Abstract
One of the best-characterized Plasmodium falciparum merozoite ligands is EBA175, whose recognition of glycophorin A is dependent upon terminal sialic acids of this erythrocyte glycoprotein. The recently solved crystal structure of EBA175 reveals that a critical factor for erythrocyte recognition is the sialic acids, which lock into pockets on the EBA175 surface. A comparison to the P. reichenowi EBA175 suggests that these interactions have a pivotal role for host specific adaptations in parasite ligands.
Invasion is a multi-step process
Plasmodium falciparum merozoites are made to infect human erythrocytes. Invasion is a multi-step process involving specific interactions between parasite ligands and host cell receptors [1]. After release from the previous schizont, the malaria merozoite readily attaches to a new erythrocyte and prepares to enter by orienting the merozoite’s apical end to appose the erythrocyte surface. Different parasite ligands and host cell receptors are involved at each step. During the process of reorientation, micronemes sequestered within the merozoite are believed to be mobilized and release some of their contents to the surface. Erythrocyte Binding Antigen 175 (EBA175), which is a member of the erythrocyte binding-like (ebl) superfamily of protein ligands, is one of several micronemal proteins that have key roles in recognizing the receptors specific to human erythrocytes and forming a junction with the host cell [2]. The model for understanding the steps of the invasion process of malaria merozoite entry into erythrocytes was largely established by in vitro studies of P. knowlesi, a primate malaria closely related to P. vivax. Both of these parasites require the presence of the Duffy blood group antigen (DARC) to invade human erythrocytes, recognizing this receptor with a Duffy binding protein (DBP), which is homologous to EBA175 and a member of the ebl superfamily. In the absence of DARC, P. vivax and P. knowlesi merozoites will attach and undergo apical reorientation, but then fail to progress beyond that step. Junction formation is a crucial step in the infection process in that it commits the parasite to try to invade that particular cell and if it is not a suitable host cell, then the parasite will not survive. Associated with junction formation is the creation of the parasitophorous vacuole that the parasite will enter, remodel and live in to complete its development to the next generation of merozoites. Molecules of the ebl superfamily all contain one or more cysteine-rich Duffy binding-like domains (DBLs) that mediate recognition of the correct erythrocyte receptor. DBL domains are also found in the P. falciparum EMP1 and hence are also implicated in cytoadherence of infected erythrocytes to the endothelial wall, emphasizing the importance of this domain structure for Plasmodium biology in general and P. falciparum pathogenesis in particular. The recently published structures of the DBL domain of PfEBA175 [3] are the first DBL domain structures to be solved in any Plasmodium species. Defining the structural basis for ligand-receptor interactions is a significant step forward for the field of Plasmodium erythrocyte invasion and cytoadherence.
Structure of Plasmodium falciparum EBA175
The natural configuration of the EBA175 ligand domain on invasive merozoites has two tandem DBL domains (referred to as F1 and F2) at the N-terminal part of the EBA175 extracellular domain in an area also known as region II (RII). The F2 DBL copy of EBA175 can bind erythrocytes independent of F1 but not nearly so well as the intact RII (F1/F2). It is the F2 DBL from which the DBL domains of PfEMP1 have evolved [4]. In the deduced three-dimensional structure of EBA175 [3], the F1 and F2 domains had very similar crystal structures and were divided by a three-helix linker. Twenty-four of the 25 cysteines were involved in intradomain disulfide bonds within F1 and F2, respectively, stabilizing each DBL domain independent of the other. Each DBL monomer is comprised mostly of α-helices, which is expected based upon the CD spectrum of F2 [5], along with two anti-parallel ββ hairpins (termed ‘β fingers’ by the authors) and a number of bound sulfate molecules.
EBA175 RII dimerizes in an anti-parallel ‘handshake interaction’ with the F1 domain of each monomer interacting with the F2 domain of the other monomer. This interlocking arrangement appears to be stabilized by interdigitation of the ‘β-finger’ from each F1 inserting into an opening in the F2 domain of the opposing monomer. Amino acids from each F2 ‘β-finger’ insert into the opposing F1 domain. The interaction between the two monomers results in the formation of two channels through the dimer separated by a β-sheet. About two-thirds of the amino acids lining the channel belong to the F2 domain, explaining its partial ability to bind erythrocytes whereas the F1 cannot bind to all.
A model for erythrocyte binding by EBA175
The authors used α-2,3-sialyllactose to examine the receptor binding surfaces of EBA175 RII. This glycan contains the essential component of the EBA175 host cell target molecule, Neu5Ac(α2,3)-Gal of glycophorin A [6]. Six glycan binding sites were observed in the dimer form of EBA175. All six were located at the dimer interface, while four were found within the channels. This suggests that dimerization is important for binding of the receptor. This was confirmed by directed mutagenesis of residues involved in dimerization. Disruption of dimerization resulted in decreased erythrocyte binding ability. Disruption of glycan binding sites also reduced erythrocyte binding.
The host cell receptor for EBA175 is glycophorin A (GpA), which exists as a dimer on the erythrocyte surface. Given that both monomers of the EBA175RII dimer are required to form the glycan binding sites and that four of those glycan binding sites are accessible only from within the channels, the authors suggest two possible mechanisms by which EBA175 dimers may bind to GpA on the erythrocyte cell surface. The EBA175 RII monomers may assemble around the receptor to form a dimer. Alternatively, they suggest that the GpA monomers may wrap around the EBA175 dimer and feed the glycans into the two channels.
Insight into parasite evolution
Parasites of the genus Plasmodium are remarkably successful and have adapted to infect vertebrate hosts from reptiles to birds to humans, with more than twenty identified Plasmodium species infecting primates alone [7]. DBL-containing proteins play a critical role in erythrocyte invasion and/or cytoadherence in every Plasmodium species investigated so far, raising the possibility that the PfEBA175 Region II structure could be directly used to predict structures for related proteins. When the P. falciparum EBA175 RII is aligned with other ebl protein products, 34 residues are invariant. Almost all of these are important for maintenance of the DBL domain fold. However, most of these DBL domains are sufficiently divergent at the primary sequence level to make modeling based on the PfEBA175 structure inappropriate, but there is one clear exception. Multiple molecular phylogenies place Plasmodium reichenowi, a chimpanzee parasite, as the closest genetic relative of P. falciparum[8,9], and P. reichenowi contains a homologue of PfEBA175 that is 83% identical to PfEBA175 at the amino acid level [10]. Using the atomic coordinates of PfEBA175 we were able to build a model PrEBA175 structure by mutating individual residues at all sites that differ between these two proteins and modeling the resulting changes using energy minimization [11].
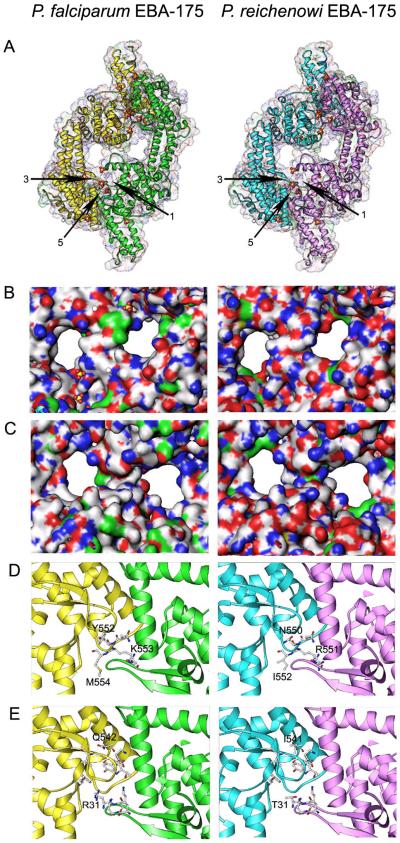
As expected, given their high sequence identity, the PfEBA175 and PrEBA175 structures are highly similar and are virtually coincident when overlayed (see Figure 1A)(some data not shown). Although the overall DBL structural features are conserved, close analysis reveals subtle but potentially significant differences in the putative receptor binding sites. The surface charge distribution in the two channels differs between PfEBA175 and PrEBA175 (see Figure 1a and 1c), presumably reflecting differences in the human and chimpanzee Glycophorin A molecules that are bound there. Glycophorins are evolving extremely rapidly across the primate lineage, and human and chimpanzee Glycophorin A differ at 11 of 64 extracellular residues [12]. Furthermore, the two structures differ at several key residues in glycan binding sites 3-6, residues which Tolia et al. determined make direct contact with the N-glycan and several of which had a marked impact on erythrocyte binding when mutated.
Figure 1. Comparison of PfEBA175 and a modeled PrEBA175 structure leads to insights into Plasmodium-primate specificity.
A PrEBA175 structure was modeled in silico using the atomic coordinates of PfEBA175 by mutating individual residues at all sites that differ between these two proteins and modeling the resulting changes using energy minimization [11]. All diagrams were created in the Ribbons program (available at www.sgce.cbse.uab.edu/ribbons).
a. Overall structures of the published PfEBA175 dimer [3] and the modeled PrEBA175 structure, showing their overall high identity. Glycan binding sites 1,3 and 5 are indicated. Because EBA175 is a dimer, glycan binding sites 1 and 2, 3 and 4, and 5 and 6 are identical; sites 2, 4 and 6 are present at the opposite end of the EBA175 dimer.
b. Close up view of the presumed Glycophorin A binding channels (view from top of images in Figure 1a), showing the surface charge distribution (Blue: basic; red: acidic; nonpolar/hydrophobic: white; polar: green). Note the charges lining the channel walls differ significantly between Pf and PrEBA175.
c. As in (b), but view from bottom of the images in Figure 1A.
d. Glycan binding pockets 3/4. All EBA175 residues that contact the glycans are shown as sticks on the ribbon backbone, with the amino acid identity of those that differ between Pf and PrEBA175 labeled. The numbering system differs between the two proteins because PrEBA175 Region II is shorter by two amino acids.
e. As in (d), but showing glycan binding pocket 5/6. Pocket 1/2 does not differ between Pf and PrEBA175.
Differences between PfEBA175 and PrEBA175 at glycan binding sites 3-6 are of particular interest because sialic acid recognition has recently been suggested to play a role in determining the host specificity of P. falciparum and P. reichenowi, which despite their close genetic similarity, are unable to efficiently infect their respective hosts. A major biochemical change in sialic acid biology has occurred in the human lineage after divergence from our common ancestor with chimpanzees [13], with the result that chimpanzee erythrocyte surface sialic acids are largely N-glycolylneuraminic acid (Neu5Gc), while human erythrocytes express solely its metabolic precursor, N-acetylneuraminic acid (Neu5Ac) [14]. In vitro binding studies have shown that PfEBA175 preferentially binds ‘human’ Neu5Ac, while PrEBA175 preferentially binds ‘chimpanzee’ Neu5Gc [15], mirroring and perhaps also explaining, at least in part, the differential host specificity of these two closely related parasites. The observed differences in the glycan binding pockets of PfEBA175 and PrEBA175 are certainly consistent with that theory. The structure of the dual DBL domains of PfEBA175 therefore has implications for the interactions between a range of Plasmodium parasites and their hosts. The structures of other DBL domain proteins will need to be solved before any clear statements about the universality of DBL domain folding can be made, but at least in the case of PrEBA175, publication of the structure of PfEBA175 alone will lead to new, directly testable models.
Acknowledgements
This work was supported in part by a grant from the National Institutes of Health R01 AI33656 to JHA and University of Notre Dame Schmitt Fellowship to AMM.
Contributor Information
D. Chattopadhyay, Division of Geographic Medicine, University of Alabama at Birmingham, Bevill Biomedical Research Building, 845 19th Street South, Birmingham, AL 35294-2170
J.C. Rayner, Division of Geographic Medicine, University of Alabama at Birmingham, Bevill Biomedical Research Building, 845 19th Street South, Birmingham, AL 35294-2170
A.M. McHenry, Department of Biological Sciences, PO Box 369, University of Notre Dame, Notre Dame, Indiana 46556-0369, USA
J. H. Adams, Department of Biological Sciences, PO Box 369, University of Notre Dame, Notre Dame, Indiana 46556-0369, USA
References
- 1.Chitnis CE, Blackman MJ. Host cell invasion by malaria parasites. Parasitol Today. 2000;16(10):411–415. doi: 10.1016/s0169-4758(00)01756-7. [DOI] [PubMed] [Google Scholar]
- 2.Adams JH, et al. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 2001;17(6):297–299. doi: 10.1016/s1471-4922(01)01948-1. [DOI] [PubMed] [Google Scholar]
- 3.Tolia NH, et al. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell. 2005;122(2):183–193. doi: 10.1016/j.cell.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 4.Michon P, et al. Evolutionary relationships of conserved cysteine-rich motifs in adhesive molecules of malaria parasites. Mol Biol Evol. 2002;19(7):1128–1142. doi: 10.1093/oxfordjournals.molbev.a004171. [DOI] [PubMed] [Google Scholar]
- 5.Pandey K, et al. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol Biochem Parasitol. 2002;123(1):23. doi: 10.1016/s0166-6851(02)00122-6. [DOI] [PubMed] [Google Scholar]
- 6.Orlandi PA, et al. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2-3)Gal-sequences of glycophorin A. J Cell Biol. 1992;116(4):901–909. doi: 10.1083/jcb.116.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coatney GR, et al. The Primate Malarias. U.S. Department of Health, Education and Welfare; 1971. [Google Scholar]
- 8.Waters AP, et al. Evolutionary Relatedness of Some Primate Models of Plasmodium. Mol Biol Evol. 1993;10(4):914–923. doi: 10.1093/oxfordjournals.molbev.a040038. [DOI] [PubMed] [Google Scholar]
- 9.Waters AP, et al. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc Natl Acad Sci U S A. 1991;88(8):3140–3144. doi: 10.1073/pnas.88.8.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozwara H, et al. Comparative analysis of Plasmodium reichenowi and P. falciparum erythrocyte-binding proteins reveals selection to maintain polymorphism in the erythrocyte-binding region of EBA-175. Mol Biochem Parasitol. 2001;116(1):81–84. doi: 10.1016/s0166-6851(01)00298-5. [DOI] [PubMed] [Google Scholar]
- 11.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 12.Baum J, et al. Natural selection on the erythrocyte surface. Mol Biol Evol. 2002;19(3):223–229. doi: 10.1093/oxfordjournals.molbev.a004075. [DOI] [PubMed] [Google Scholar]
- 13.Chou HH, et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci U S A. 2002;99(18):11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muchmore EA, et al. A structural difference between the cell surfaces of humans and the great apes. Am J Phys Anthropol. 1998;107(2):187–198. doi: 10.1002/(SICI)1096-8644(199810)107:2<187::AID-AJPA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Martin MJ, et al. Evolution of human-chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci U S A. 2005;102(36):12819–12824. doi: 10.1073/pnas.0503819102. [DOI] [PMC free article] [PubMed] [Google Scholar]