Abstract
We studied cardiac function in young and old, wild-type (WT), and longer-living Little mice using cardiac flow velocities, echocardiographic measurements, and left ventricular (LV) pressure (P) to determine if enhanced reserves were in part responsible for longevity in these mice. Resting/baseline cardiac function, as measured by velocities, LV dimensions, +dP/dtmax, and −dP/dtmax, was significantly lower in young Little mice versus young WT mice. Fractional shortening (FS) increased significantly, and neither +dP/dtmax nor −dP/dtmax declined with age in Little mice. In contrast, old WT mice had no change in FS but had significantly lower +dP/dtmax and −dP/dtmax versus young WT mice. Significant decreases were observed in the velocity indices of old Little mice versus old WT mice, but other parameters were unchanged. The magnitude of dobutamine stress response remained unchanged with age in Little mice, while that in WT mice decreased. These data suggest that while resting cardiac function in Little mice versus WT mice is lower at young age, it is relatively unaltered with aging. Additionally, cardiac function in response to stress was maintained with age in Little mice but not in their WT counterparts. Thus, some mouse models of increased longevity may not be associated with enhanced reserves.
DWARF mice (1,2) and rats (3) have increased longevity under laboratory conditions and therefore are of interest in gerontology. However, many of these mice have complex lesions with multiple endocrine changes. For example, the Snell and Ames dwarf mice have hypothyroidism (1), which is well known to alter cardiac function (4,5).
The Little mouse is generated by a missense mutation in the growth hormone (GH)–releasing hormone receptor (Ghrhr) (6), but, importantly, the rest of its pituitary is intact and it has normal thyroid function (1). These mice have reduced levels of circulating insulin-like growth factor 1 (IGF-I) (7) and have about 1% of normal levels of circulating GH (1,2). As a result, the Little (Ghrhrlit/lit) mice weigh about a third less (1) and live a third longer than their wild-type (Ghrhrlit/+) littermates (2). It has been shown that Little mice, despite having significant deficiencies in GH, have normal hematopoiesis and normal thyroid function (8), but delayed immune and collagen aging (2). Although the anabolic effects of GH/IGF-I in general are well recognized, the specific effects of GH and IGF-I deficiency in isolation on mouse cardiac function are not well characterized. Studies in young dwarf rats showed reduced left ventricular (LV) weight and impaired cardiac performance in vitro with significantly impaired systolic function and reduced LV diastolic distensibility (9). Spontaneous dwarf rats have no detectable circulating GH levels and have IGF-I levels at <10% of normal rats (10). Impaired IGF-I signaling causes cardiomyocyte apoptosis in spontaneously hypertensive models of rats (11), and IGF-I–deficient mice have increased rates of apoptosis following myocardial infarction (12). In contrast, elevated levels of IGF-I augment myocardial contractility by sensitizing myofilaments to calcium in rat hearts (13) and retard apoptosis in heart failure models of mice (14) and in cultured rat cardiomyocytes (15). Cardiac-specific and supraphysiologic IGF-I levels in SIS2 mice produced enhanced function in youth and impaired function and hypertrophy in old age (16). In human adults with GH deficiency, GH replacement leads to augmentation of LV mass, intraventricular septum thickness, and stroke volume (17). These data would suggest that GH deficiency is associated with impairment in cardiac function and that correcting this deficiency by replacement of GH can improve cardiac function.
However, it is also known that GH and IGF-I–deficient animals live much longer than their wild-type (WT) counterparts (2,18–20), which may be in part due to maintenance of cardiac function with age in these animals. So, the following questions need to be answered: (i) Is cardiac function in dwarf mice different from that of WT mice? (ii) How does the cardiac function change with age in dwarf animals? and (iii) Are there augmented cardiac reserves available to the dwarf animals that could contribute to their extended longevity? To address these questions, we studied and compared cardiac function in young and old Little mice with that of their age-matched WT littermates.
Methods
Animal Preparation
Twenty-four young mice (aged 4 months; 11 Little and 13 WT) and 17 old mice (aged 30 months; 8 Little and 9 WT) were used in the study. Animals were kept in rooms with controlled temperature (24°C) and lighting (14:10 hour light/dark cycle) with free access to food and water. The diets of both groups of mice consisted of normal chow.
All studies (invasive and noninvasive) were performed on anesthetized mice. The mice were anesthetized with 1% isoflurane (in 100% oxygen) administered at a continuous flow rate of 20 mL/min (VetEquip, Inc., Pleasanton, CA) for noninvasive Doppler measurements. Pentobarbital cocktail (1.8 mL of pentobarbital sodium at 50 mg/mL, 4.0 mL of 200 proof ethyl alcohol, and 16.0 mL of 0.9% saline) administered intraperitoneally at 10 μL/g of body weight (BW) was used for the invasive LV pressure (LVP) measurements. The anesthetized mouse was placed on the electrocardiogram (ECG)/heater board with the board temperature adjusted to maintain mouse body temperature at 37 ± 1°C. The limbs of the mouse were taped to the four electrodes, the quality of ECG assessed, and the electrode contact optimized as needed. All animal protocols were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (DHHS Publication No. 85-23, Revised 1985, Office of Science and Health Reports, Bethesda, MD).
Cardiac Doppler Measurement
Aortic and mitral blood flow velocities were measured with a 10 MHz pulsed Doppler probe in all mice. The probe was placed just below the sternum using minimal pressure and angled toward the ventricular inflow and outflow tracks, respectively (21). At each site, the sample volume depth and probe position were adjusted to record the maximum velocity with waveform, direction, and timing consistent with mitral or aortic velocity. We have verified in previous studies (22,23) that consistent and reproducible signals are obtainable from these sites in mice without image guidance. Typical depth setting was 4–7 mm for transmitral recordings and 6–9 mm for transaortic recordings (23). Two-second segments of the Doppler signals along with ECG were acquired and stored using a Doppler signal processing workstation (DSPW; Indus Instruments, Houston, TX).
Echocardiography Measurement
Echocardiograph measurements were made in 21 young (11 Little, 10 WT) and in 11 old (5 Little, 6 WT) mice using an 8 MHz to 13 MHz probe (Sequoia C256; Acuson Siemens, Mountain View, CA). Short-axis M-mode was used for measurement of LV systolic and diastolic dimensions. Percent fractional shortening (%FS) was calculated as [(LVEDD − LVESD)/LVEDD)] × 100, where LVESD is LV end-systolic dimension and LVEDD is LV end-diastolic dimension.
LVP Measurement
We were able to measure LVP in 15 young (7 Little, 8 WT) and in 13 old (6 Little, 7 WT) mice using a 0.36-mm-diameter pressure catheter. The neck area of the anesthetized mouse was shaved, and using blunt surgical procedures, the right carotid artery was isolated. The artery was tied off distally, and the proximal end was temporarily closed. A small cut was made in the artery wall, and a modified RADI PressureWire3 (RADI Medical Systems, Upsala, Sweden) was inserted and held in place with a suture tied over the artery–catheter overlap region (21). The proximal end of the artery was then opened, and the catheter was advanced into the ascending aorta and then into the LV. LVP was measured at baseline and after intraperitoneal administration of dobutamine (1.5 μg/g of BW as reported in Tanaka and colleagues, 24). Again, the signals were acquired using DSPW and stored for offline analysis.
Data Analysis
Noninvasive cardiac parameters such as peak and mean aortic flow velocities, mean aortic acceleration, peak early flow velocity, and isovolumic contraction (ICT) and relaxation (IRT) times were extracted from aortic and mitral flow velocity signals. Tei index was calculated as (ICT + IRT)/systolic ejection time (ET) as defined by Tei and colleagues (25). Maximal rate of LV contractility (+dP/dtmax; mmHg/s), maximal rate of LV relaxation (−dP/dtmax; mmHg/s), and τ (ms; time constant of −dP/dt) were calculated from the LVP signal. All the parameters are presented in the form of mean ± standard error (SE), and comparisons were made using paired and unpaired Student's t test using a significance level of .05.
Results
Little mice are clearly smaller. Both young and old Little mice weighed significantly less than their WT counterparts (WT vs Little—young: 22.8 ± 0.3 vs 13.3 ± 0.4 g, p < .05; old: 30.7 ± 0.8 vs 18.4 ± 0.8 g, p < .05), respectively. This difference in BW represented about 42% lower weight in young Little mice and 40% lower weight in old Little mice compared to their respective WT counterparts. No significant differences were observed between the baseline heart rates of the two groups with either of the anesthetics. The data are summarized in Table 1.
Table 1.
Baseline (Resting Values) Parameters Extracted From Noninvasive Cardiac Doppler Flow Velocity Signals and Invasive Left Ventricular (LV) Pressure
| Parameters | Young Mice | Old Mice | ||
|---|---|---|---|---|
| WT (11) | Little (10) | WT (9) | Little (8) | |
| General | ||||
| Heart rate (beats/min) | 414 ± 12 | 380 ± 16 | 409 ± 14 | 371 ± 11 |
| Body weight, g | 22.8 ± 0.3 | 13.3 ± 0.4* | 30.7 ± 0.8† | 18.4 ± 0.8‡,§ |
| Cardiac Doppler indices | ||||
| Peak aortic flow velocity, cm/s | 88.5 ± 2.1 | 73.7 ± 3.0* | 78.6 ± 3.2† | 65.0 ± 2.5‡,§ |
| Mean aortic flow velocity, cm/s | 21.4 ± 0.6 | 17.6 ± 1.1* | 19.8 ± 1.1 | 15.5 ± 0.6‡,§ |
| Mean aortic acceleration, cm/s2 | 7254 ± 257 | 5943 ± 434* | 6714 ± 485 | 5304 ± 304‡,§ |
| Peak E-flow velocity, cm/s | 75.1 ± 1.6 | 61.4 ± 3.5 | 62.8 ± 5.4† | 52.5 ± 3.7 |
| Isovolumic contraction time, ms | 12.3 ± 0.4 | 11.5 ± 0.7 | 13.5 ± 0.6† | 12.0 ± 0.5 |
| Isovolumic relaxation time, ms | 18.4 ± 0.5 | 19.2 ± 1.2 | 20.5 ± 1.1 | 21.2 ± 1.0 |
| Tei index | 0.56 ± 0.01 | 0.55 ± 0.03 | 0.66 ± 0.11 | 0.61 ± 0.02 |
| WT (8) | Little (7) | WT (7) | Little (6) | |
| LV pressure indices | ||||
| Heart rate, beats/min | 392 ± 18 | 363 ± 32 | 392 ± 8 | 385 ± 6 |
| Maximal +dP/dt, mmHg/s | 6819 ± 319 | 5079 ± 449* | 5062 ± 557† | 5153 ± 375 |
| Maximal −dP/dt, mmHg/s | −6708 ± 521 | −4941 ± 434* | −4291 ± 501† | −5028 ± 601 |
| Tau, τ, ms | 11.6 ± 0.9 | 10.2 ± 0.8 | 11.0 ± 1.2 | 9.3 ± 1.8 |
Notes: All values are mean ± standard error.
Comparisons at p < .05:
Young WT versus young Little.
Young WT versus old WT.
Old WT versus old Little.
Young Little versus old Little.
WT = wild-type.
Doppler Studies
We studied systolic and diastolic function noninvasively with pulsed Doppler in Little mice and their WT littermates. Indices of systolic function such as peak and mean aortic flow velocities and mean aortic acceleration were significantly lower in the young Little mice than in the young WT mice. Also, peak early mitral velocity in young Little mice was significantly lower than that of young WT mice, but no significant differences were observed either in ICT, IRT, or Tei index between young Little and WT mice (see Table 1).
We then examined the old Little mice compared to the old WT mice. Differences were found in systolic indices with significantly lower peak and mean aortic flow velocities and mean aortic acceleration in the old Little mice. No significant differences were observed in peak mitral flow velocity, ICT, IRT, or Tei index between the old Little and WT mice.
Peak and mean aortic velocity and mean aortic acceleration were decreased significantly in old versus young Little mice. In WT mice, peak aortic velocity and peak early mitral velocity were decreased significantly in the old versus young mice. The Tei index was significantly higher in the old WT than in the young WT mice, which is consistent with overall dysfunction with age. In general, the values for the young Little mice were slightly lower but not significantly different from those in old WT mice (Table 1).
Echocardiography Studies
Echocardiograph measurements (Figure 1) showed that Little mice had significantly lower LV systolic and diastolic dimensions than their respective WT littermates, but %FS remained the same in Little and WT mice within each age group. Additionally, %FS did not change significantly with age in WT mice, but old Little mice had significantly higher %FS than did young Little mice.
Figure 1.
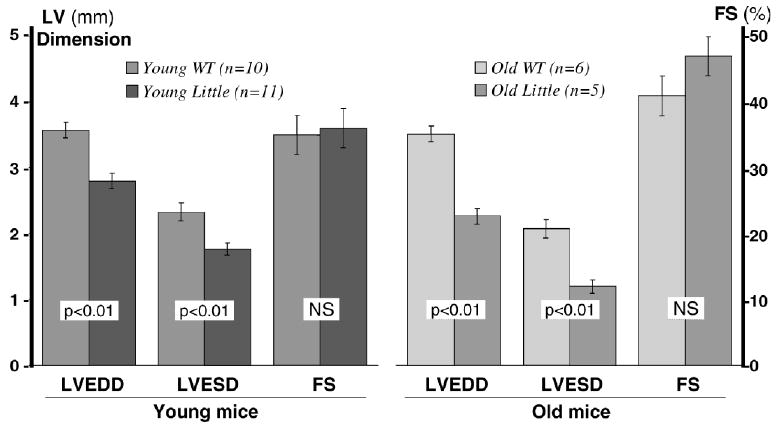
Echocardiographic measurement of left ventricular (LV) dimensions (LV end-diastolic dimension [LVEDD] and LV end-systolic dimension [LVESD]) and percent fractional shortening (%FS) in young and old Little and wild-type (WT) mice.
Invasive Studies
The parameters of +dP/dtmax, −dP/dtmax, and τ (time constant of relaxation) were derived from the invasive LVP measurements. Both +dP/dtmax and −dP/dtmax were significantly lower in the young Little mice compared to their young WT littermates, but τ was similar. In old mice, however, the differences in +dP/dtmax and −dP/dtmax between Little and WT mice disappeared.
Both +dP/dtmax and −dP/dtmax were significantly lower in old WT mice compared to young WT mice (Table 1). However, aging did not diminish the heart function in old Little mice compared to young Little mice. We found no significant differences in τ between any of the groups.
Responses to Stress (Dobutamine)
Heart rate, +dP/dtmax, and −dP/dtmax increased significantly, and τ decreased significantly, from their respective baseline values in the young Little and WT mice following the administration of dobutamine. Although the trend was similar in both groups of young mice in response to dobutamine, the percent change in magnitude from baseline showed that +dP/dtmax increased by 98% in WT and by 76% in Little mice, −dP/dtmax increased by 28% in WT and 40% in Little mice, and τ decreased by 28% in WT and 34% in Little mice. The absolute baseline and postdobutamine values of +dP/dtmax, −dP/dtmax, and τ in young mice are summarized in Figure 2. In old mice the percent change in magnitude from baseline showed that +dP/dtmax increased by only 65% in WT and increased by 91% in Little mice, −dP/dtmax increased by 37% in WT and 30% in Little mice, and τ decreased by 22% in WT and 31% in Little mice. The absolute baseline and post-dobutamine values of +dP/dtmax, and −dP/dtmax in old mice are shown in Figure 3.
Figure 2.
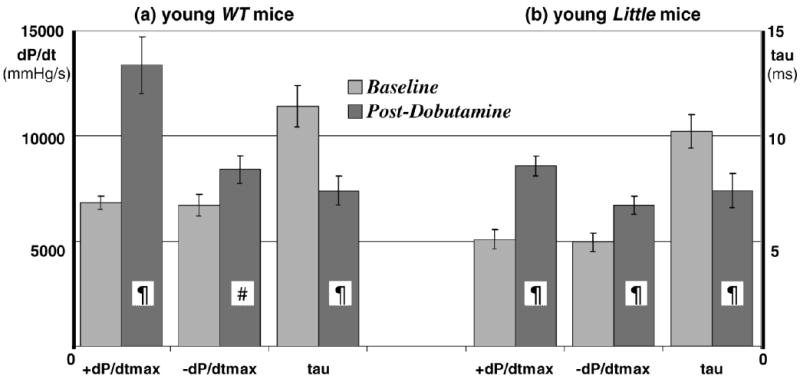
Baseline and postdobutamine left ventricular pressure parameters in 8 young wild-type (WT) (a) and 7 young Little mice (b) (¶p < .01, #p < .05 postdobutamine vs baseline). The heart rates at (WT vs Little) were 392 ± 18 versus 363 ± 32 beats/min at baseline and 541 ± 9 versus 535 ± 10 beats/min postdobutamine.
Figure 3.
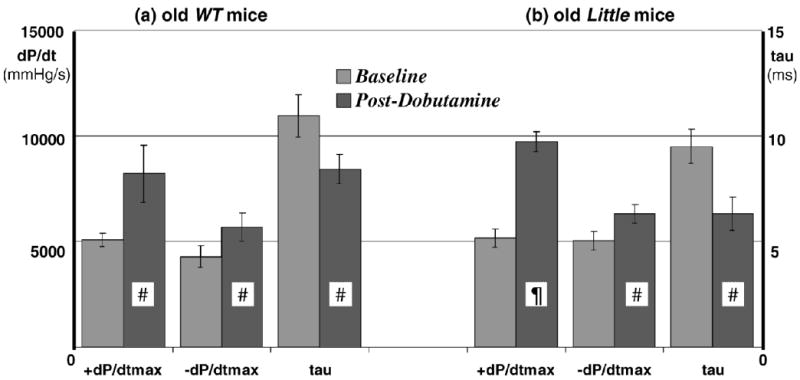
Baseline and postdobutamine left ventricular pressure parameters in 7 old wild-type (WT) (a) and 6 old Little mice (b) (¶p < .01, #p < .05 postdobutamine vs baseline). The heart rates at (WT vs Little) were 392 ± 8 versus 385 ± 6 beats/min at baseline and 559 ± 25 versus 515 ± 25 beats/min postdobutamine.
When compared for effects of aging within the groups, the magnitude of responses of young WT mice was significantly higher than that of the old WT mice, whereas the magnitude of responses of young and old Little mice was similar.
Discussion
Dwarf mice, including the Little mouse, live much longer than WT mice. This extended life span makes dwarf mice of interest in the field of geriatrics, but very little is known about the cardiovascular function of these mice and how it is altered as they age. Many of the available models of dwarfism have significant global pituitary dysfunction including loss of thyroid regulation and lack of prolactin in addition to the deficit in GH production (2). Thyroid hormone plays a fundamental role in cardiovascular homeostasis, and any alterations in thyroid function lead to altered cardiac function, including changes in heart rate (4,5). Therefore, it would be no surprise that altered cardiac function would occur in the Snell or Ames dwarf mice where very low thyroid hormone levels are part of the phenotype. The Little mouse model of dwarfism has no active GH-releasing hormone receptor, but the rest of its anterior pituitary is intact and has normal thyroid function (1). Therefore, we hypothesized that cardiac function in these mice might be robust and not deteriorate with age.
Body Weight
GH deficiency is associated with significant reduction in BW in humans (26,27), rats (9,28,29), and mice (2,30,31). Our study confirmed that the young Little mice have significantly decreased BW compared to their WT littermates. The percent difference in BW with age is maintained in these mice (WT increased 35% and Little by 38%). Thus Little mice have aging-related obesity as do WT mice under normal dietary conditions. Miller and colleagues (30) reported that low BW at a young age is a significant predictor of prolonged life span in mice. We killed our animals after the invasive studies; therefore, we were unable to record either their life span or weight beyond 30 months.
Cardiac Function in Young Little Mice
Abnormalities in cardiac function have been a component of human and animal models deficient in GH. In GH-deficient humans, cardiac performance (as measured by ejection fraction and cardiac index) is significantly depressed (32), and several abnormalities in cardiac structure and function have been reported (33,34). Cardiac abnormalities in dwarf rats include reduced LV weight, smaller size of myocytes, impaired myocardial contractility and distensibility (9), reduced LV diastolic volumes (indexed to tibial length), depressed cardiac index, and abnormal stress-shortening relationships (34). Although the effects of GH deficiency are well established in dwarf mice (17), there are very few reports on cardiac function in these animals. Ren and Brown-Borg (31) reported that dwarf mice had lower heart weight and that excitation–contraction coupling in ventricular myocytes was impaired consistent with low levels of thyroid hormone. In our study, noninvasive cardiac Doppler indices showed significantly decreased systolic and diastolic function, and echocardiography studies showed decreased systolic and diastolic dimensions in young Little mice under light isoflurane anesthesia (baseline). The relative decreases in systolic and diastolic dimensions, however, kept the %FS similar in young Little and WT mice. Importantly, heart rates under these conditions were not different, consistent with the absence of thyroid differences between the two groups. These observations in Little mice at baseline were also confirmed by the reduced values of +dP/dtmax and −dP/dtmax obtained from LVP, despite slight decreases in heart rate under pentobarbital anesthesia. We acknowledge that anesthetics influence hemodynamic measurements; however, mouse heart rates of 350–400 beats/min were reported in sleeping mice when monitored using telemetry (35). While the heart rates with isoflurane were about 400 beats/min, they were slightly lower than with pentobarbital but still >350 beats/min. This would make the ±dP/dt values lower than those reported by others. The assumption that the effect of anesthesia is similar on both groups of mice allowed us to evaluate and compare the relative performance of the two groups of mice. The findings are consistent with studies in human and animal models of dwarfism that have shown that isolated GH deficiency is associated with impairment in cardiac function (9,13,17,33), but are in contrast to the findings of Lembo and colleagues (36), who reported that “midi” mice with 30% of normal levels of IGF-I (total IGF-I=51 ng/mL) had better myocardial contractility perhaps enhanced due to elevated serum GH levels. Similarly, patients of Laron syndrome who have high levels of serum GH have normal LV contractile reserve (37). However, the levels of IGF-I in the Little mouse are even lower at 10% of normal (7) or 9%–23% of normal, as reported by Liang and colleagues (1) and have about 1% of normal levels of circulating of GH (1,2), thus resulting in diminished cardiac function in these mice.
On the basis of our findings, we considered that the lowered function at baseline in Little mice might permit enhanced physiologic reserves in later life for dealing with potential challenges. To test this hypothesis, we used dobutamine to maximally stimulate the heart (24) of young Little and WT mice. We found no differences in the relative peak responses of either systolic or diastolic function between the young Little and WT mice as measured by LVP (Figure 2). Thus, the young Little mice had neither enhanced nor decreased cardiac contractile reserve under stress when compared to their WT littermates.
Cardiac Function in Old Little Mice
The noninvasive cardiac Doppler indices of systolic function were lower in old Little mice at rest (baseline) compared to old WT mice. This finding was consistent with LV dimensions, but again the relative decreases in systolic and diastolic dimensions kept the %FS similar in young Little and WT mice. With age, however, %FS was significantly higher in Little mice than in WT mice (Figure 1). The findings of reduced dimensions and function with age as measured noninvasively were not confirmed by invasive studies (+dP/dtmax and −dP/dtmax). Importantly, there were no age-related differences in +dP/dtmax and −dP/dtmax of Little mice from 4 months to 30 months of age, and the increase in %FS in old Little mice may support this finding that the cardiac contractility in these mice is unaltered if not improved. In contrast, +dP/dtmax and −dP/dtmax decreased significantly in WT mice with age (see Table 1). The significantly higher value of the Tei index in the old WT mice was consistent with the presence of cardiac dysfunction.
Despite the diminished baseline cardiac function in the young Little mice when compared to their WT littermates, their response to stress on a percentage or absolute basis did not change with age (see Figure 3). We speculated that the low levels of circulating IGF-I responsible for the diminished baseline cardiac function at a young age in Little mice could have played a cardioprotective role. Delaughter and colleagues (16) have shown that chronic expression of local IGF-I in the SIS2 transgenic mouse resulted in diminished systolic performance in old age, despite enhanced systolic performance at a young age. Although adults with Laron syndrome or Lembo and colleagues' (36) “midi” mouse live long perhaps due to high serum levels of GH, humans with GH and several other pituitary hormone deficiencies are known to have lived up to the age of 91 (38). It is reported that patients with adult onset of pituitary hormone deficiencies could have died early of complications from tumors, surgical procedures, or radiation treatments (37). While we can speculate that normal levels of IGF-I in the WT mouse may have caused its heart to hypertrophy with age and diminish systolic performance (see Figure 4), it is also known that IGF-I levels can decrease substantially from 4 months of age to 30 months of age in WT mice (39), and yet these mice have reduced contractility. It has been shown that serum IGF-I levels are decreased by as much as 50% in 80- to 90-year-old humans (40). Despite the reduction, the circulating levels of IGF-I are still high in WT mice compared to those in dwarf animals. Thus our hypothesis of Figure 4 may be valid; therefore, the role of the reduced circulating levels of IGF-I on the myocardial performance with age needs to be further investigated.
Figure 4.
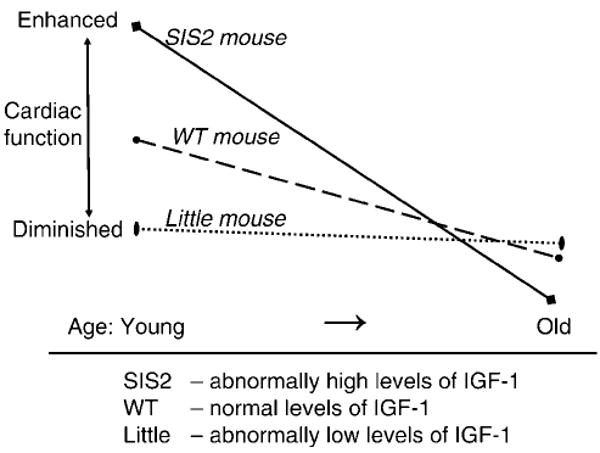
Plot of hypothetical curves concerning cardiac function in mice with different levels of insulin-like growth factor 1 (IGF-I): SIS2 mouse (high), wild-type (WT) mouse (normal), and Little mouse (low).
A basic mechanism of aging is the accumulation of oxidative damage. It has been reported that Little mice have increased stress resistance indices as observed through the up-regulation of several genes involved in reactive oxygen species detoxification (41). In Little mice, this could slow the aging of cardiac tissue through the reduction in the cumulative effect of oxidative damage caused by the presence of toxic free radicals that are generated from normal metabolism. Thus, dwarf mice may have enhanced antioxidative defense systems, especially if they live in a relatively stress-free environment (17,42), but may not have enhanced physiologic reserves to respond to environmental challenges. Also, higher measures of hematopoetic stem cells in Little mice (8) means that the damaged blood cells may be more readily replaced with new ones (albeit less efficiently in old age) to maintain function. All these potentially protective mechanisms may contribute to longevity in Little mice observed in laboratory environments.
Conclusion
Dwarf mice, which live much longer than their wild-type counterparts, might be aided by maintenance of cardiac function with age. We studied and compared the cardiac function in young and old Little (dwarf) mice to that of their WT littermates. Our results show that Doppler-derived systolic and diastolic measurements, Echocardiograph measurements of systolic and diastolic dimensions, and LVP-derived +dP/dtmax and −dP/dtmax at baseline were decreased in the young Little mice compared to their WT littermates. Although cardiac function in young Little mice is diminished, further deterioration did not occur with age (unlike that in WT mice). Stress-induced changes in LVP were similar in young Little and WT mice when compared to their respective baseline values. In the old Little and WT mice, the changes in −dP/dtmax to stress were similar when compared to their respective baseline values. However, with aging, the increase in +dP/dtmax to stress was slightly enhanced in Little mice and slightly diminished in WT mice. Neither of our hypotheses—(i) enhanced baseline function in Little mice or (ii) increased reserve in Little mice—proved to be correct. Instead, we found that the status of cardiac function at baseline and under stress, although reduced, was maintained with age in Little mice but not in their WT counterparts. This maintenance of cardiac function with age suggests that some models of increased longevity may not be associated with enhanced reserves.
Acknowledgments
This work was funded in part by the National Institutes of Health (AG17899 [Taffet], AG19254, AG20752, and DK56338 [Darlington], GM069234 and HL22512 [Hartley], HL73041 [Reddy]), the DeBakey Heart Center, the Medallion Foundation, the Hankamer Foundation, and the Ellison Medical Foundation.
We thank Thuy T. Pham, Jennifer Pocius, and Fazia Huq for their contributions to this work and Jim Brooks for editorial review.
References
- 1.Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp Gerontol. 2003;38:1353–1364. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimokawa I, Higami Y, Tsuchiya T, et al. Life span extension by reduction of growth hormone-insulin-like growth factor-1 axis: relation to caloric restriction. FASEB J. 2003;17:1108–1109. doi: 10.1096/fj.02-0819fje. [DOI] [PubMed] [Google Scholar]
- 4.Gallowitsch HJ. Thyroid and cardiovascular system. Wein Med Wochenschr. 2005;155:436–443. doi: 10.1007/s10354-005-0218-9. [DOI] [PubMed] [Google Scholar]
- 5.Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 2005;112:3122–3130. doi: 10.1161/CIRCULATIONAHA.105.572883. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of Little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4:227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- 7.Donahue LR, Beamer WG. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor binding protein 3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- 8.Sharma Y, Flurkey K, Astle CM, Harrison DE. Mice severely deficient in growth hormone have normal hematopoiesis. Exp Hematol. 2005;33:776–783. doi: 10.1016/j.exphem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Cittadini A, Strömer H, Vatner DE, et al. Consequences of growth hormone deficiency on cardiac structure, function, and β-adrenergic pathway: studies in mutant dwarf rats. Endocrinology. 1997;138:5161–5169. doi: 10.1210/endo.138.12.5591. [DOI] [PubMed] [Google Scholar]
- 10.Park S, Sohn S, Kineman RD. Fasting-induced changes in the hypothalamic-pituitary-GH axis in the absence of GH expression: lessons from the spontaneous dwarf rat. J Endocrinol. 2004;180:369–378. doi: 10.1677/joe.0.1800369. [DOI] [PubMed] [Google Scholar]
- 11.Kuo WW, Chu CY, Wu CH, et al. Impaired IGF-I signalling of hypertrophic hearts in the developmental phase of hypertension in genetically hypertensive rats. Cell Biochem Funct. 2005;23:325–331. doi: 10.1002/cbf.1244. [DOI] [PubMed] [Google Scholar]
- 12.Palmen M, Daemen MJ, Bronsaer R, et al. Cardiac remodeling after myocardial infarction is impaired in IGF-1 deficient mice. Cardiovasc Res. 2001;50:516–524. doi: 10.1016/s0008-6363(01)00237-1. [DOI] [PubMed] [Google Scholar]
- 13.Delafontaine P, Brink M. The growth hormone and insulin-like growth factor 1 axis in heart failure. Ann Endocrinol (Paris) 2000;61:22–26. [PubMed] [Google Scholar]
- 14.Samarel AM. IGF-1 overexpression rescues the failing heart. Circ Res. 2002;90:631–633. doi: 10.1161/01.res.0000015425.11187.19. [DOI] [PubMed] [Google Scholar]
- 15.Maldonado C, Cea P, Adasme T, et al. IGF-1 protects cardiac myocytes from hyperosmotic stress-induced apoptosis via CREB. Biochem Biophys Res Commun. 2005;336:1112–1118. doi: 10.1016/j.bbrc.2005.08.245. [DOI] [PubMed] [Google Scholar]
- 16.Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 1999;13:1923–1929. doi: 10.1096/fasebj.13.14.1923. [DOI] [PubMed] [Google Scholar]
- 17.Maison P, Chanson P. Cardiac effects of growth hormone in adults with growth hormone deficiency: a meta-analysis. Circulation. 2003;108:2648–2652. doi: 10.1161/01.CIR.0000100720.01867.1D. [DOI] [PubMed] [Google Scholar]
- 18.Brown-Borg HM, Rakoczy SG. Growth hormone administration to long-living dwarf mice alters multiple components of the antioxidative defense system. Mech Ageing Dev. 2003;124:1013–1024. doi: 10.1016/j.mad.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 20.Kopchick JJ, Laron Z. Is the Laron mouse an accurate model of Laron syndrome? Mol Genet Metab. 1999;68:232–236. doi: 10.1006/mgme.1999.2890. [DOI] [PubMed] [Google Scholar]
- 21.Reddy AK, Taffet GE, Li YH, et al. Pulsed Doppler signal processing for use in mice: applications. IEEE Trans Biomed Eng. 2005;52:1771–1783. doi: 10.1109/TBME.2005.855709. [DOI] [PubMed] [Google Scholar]
- 22.Hartley CJ, Michael LH, Entman ML. Noninvasive measurement of ascending aortic blood velocity in mice. Am J Physiol Heart Circ Physiol. 1995;268:H499–H505. doi: 10.1152/ajpheart.1995.268.1.H499. [DOI] [PubMed] [Google Scholar]
- 23.Taffet GE, Hartley CJ, Wen X, Pham TT, Michael LH, Entman ML. Noninvasive indexes of cardiac systolic and diastolic function in hyperthyroid and senescent mouse. Am J Physiol Heart Circ Physiol. 1996;270:H2204–H2209. doi: 10.1152/ajpheart.1996.270.6.H2204. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka N, Dalton N, Mao L, et al. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation. 1996;94:1109–1117. doi: 10.1161/01.cir.94.5.1109. [DOI] [PubMed] [Google Scholar]
- 25.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik J, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–664. doi: 10.1016/0735-1097(96)00202-1. [DOI] [PubMed] [Google Scholar]
- 26.Thomas M, Massa G, Craen M, et al. Prevalence and demographic features of childhood growth hormone deficiency in Belgium during the period of 1986–2001. Eur J Endocrinol. 2004;151:67–72. doi: 10.1530/eje.0.1510067. [DOI] [PubMed] [Google Scholar]
- 27.Parra A, Argote RM, Garcia G, Cervantes C, Alatorre S, Perez-Pasten E. Body composition in hypopituitary dwarfs before and during human growth hormone therapy. Metabolism. 1979;28:851–857. doi: 10.1016/0026-0495(79)90212-9. [DOI] [PubMed] [Google Scholar]
- 28.Daugaard JR, Laustsen JL, Hansen BS, Richter EA. Insulin action in growth hormone-deficient and age-matched control rats: effect of growth hormone treatment. J Endocrinol. 1994;160:127–135. doi: 10.1677/joe.0.1600127. [DOI] [PubMed] [Google Scholar]
- 29.Umezu M, Kagabu S, Jiang J, Sato E. Evaluation and characterization of congenital hypothyroidism in rdw dwarf rats. Lab Anim Sci. 1998;48:496–501. [PubMed] [Google Scholar]
- 30.Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- 31.Ren J, Brown-Borg HM. Impaired cardiac excitation-contraction coupling in ventricular myocytes from Ames dwarf mice with IGF-1 deficiency. Growth Horm IGF Res. 2002;12:99–105. doi: 10.1054/ghir.2002.0267. [DOI] [PubMed] [Google Scholar]
- 32.Cittadini A, Cuocolo A, Merola B, et al. Impaired cardiac performance in GH-deficient adults and its improvement after GH replacement. Am J Physiol. 1994;267(2 Pt 1):E219–E225. doi: 10.1152/ajpendo.1994.267.2.E219. [DOI] [PubMed] [Google Scholar]
- 33.Merola B, Cittadini A, Colao A, et al. Cardiac structural and functional abnormalities in adult patients with growth hormone deficiency. J Clin Endocrinol Metab. 1993;77:1658–1661. doi: 10.1210/jcem.77.6.8263155. [DOI] [PubMed] [Google Scholar]
- 34.Longobardi S, Cuocolo A, Merola B, et al. Left ventricular function in young adults with childhood and adulthood onset growth hormone deficiency. Clin Endocrinol. 1998;48:137–143. doi: 10.1046/j.1365-2265.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 35.Kramer K, Voss HP, Grimbergen JA, et al. Telemetric monitoring of blood pressure in freely moving mice: a preliminary study. Lab Anim. 2000;34:272–280. doi: 10.1258/002367700780384663. [DOI] [PubMed] [Google Scholar]
- 36.Lembo G, Rockman HA, Hunter JJ, et al. Elevated blood pressure and enhanced myocardial contractility in mice with severe IGF-1 deficiency. J Clin Invest. 1996;98:2648–2655. doi: 10.1172/JCI119086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinberg MS, Scheinowitz M, Laron Z. Echocardiographic dimensions and function in adults with primary growth hormone resistance (Laron syndrome) Am J Cardiol. 2000;85:209–213. doi: 10.1016/s0002-9149(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 38.Krzisnik C, Kolacio Z, Battelino T, Brown M, Parks JS, Laron Z. The ‘Little People’ of the island of Krk – revisited. J Endocr Genet. 1999;1:9–19. [Google Scholar]
- 39.Xu X, Bennett SA, Ingram RL, Sonntag WE. Decreases in growth hormone receptor signal transduction contribute to the decline in insulin-like growth factor I gene expression with age. Endocrinology. 1995;136:4551–4557. doi: 10.1210/endo.136.10.7664676. [DOI] [PubMed] [Google Scholar]
- 40.Lamberts SWJ, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 41.Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3:423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 42.Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. TRENDS Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]


