3272. [PubMed] [Google Scholar]
- KELLENBERGER E., LARK K. G., BOLLE A. Amino acid dependent control of DNA synthesis in bacteria and vegetative phage. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1860–1868. doi: 10.1073/pnas.48.10.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANGALO R., WACHSMAN J. T. Effect of 8-azaguanine on growth and viability of Bacillus megaterium. J Bacteriol. 1962 Jan;83:27–34. doi: 10.1128/jb.83.1.27-34.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI T., OKAZAKI R. Studies of deoxyribonucleic acid synthesis and cell growth in the deoxyriboside requiring bacteria, Lactobacillus acidophilus. II. Deoxyribonucleic acid synthesis in relation to ribonucleic acid and protein synthesis. Biochim Biophys Acta. 1959 Oct;35:434–445. doi: 10.1016/0006-3002(59)90393-2. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J., PERKINS H. R. 5-Fluorouracil and mucopeptide biosynthesis by Staphylococcus aureus. Biochem J. 1960 Dec;77:448–459. doi: 10.1042/bj0770448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Borek E. THE MECHANISM OF BACTERIAL FRAGILITY PRODUCED BY 5-FLUOROURACIL: THE ACCUMULATION OF CELL WALL PRECURSORS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):324–327. doi: 10.1073/pnas.46.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACHSMAN J. T., MANGALO R. Use of 8-azaguanine for the isolation of auxotrophic mutants of Bacillus megaterium. J Bacteriol. 1962 Jan;83:35–37. doi: 10.1128/jb.83.1.35-37.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE P. J., NICHOL C. A. Effects of uracil and thymidine on the development of resistance to 5-fluorouracil in Pediococcus cerevisiae. J Bacteriol. 1963 Jan;85:97–105. doi: 10.1128/jb.85.1.97-105.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


