Abstract
Background
Live attenuated influenza vaccines (LAIV) are being developed and tested against a variety of influenza viruses with pandemic potential. We describe the results of an open label Phase I trial of a live attenuated H7N3 virus vaccine.
Methods and Findings
The H7N3 BC 2004/AA ca virus is a live attenuated, cold-adapted, temperature-sensitive influenza virus derived by reverse genetics from the wild-type low pathogenicity avian influenza virus A/chicken/British Columbia/CN-6/2004 (H7N3) and the A/AA/6/60 ca (H2N2) virus that is the Master Donor Virus of the live, intranasal seasonal influenza vaccine. We evaluated the safety, infectivity, and immunogenicity of two doses of 107.5 TCID50 of the vaccine administered by nasal spray 5 weeks apart to normal healthy seronegative adult volunteers in an inpatient isolation unit. The subjects were followed for 2 months after 1 dose of vaccine or for 4 weeks after the second dose.
Twenty-one subjects received the first dose of the vaccine, and 17 subjects received two doses. The vaccine was generally well tolerated. No serious adverse events occurred during the trial. The vaccine was highly restricted in replication: 6 (29%) subjects had virus recoverable by culture or by rRT-PCR after the first dose. Replication of vaccine virus was not detected following the second dose. Despite the restricted replication of the vaccine, 90% of the subjects developed an antibody response as measured by any assay: 62% by hemagglutination inhibition assay, 48% by microneutralization assay, 48% by ELISA for H7 HA-specific serum IgG or 71% by ELISA for H7 HA-specific serum IgA, after either one or two doses. Following the first dose, vaccine-specific IgG secreting cells as measured by ELISPOT increased from a mean of 0.1 to 41.6/106 PBMCs; vaccine specific IgA secreting cells increased from 2 to 16.4/106 PBMCs. The antibody secreting cell response after the second dose was less vigorous, which is consistent with the observed low replication of vaccine virus after the second dose and consequent lower antigenic stimulation.
Conclusion
The live attenuated H7N3 vaccine was generally well tolerated but was highly restricted in replication in healthy seronegative adults. Despite the restricted replication, the vaccine was immunogenic, with serum IgA being the most sensitive measure of immunogenicity. Further development of this vaccine is warranted. (ClinicalTrials.gov Identifier: NCT00516035)
Keywords: avian, influenza, H7N3 vaccine
INTRODUCTION
In addition to yearly outbreaks and epidemics, influenza A viruses cause periodic pandemics, in which viruses containing novel hemagglutinins (HAs) are introduced into susceptible human populations. In the last century, pandemics caused by H1N1, H2N2 and H3N2 influenza viruses each took a considerable toll on human life, with the greatest mortality associated with the 1918 H1N1 pandemic [1]. Novel HAs are introduced into the human population from the reservoir of avian influenza (AI) viruses in waterfowl and shorebirds. The increasing incidence of human infections with AI strains has made developing vaccines against AI viruses a global public health priority to prepare for the next pandemic.
Although considerable attention has been focused on influenza A H5N1 viruses, the identity of the next pandemic influenza virus cannot be predicted. Of the remaining AI virus subtypes, H7 viruses currently pose a significant threat. Like H5 AI viruses, H7 viruses transmitted from wild birds to poultry can acquire the ability to become highly pathogenic [2, 3].
H7 influenza viruses have been known to cause sporadic cases of human infection for many years. In 1959, fowl plague virus (H7 influenza) was isolated from the blood of an American man with an extensive travel history, although he remained seronegative [4, 5]. From that time until 2003, rare cases of H7 infection were reported [6–10]. Most of these individuals had conjunctivitis or were asymptomatic but had contact with H7-infected poultry or seals. In February 2003, a large H7N7 HPAI outbreak occurred in poultry in The Netherlands. Of the 4500 people exposed, 453 had conjunctivitis or influenza-like illness, and 89 had laboratory-confirmed H7N7 infection. Three culture-confirmed cases of secondary transmission were documented [11]. A veterinarian who initially developed conjunctivitis died from pneumonia and acute respiratory distress syndrome [2, 11]. H7 influenza virus was detected by rRT-PCR on bronchoscopy and virus was isolated from post-mortem lung samples [2]. Since 2003, there have been additional documented human cases of H7 influenza infection in Canada [12], the UK [13] and the US [14, 15], and serosurveillance studies have shown that people in frequent contact with birds often have serologic evidence of infection with H7 viruses [15].
In preparation for the next influenza pandemic, a number of strategies to develop potential pandemic vaccines are underway including live attenuated vaccines [3, 16]. Live attenuated influenza vaccines (LAIV) bearing the HA and NA of the viruses of interest and the remaining genes from the A/Ann Arbor/6/60 ca virus (AA ca) have several potential benefits as pandemic vaccines; the vaccines are based on licensed technology, can be produced at high yield, and elicit antibodies (systemic and mucosal) and cell-mediated immune responses. Seasonal LAIVs have been shown to be more efficacious than inactivated vaccines in seronegative (or immunologically naïve) individuals, especially children [17–19] and to afford greater protection against drifted strains [20, 21]. In seronegative young children LAIV is immunogenic [22] and efficacious even when a single dose is administered [23]. In addition, since LAIVs are given intranasally, they could be administered easily in a situation that required rapid mass vaccination.
Several reassortant or recombinant vaccines containing avian HA and NA genes and the AA ca internal protein genes have been developed to date, including candidate vaccines for H9N2, H5N1 and H7N3 viruses. These vaccines were shown to be attenuated and immunogenic in preclinical studies [24–26]. Clinical trials have been performed with a live attenuated H9N2 G9/AA ca reassortant vaccine virus[27], and with live attenuated H5N1 Viet Nam 2004/AA ca and Hong Kong 2003/AA ca vaccine viruses (Karron et al, manuscript in preparation) [28]. Here we report the Phase I evaluation of live attenuated H7N3 A/British Columbia/2004 ca vaccine (H7N3 BC 2004/AA ca), which represents the first clinical trial of a live attenuated H7 influenza vaccine.
SUBJECTS, MATERIALS AND METHODS
Vaccine virus
H7N3 BC 2004/AA ca is a live attenuated, cold-adapted, temperature-sensitive influenza virus with the HA and NA gene segments derived from wild-type LPAI virus A/chicken/British Columbia/CN-6/2004 (H7N3) and 6 internal protein gene segments from the AA ca (H2N2) Master Donor Virus of the seasonal intranasal LAIV [26, 29]. The parent H7N3 influenza virus is of the LP phenotype and does not contain the multi-basic cleavage site associated with HP in avian species, so modification of the HA cleavage site was not required. The vaccine was produced by plasmid-based reverse genetics as previously described and was found to be attenuated, immunogenic and efficacious in mice and ferrets [26].
Clinical trial material was manufactured at MedImmune (Santa Clara, CA). The vaccine virus was amplified in specific pathogen free eggs, filtered and stabilized with sucrose-phosphate buffer (SP) and filled into Accuspray™ Nasal Spray Systems (Becton-Dickinson, Franklin Lakes, NJ) at a volume of 0.5 mL per sprayer. Filled sprayers were stored frozen at −60 °C or below.
Study Population
This Phase 1 clinical trial was conducted at the Center for Immunization Research (CIR) isolation unit at the Johns Hopkins Bayview Medical Center. Twenty-one healthy adult subjects from the Baltimore metropolitan area were recruited and enrolled in the fall of 2007. The clinical protocol was reviewed and approved by the Western Institutional Review Board (WIRB). Informed, witnessed, written consent was obtained from each subject. Healthy adult men and non-pregnant women between the ages of 18 and 49 years of age were enrolled in the clinical trial if they met eligibility criteria and were willing to remain on the isolation unit for the duration of the inpatient portion of the trial. Screening and follow-up visits were conducted at the CIR outpatient clinic on the Johns Hopkins East Baltimore Campus.
Study design
This study was conducted between April 1st and December 20th when wild-type (wt) influenza would be unlikely to be circulating in the local community. To further reduce the risk of reassortment between a naturally occurring epidemic wt influenza virus and the vaccine virus, the presence of wt influenza viruses in the community was monitored between April 1st–May 1st and October 1st–December 20th by obtaining information regarding influenza virus detection from the Diagnostic Virology Laboratory and Department of Hospital Epidemiology and Infection Control at Johns Hopkins Hospital. Participants were not enrolled if there were >3 influenza cases documented in the week preceding the planned vaccination.
This study was conducted as an open-label Phase 1 inpatient trial with all subjects receiving vaccine. Subjects were screened to establish health status and, if eligible, were given one or two doses of vaccine as a nasal spray using the Accuspray™ device and were examined daily while on the inpatient unit by a health care provider (physician, nurse practitioner, or physician’s assistant).
Isolation Unit and Staffing
The CIR Isolation Unit is a non-smoking, dormitory-like setting designed to house up to 40 adult men and women. The isolation unit has self-contained ventilation and hot water systems as well as an externally vented HEPA filtered exhaust. It also has four bedrooms with separate bathroom and shower areas, a clinical specimen processing area, nursing station, laundry facility, kitchen area, computers, and two large lounges. Daily activities and catered meals were provided for the participants.
Registered nurses and medical technicians staffed the isolation unit for 24 hours each day for the duration of the inpatient portion of the trial. As a prerequisite for working on the inpatient portion of the study, each staff member was required to sign a written agreement intended to minimize the potential for transmission of vaccine virus from participants to staff members and from staff members to others in the community. Staff members were vaccinated for seasonal influenza within 6 months of the start of the study. The staff agreement included practicing respiratory precautions from the time vaccine was administered until discharge, not working in other inpatient care settings, disclosing personal febrile or respiratory illness and, in the event of such illness, agreeing to nasal swab sampling and to initiation of therapy with oseltamivir pending testing of the nasal swab specimen for the presence of vaccine virus by real-time reverse transcription polymerase chain reaction (rRT-PCR).
Study procedures
To establish the health status of potential participants, CIR staff elicited medical histories, including menstrual and contraceptive history and/or history of surgical sterility for female participants, and administered physical examinations. Potential participants were screened for tuberculosis (using PPD tests), for viral hepatitis, HIV infection, and antibody to H7N3 influenza A viruses using serologic assays. Blood specimens were obtained for hematology and serum biochemistries, and urinalyses were performed via dipstick. Potential participants who were female were tested for pregnancy using urine β-HCG assays and were counseled to avoid becoming pregnant during the study.
Participants were admitted to the isolation unit two days prior to vaccination for orientation and were monitored for signs or symptoms of acute illness that would preclude their participation in the study. Those who manifested any signs or symptoms of illness or who were uncomfortable with the isolation unit procedures were discharged prior to vaccination. Despite our best efforts, adventitious agents are occasionally introduced into the unit that may not be detected in the 2 days prior to immunization, and the dorm-like setting can facilitate transmission of respiratory viruses. Therefore, in addition to testing for vaccine virus, we test for adventitious viral agents when a subject develops symptoms of respiratory tract illness. Serum β-HCG testing was performed on female participants 2 days prior to vaccination and urine β-HCG testing was performed on the day of vaccination. A positive pregnancy test made the subject ineligible. Additional inclusion and exclusion criteria were re-reviewed prior to vaccination.
On study day 0, each healthy adult volunteer received a dose of 0.5 mL of vaccine delivered as a nasal spray (0.25 mL per nostril) using the Accuspray™ device. Volunteers were observed for 30 minutes post-vaccination and were asked to report any symptoms occurring during their inpatient stay to the nursing staff. While on the isolation unit, the staff was required to observe standard respiratory precautions and to wear gowns, gloves, and masks from just prior to vaccination until the day of discharge.
For the duration of the inpatient portion of the study, symptoms were recorded and reported to the study physician. In addition, an examination of the head, eyes, ears, nose, throat, heart and lungs was performed daily, and vital signs were obtained twice daily. Fever, rhinorrhea, pharyngitis, cough, headache, systemic illness (myalgia or chills), and lower respiratory tract illness were defined as previously described [30, 31]. Nasal washes were collected daily during the inpatient portion of the study and were tested for vaccine virus (see below). In the event of a respiratory or febrile illness, nasal wash specimens were also cultured for adventitious respiratory viruses, including wt influenza viruses, parainfluenza viruses types 1, 2, and 3, respiratory syncytial virus, and adenovirus. None of these viruses were detected in our subjects. rRT-PCR of nasal wash was done to detect enterovirus (which was not found) and rhinovirus in ill subjects (methods are described below). Blood was collected one day before and on day 7 after administration of vaccine for antibody secreting cell (ASC) measurement.
Discharge from the isolation unit was contingent upon absence of vaccine virus as detected by rRT-PCR from nasal washes obtained for 3 consecutive days prior to discharge day, starting with day 6. None of the participants was required to stay on the isolation unit longer than anticipated.
Participants were asked to return to the CIR clinic for an outpatient visit after discharge from the isolation unit. The visits were scheduled on study day 28 following each dose of vaccine (and day 61 after the first dose, if the subject only received one dose). At each visit, staff obtained vital signs, reviewed interim histories, and obtained blood and nasal wash samples for antibody testing. Subjects were readmitted on study day 33 for the second dose of vaccine, which they received on day 35 after the first dose.
Safety Assessments
In addition to the daily clinical assessments, subjects had blood drawn after vaccination to assess potential toxicity. Serum alanine aminotransferase (ALT) levels were tested prior to dosing (baseline), on day 3 and on day 7 after each vaccination and were followed to resolution if elevated. A grade 1 elevation in ALT was defined as 1.51 – 3 times the upper limit of normal (ULN) for our assay (40 for females, 60 for males); grade 2, 3.1–6 times ULN; grade 3, 6.1–10 times ULN, and Grade 4 >10 times ULN. A complete blood count was done at baseline and on day 7. A grade 1 decrease in absolute neutrophil count (ANC) was defined as 1000–1300 cells/μL; grade 2 ANC 750–999 cells; grade 3 ANC 500–749 cells and grade 4 ANC <500 cells/μL.
Isolation, quantitation and identification of the H7N3 virus
Nasal washes were obtained prior to vaccination and then daily from the day of vaccination until the day of discharge. Specimens were tested for the presence of vaccine virus by quantitative viral culture on Madin Darby Canine Kidney (MDCK) cells [30]. For the purposes of calculation, specimens that were culture negative were assigned a titer of 0.6 TCID50/mL. Specimens were also tested for influenza A viruses using an rRT-PCR assay that amplified a portion of the M2 gene. This assay was based upon a modification of previously published methods [32] in which we used the Nuclisens MiniMAG system (bioMerieux, Durham, NC) for RNA extraction. The starting volume of nasal wash tested was 1.0 mL. Using these methods, we found that the sensitivity of the rRT-PCR assay for vaccine virus was approximately 100.5 TCID50/mL of nasal wash.
Immunologic Assays
Receptor-destroying enzyme treated sera were tested for hemagglutination inhibiting (HI) antibodies to H7N3 as previously described [33] with the following modifications: a 1% suspension of horse red blood cells (RBC) was used, instead of turkey RBC, and 2 or 4 HA units (HAU) of H7N3 BC 2004/AA ca were used in separate assays [14, 34]. Antigen and sera were incubated together for 1 hour, and then with the RBC for 30–45 minutes. The sera were also tested for neutralizing antibodies using a modified version of a published microneutralization assay [35]. Briefly, our assay differed from the previously published microneutralization assay in the following respects: 1) MDCK cells were originally obtained from ECACC (European Collection of Cell Cultures); 2) our test virus was not wt H7N3, but rather the vaccine virus, H7N3 BC 2004/AA ca; 3) because the test virus is temperature sensitive, incubation was carried out at 32°C rather than at 37°C; 4) the number of cells used per well was 2 ×104, rather than 1.5 ×104.
Sera were also tested for IgA and IgG antibody to the H7 HA by ELISA. Nunc polysorb 96 well plates (Fisher Scientific Pittsburgh, PA) were coated with 30ng/well of recombinant H7 (rH7) HA protein from a heterologous A/Netherlands/219/03 (H7N7) virus that was expressed in a baculovirus vector in insect cells (Protein Sciences, Meriden, CT). The HA of the H7N3 BC 2004 and H7N7 Netherlands 2003 are 77% similar at the nucleotide level and 85% identical at the amino acid level. The ELISA was performed using endpoint titration in which the cutoff for positive was greater or equal to 0.2 OD [33]. Nasal wash specimens were concentrated 10-fold (using Aquacide (Calbiochem, San Diego, CA) and dialysis tubing (Fisher Scientific) to adsorb the water) and tested using the same H7 antigen to measure vaccine-specific IgA, expressed as a percent of total IgA, as previously described [30].
Total and influenza vaccine-specific IgG and IgA antibody secreting cells (ASC) were measured using an enzyme-linked immunospot (ELISPOT) assay based on an assay described by Sasaki et al. [36]. Briefly, our assay differed from the published assay in that the wells were coated with one of the following: 1) beta-propiolactone (BPL) treated H7N3 BC 2004/AA ca virus diluted to 5000 HAU/mL in Dulbecco’s phosphate-buffered saline (D-PBS, Invitrogen); 2) rH7 HA protein diluted to 10μg/mL in D-PBS; 3) BPL-treated cold-adapted A/Ann Arbor/6/60 (H2N2) virus diluted to 5000 HAU/mL; or 4) purified goat anti-human IgA + IgG + IgM (Kirkegaard & Perry Laboratories, Gaithersburg, MD) at a concentration of 5μg/mL in D-PBS. PBS alone and human CCRF-CEM cells (human T-lymphoblasts; ATCC, Manassas, VA) cells were used as negative controls; human IM9 cells (human IgG+ lymphoblasts; ATCC) were used as a positive control. Plate images were recorded and counted using ImmunoSpot 4 software (Cellular Technologies Ltd., Shaker Heights, OH). Human IgA ASC were visualized as red spots and IgG ASC were visualized as blue spots. The number of ASC was expressed per 106 total IgG or IgA ASC.
Rhinovirus rRT-PCR
As several of the subjects developed upper respiratory tract symptoms not associated with vaccine virus shedding, a TaqMan rRT-PCR assay was performed using primers and probe from the 5′-untranslated region of the human rhinovirus (hRV) genome and the universal one step RT-PCR master mix (Applied Biosystems) and run on an Applied Biosystems 7300 real-time PCR system. Viral RNA was extracted from 1000μl of nasal wash specimen using the Nuclisens MiniMAG system (bioMerieux) as described above. Appropriate positive (hRV grown in HeLa cells) and negative uninfected (LLC-5 cells) controls were run with each batch of clinical specimens. The forward primers (sequences 5′-3′: TGT GAA GAG CCC CGT GTG and TGT GAA GAC TCG CAT GTG) were used at a final concentration of 900 nM. The reverse primer, GTA GTC GGT CCC ATC CC was used at 200 nM and the probe, ’−6FAM-TCCTCCGGCCCCTGAATGCG-TMR at a final concentration of 0.2 μM [37].
Data Analysis
Infection with the H7N3 BC 2004/AA ca vaccine virus was defined as: 1) shedding of vaccine virus detected by culture and/or; 2) shedding of vaccine virus detected by rRT-PCR any time after study day 1 and/or; 3) a ≥ 4-fold rise in serum HI, neutralizing, or rH7-specific IgG or IgA serum antibodies as measured by ELISA. Subjects whose nasal washes were rRT-PCR positive on study day 1 but who were without other evidence of infection were not considered infected because we could not exclude the possibility that input virus, rather than replicating virus, was being detected. Log2-transformed reciprocal HI, neutralizing and ELISA antibody titers were used to calculate mean titers. Pearson’s correlations (coefficients adjusting for nonindependent data) were performed on the results of the immunological assays to determine the level of correlation of the titers.
RESULTS
Study subjects
Sixty-four potential subjects were screened for the H7N3 BC 2004/AA ca virus vaccine trial. Seven subjects were found to be ineligible prior to collection of serum for HI testing; the remaining 57 had sera obtained for measurement of HI antibody titers against the H7N3 virus, and all were seronegative. During the screening process, 29 of these subjects were either deemed ineligible or chose not to participate in the study. Informed consent was obtained from 28 subjects who were admitted to the isolation unit. Seven of the admitted subjects were discharged before vaccination for medical reasons. Twenty-one individuals were vaccinated in September 2007. The age of the subjects ranged from 19 to 49 years (mean age 32.3 years, SD 8.6); all were Black and 14 were male. Seventeen of the 21 subjects received a second dose of vaccine five weeks later: two were unable to return for personal reasons, and two were not revaccinated for reasons of social incompatibility with the cohort. Three of the 4 subjects who received a single dose of vaccine completed their day 28 and day 61 follow up visits, and one completed the study day 61 follow-up visit only.
Reactogenicity
The vaccine was generally well tolerated, although a number of minor illnesses were reported, particularly following the first dose. One volunteer was discharged prior to receiving a first dose of vaccine because he had rhinorrhea. Despite his being discharged, his illness appeared to have spread to others: 8 volunteers reported combinations of respiratory tract symptoms that included nasal congestion, rhinorrhea, conjunctival suffusion and sore or scratchy throat. One subject who complained of nasal congestion on day 3 after vaccination had vaccine virus detected by rRT-PCR on days 1 and 4. The remaining symptomatic volunteers did not have vaccine virus recovered by culture or rRT-PCR at the time of their symptoms, and cultures for adventitious viruses from these subjects were negative. However, rhinovirus was detected by rRT-PCR (performed after the completion of the study) in the prevaccination nasal wash from the individual who was discharged prior to vaccination, as well as in 2 asymptomatic volunteers who remained on the unit. Additionally, nasal wash specimens from 6 of the 7 volunteers who had respiratory symptoms were positive for rhinovirus by rRT-PCR during the time in which they were symptomatic. Thus, we believe that these illnesses likely resulted from rhinovirus infection. All but 1 of these individuals had mild symptoms, with minimal impact on daily activity-- one volunteer had more moderate symptoms of nasal congestion, rhinorrhea and myalgia that required symptomatic treatment and limited his activity somewhat (Table 1). This subject with rhinovirus and URI symptoms without apparent lower airway involvement also had transient tachypnea.
Table 1.
Safety Assessment and Virologic Response to 2 Doses of H7N3 BC 2004/AA ca vaccine
| Isolation of Vaccine in Nasal Wash |
Detection of Vaccine Virus by rRT-PCR in Nasal Wash |
SYMPTOMS REPORTED % with Indicated Illness c |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | No. of subjects |
% Shedding Virus |
Peak Titer (mean ± SD log10TCID50)a |
% rRT-PCR+ on Day 1b |
% rRT-PCR+ after Day 1 |
Duration of shedding (mean ± SD) days |
Fever | URT symptomsd |
LRI | Cough | Ha | My | Other | Lab abnormality |
| First Dose | 21 | 24 | 1.2 ± 0.6 | 81 | 19 | 3.0 ± 1.8 | 0 | 38 | 0 | 0 | 19 | 10 | 76e | 19f |
| Rhinovirus+ | 6 | 17 | 1.0 ± 0.0 | 67 | 0 | 1.0 ± 0.0 | 100 | 50 | 33 | 83 | 17 | |||
| Rhinovirus − | 15 | 27 | 1.3 ± 0.7 | 87 | 27 | 3.4 ± 1.4 | 13 | 7 | 0 | 73 | 20 | |||
| Second Dose | 17 | 0 | 20.6 ± 0.0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 12 | 0 | 35g | 12h |
Abbreviations used are as follows: TCID 50=50% tissue culture infectious dose, SD=standard deviation, URT=upper respiratory tract, LRI=lower respiratory tract illness, Ha=headache, My=myalgia. Transient abnormalities in blood pressure, pulse and respiratory rate are not included here.
Viral titers are expressed as log 10 TCID50/ml. Mean peak titers were calculated for infected subjects for the first dose. Peak titers of 0.6 TCID 50/ml were assigned to culture-negative samples.
13 subjects (62%) had virus recoverable by rRT-PCR on day 1 only.
All adverse events were grade 1 or mild except 1 subject who had nasal congestion, rhinorrhea and myalgias of moderate severity, and the leg abscess which required drainage and oral antibiotics.
Defined as nasal congestion, rhinorrhea, runny nose, transient tachypnea or throat discomfort (sore, itchy or scratchy throat).
4 subjects had nausea, 4 with lightheadedness, 3 with muscle pain, 2 subjects with tinea corporis, rash, elevated diastolic blood pressure, 1 subject had conjunctival suffusion (not conjunctivitis), tragal pain, loose stools, rectal pain, blood tinged sputum, tooth pain, gas, heartburn, tachycardia, cold sore, flushing, lip swelling, elevated systolic blood prssure, papule on neck, leg pustule and MRSA leg abscess.
One participant had a low ANC on day 7 of 1073 but when repeated on day 8, the value was 1896. 3 participants had grade 1 ALT elevations on day 7 that resolved.
2 subjects had heartburn and vaginal discharge, 1 subject had flushing, allergic rhinitis, pruritis, elevated diastolic blood pressure, lightheadedness and rash.
2 participants had grade 1 ALT elevations on day 7 that resolved.
Among the rhinovirus-shedding subjects (n=6), other illnesses reported following the first dose of vaccine include headache (n=3), lightheadedness (n=1) vomiting (n=1) and systemic illness manifesting as generalized myalgia (n=2), none of which coincided with vaccine virus recovery or detection. Among the rhinovirus-negative subjects (n=15), other illnesses reported following the first dose of vaccine include: headache (n=1), nausea (n=4), lightheadedness (n=3) (Table 1). This was not associated with vaccine virus shedding.
One subject expectorated blood tinged material shortly after a nasal wash; this was attributed to trauma from the nasal wash and did not occur again. Several other illness were reported that we felt were unlikely or not related to the vaccine, such as focal muscle pain which did not meet the case definition for myalgia, tinea corporis, rash, tooth pain, and a leg abscess (Table 1). Following the second dose of vaccine, 2 subjects reported headache, and one additional subject had nasal congestion and nasal discharge. Vaccine virus was not recovered from any of these individuals (Table 1).
Five instances of elevation in serum ALT were observed in 4 subjects: in 3 following the first dose of vaccine, and in 2 following the second dose. These were of grade 1 severity, resolved within 1 week, and were not associated with vaccine viral replication as detected by rRT-PCR or culture, although 4 of 5 of the instances were associated with serological responses to the vaccine (one of the subjects was positive for rhinovirus). One subject had a grade 1 neutropenia (ANC=1073) on day 7 following the first dose of vaccine that resolved by day 8 (ANC= 1896). This was also not temporally associated with viral replication; although the subject had vaccine virus detected by rRT-PCR on days 1 and 4 and had a serological response to the vaccine virus (Table 1).
Vaccine Virus Replication
The H7N3 BC 2004/AA ca vaccine virus was restricted in replication (Table 1). After the first dose, 5/21 (24%) subjects had vaccine virus recovered by culture from nasal wash: 1 subject on days 1 through 5, 1 on days 2 through 4, and 3 on day 1 only. Peak titers ranged from 100.75 to 102.25 TCID50/mL. When the nasal wash specimens were tested by rRT-PCR, vaccine virus was detected in 17 (81%) individuals following the first dose of vaccine: 2 individuals on days 1 through 5, 1 individual on days 1 and 4, 1 on days 1 and 2, and 13 subjects on day 1. All specimens that were culture-positive were also rRT-PCR positive. Vaccine virus was not recovered by culture or rRT-PCR from any nasal wash specimen following the second dose of vaccine (Table 1).
Immune Responses
HI assays were initially performed using 4 HAU and after two doses of vaccine, only 43% of the subjects had 4-fold or greater rise in serum antibody titer (Tables 2 and 3). Since a previous study showed that the sensitivity of the HI assay for H7 viruses could be improved using 2 HAU, the assay was repeated using this method [14]. When sera were tested using this modified HI assay, 3 of 21 (14%) subjects had a 4-fold or greater rise in HI titer after the first dose, and 7 of 17 (41%) subjects had a 4-fold or greater rise in HI titer after the second dose. Two additional subjects had a gradual rise, achieving a 4-fold increase in titer between the pre-vaccination specimen and the specimen obtained after the second dose. In addition, 1 of the 4 subjects who received only 1 dose (and who did not return for the day 28 visit) had a 4-fold rise in antibody detected at day 61 after vaccination. In total, 62% (13 of 21) of those who received any dose of the vaccine had a 4-fold or greater rise in HI serum antibody titers measured using 2 HAU of the vaccine virus (Table 2).
Table 2.
Immunological Response to 2 Doses of H7N3 BC 2004/AA ca vaccine
| Reciprocal mean ± SD log 2 antibody |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HI using 4 HAU and horse RBC |
HI using 2 HAU and horse RBC |
Serum Neut Ab Response |
Serum HA-IgG ELISAa |
Serum HA-IgA ELISAa |
Nasal Wash HA-IgA ELISAa |
||||||||||||||
| Dose | Number of vaccinees |
Pre | Postb | % with 34-fold rise |
Pre | Post | % with 34-fold rise |
Pre | Post | % with 34-fold rise |
Pre | Post | % with 34-fold rise |
Pre | Post | % with 34-fold rise |
Pre | Post | % with 34-fold rise |
| First Dose | 21c | 1.0 ± 0.0 | 1.3 ± 0.8 | 10 | 1.1 ± 0.4 | 1.9 ± 1.3 | 14 | 2.5 ± 0.4 | 3.0 ± 0.8 | 10 | 6.7 ± 1.6 | 7.3(1.6) | 29 | 7.6 ± 2.1 | 8.8 ± 1.9 | 52 | 6.9 ± 1.7 | 7.8 ± 1.5 | 24 |
| Second Dose | 17 | 1.3 ± 0.8 | 2.2 ± 1.5 | 29 | 1.9 ± 1.3 | 3.0 ± 1.3 | 41 | 3.0 ± 0.9 | 4.0 ± 1.8 | 41 | 6.9 ± 1.4 | 7.6(1.4) | 24 | 8.4 ± 1.4 | 9.0 ± 1.5 | 29 | 7.7 ± 1.4 | 8.2 ± 2.3 | 12 |
| Any dose | 21 | 1.0 ± 0.0 | 2.2 ± 1.4 | 43d | 1.1 ± 0.4 | 3.0 ± 1.2 | 62e,f | 2.5 ± 0.4 | 4.0 ± 1.7 | 48d | 6.7 ± 1.6 | 7.7(1.5) | 48e | 7.6 ± 2.1 | 9.2 ± 1.9 | 71 | 6.9 ± 1.7 | 8.2 ± 2.1 | 33g |
Abbreviations used are as follows: SD= standard deviation, HI= hemagglutinin Inhibition assay, RBC=r ed blood cell, Neut= neutralizing, Ab= antibody, HA= hemagglutinin, HAU=hemagglutinin units, ELISA= Enzyme Linked Immunosorbent Assay, No.= Number.
ELISA performed utilizing recombinant H7 HA protein from a heterologous A/Netherlands/219/03 (H7N7) virus expressed in a baculovirus vector.
Post values drawn on day 28 for dose 1, day 27 for dose 2.
Only 20 subjects had results available after dose 1; 1 subject did not return for the day 28 visit.
2 individuals (11%) who did not receive dose 2 were found to have a >4 fold rise in titers at day 61.
1 person (5%) who did not have blood drawn at day 28 and who did not receive dose 2 was found to have a 4 fold rise in titers on day 61.
2 individuals had a gradual rise in titer between dose 1 and dose 2.
1 individual had a gradual rise in titer between dose 1 and dose 2.
Table 3.
Summary of Immunological Responses to any Dose of H7N3 BC 2004/AA ca vaccine
| Results of antibody and ELISPOT assays | |||||||
|---|---|---|---|---|---|---|---|
| Subject No. | HI using | Neut Ab | serum IgGc | serum IgAc | NW IgAc | IgG ASCc,d | |
| 4 HAU antigen | 2 HAU antigen | ||||||
| 1 | − | − | − | + | + | + | + |
| 2b | − | − | + | − | − | − | − |
| 4*,a | + | + | + | + | + | − | + |
| 5 | − | − | − | − | − | − | − |
| 6b | + | + | − | + | − | − | + |
| 7 | − | + | + | − | + | − | − |
| 8* | − | − | − | + | + | + | − |
| 9 | + | + | − | − | − | − | + |
| 10 | + | + | − | + | + | − | N/A |
| 11b | + | + | + | + | + | + | + |
| 12 | − | − | + | + | + | − | − |
| 13 | − | + | + | − | + | − | − |
| 14 | + | + | + | − | + | − | − |
| 15* | + | + | + | − | − | + | + |
| 16 | − | − | − | − | + | + | + |
| 17*,b | − | − | − | − | − | − | + |
| 18* | − | + | − | − | + | + | + |
| 19 | + | + | + | + | + | + | + |
| 20 | + | + | − | + | + | − | + |
| 21 | − | − | − | + | + | − | − |
| 22* | − | + | + | − | + | − | + |
| total (%) | 9/21 (43%) | 13/21 (62%) | 10/21 (48%) | 10/21 (48%) | 15/21 (71%) | 7/21 (33%) | 12/21 (57%) |
Abbreviations used are as follows: No.= number, HAU=hemagglutin unit, HI= Hemagglutination inhibition assay, Neut= microneutralization assay, Ab=Antibody NW= Nasal wash, ASC= Antibody Secreting Cell, N/A=Not Available.
represents < a 4 fold increase in antibody titer or an ASC response.
represents a 4 fold or greater increase in antibody titer or an ASC response.
these subjects were positive by rRT-PCR for rhinovirus.
Subject 3 developed URI symptoms prior to vaccination and was discharged from the study without receiving the vaccine.
These subjects received only 1 dose of vaccine.
ELISA and ELISPOT assays performed utilizing recombinant H7 HA protein from a heterologous A/Netherlands/219/03 (H7N7) virus expressed in a baculovirus vector.
subjects who had a >5 cell increase in the number of vaccine-specific IgG secreting cells/106 PBMCs after the first vaccination.
Overall, 19 subjects (90%) had a 4 fold or greater increase in antibody titers by either HI, Neut assay or ELISA to the H7N3 BC 2004/AA ca.
Serum neutralizing antibodies to the H7N3 BC 2004/AA ca vaccine virus were also assessed. Four-fold or greater rises in antibody titer were observed in 2 (10%) volunteers following the first dose of vaccine and in 7 (41%) following the second dose of vaccine (including 1 who also had a response to the first dose). Again, 2 of 4 subjects who received only 1 dose had a response detected at day 61 after vaccination (Table 2). In all, 10 of 21 (48%) had a 4-fold or greater rise in neutralizing antibody titer following any dose of vaccine (Table 2).
Serum IgG and IgA and nasal wash IgA antibodies to a rH7 HA protein were measured by ELISA. Four-fold or greater rises in serum IgG titer were observed in 6 (29%) volunteers after the first dose and 4 (24%) volunteers after the second dose of vaccine (including 1 person who responded to both dose 1 and 2 with a 4-fold rise in titer). One subject who only received 1 dose of the vaccine had a 4-fold rise in titer by day 61. In all, 10 of 21 (48%) of the volunteers had an antibody response after any dose of vaccine. Surprisingly, serum IgA appeared to be the most sensitive measure of immune response to this vaccine virus: 11 (52%) volunteers responded after the first dose and an additional 5 (29%) volunteers (1 of whom also responded to the first dose) had a 4-fold or greater increase in titer after the second dose. In all, serum IgA responses were observed in 15 of 21 (71%) individuals. Conversely, the nasal wash IgA was the least sensitive measure of an immune response: overall, only 7 subjects (33%) had a 4-fold or greater rise in nasal wash IgA titers after any dose (Table 2). As a measure of specificity of the IgA assay, the sera from the subjects were analyzed for serum IgA for H1 HA; only 1/15 subjects who had a 4-fold or greater rise in titer to the H7 HA also had a 4-fold increase in IgA titer.
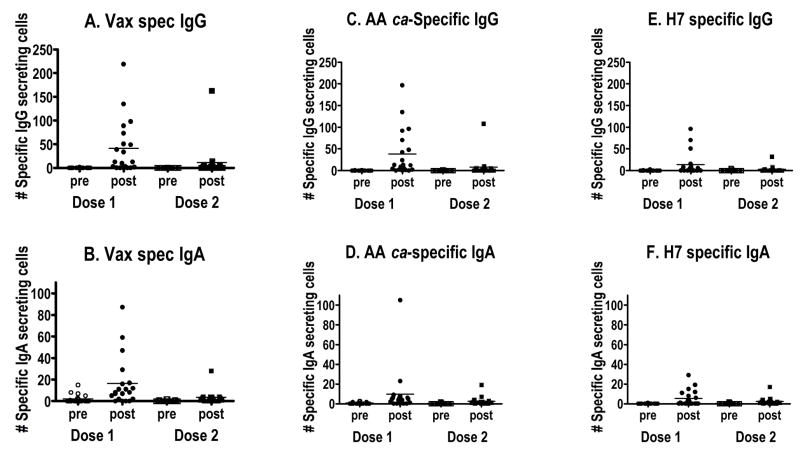
The development of ASC responding to vaccine virus (vaccine-specific), responding to the internal protein genes of AA ca (AA ca-specific), and to the HA alone (rH7 HA-specific) is shown in Figure 1. When vaccine-specific ASC were measured prior to immunization, 1 subject had vaccine-specific IgG secreting cells at a level of 2/106 PBMCs, and vaccine-specific ASC were not detected in any of the other subjects. Seven days after the first vaccination, the mean (SD) number of vaccine-specific IgG ASC was 41.6(57.4)/106 PBMCs, and 12 of 20 (60%) subjects had an increase of vaccine-specific IgG ASC of >5/106 PBMCs. By the time of the second dose, vaccine-specific IgG ASC were nearly undetectable. Although ASC were detected in a small number of subjects after the second dose, the mean [SD] number of vaccine specific IgG ASC was less than those detected after the first dose (11.5 [39.2], Figure 1, panel A). This general pattern of a response to the first dose of vaccine, followed by a return to near baseline levels prior to the second dose of vaccine, and a less vigorous response to the second dose of vaccine was also observed when vaccine-specific IgA, AA ca-specific IgG and IgA, and rH7-specific IgG and IgA ASCs were measured. Vaccine-specific IgA responses were of lower magnitude than vaccine-specific IgG responses: the mean numbers (SD) of IgA ASCs/106 PBMCs were 2.0 (3.9) prior to the first dose, 16.4 (22.8) after the first dose, 0.4 (0.7) before the second dose, and 3.5 (6.5) after the second dose (Figure 1, panel B). ASC responses to the AA ca virus were similar to the vaccine-specific responses (Figure 1, panels C and D), suggesting that the ASC assay detected antibodies to internal proteins as well as the H7 and N3 glycoproteins. ASC responses to rH7 HA were less than those to the vaccine or the AA ca (which may reflect the heterologous source of the H7 HA): following dose 1, H7 HA-specific IgG increased from 0.2 (0.7) to 13.6 (26.7) ASC/106 PBMCs and IgA from 0.0 (0.2) to 5.4 (8.1) ASC/106 PBMCs (Figure 1, panels E and F).
Figure 1.
ELISPOT data showing vaccine specific IgG (A) and IgA (B) secreting cells in blood. C = IgG and D = IgA secreting cells using the AA ca virus as the test antigen. E = rH7 HA specific IgG ASC; F = rH7 specific IgA ASC. All numbers expressed per 106 PBMCs. Vax = Vaccine; pre = before each dose was given; post = day 7 after each dose was given.
A comparison of the various immunological assays performed in each individual subject is summarized in Table 3. These results demonstrate the improvement in sensitivity when 2 HAU rather than 4 HAU of virus were used in the HI assay and the sensitivity of the serum IgA assay. The individuals who shed rhinovirus by rRT-PCR after dose 1 are identified with an asterisk. There was no apparent inhibition of the immune response to the H7N3 vaccine virus in individuals infected with rhinovirus. A Pearson’s correlation was performed on the results of the immunological assays. A statistically significant correlation was not found between the assays. However, the serum IgG and IgA correlated with each other (correlation coefficient ρ=0.52 95% CI 0.11–0.78; p= 0.017) and the serum and nasal IgA results correlated with each other (ρ=0.46 CI 0.03–0.74; p=0.036).
DISCUSSION
We have reported the first human trial of a live attenuated H7 influenza A virus vaccine. The live attenuated, cold-adapted, temperature sensitive vaccine, H7N3 BC 2004/AA ca elicited adverse events that were generally mild, was restricted in replication and immunogenic in seronegative healthy adult volunteers.
Several other H7 vaccines have been developed and evaluated in pre-clinical studies [38–41] and several H7 vaccines have also been developed for use in chickens [42–45]. All of these are inactivated whole or split virus vaccines that contain the HA and NA from avian H7 viruses and the internal protein genes from PR8 vaccine donor virus. To date, 2 H7N7 vaccines, an H7N1 vaccine, and an H7N2 vaccine have been studied, including both non-adjuvanted vaccine preparations and preparations containing alum or immune stimulatory complexes (ISCOMs). In animal studies, these vaccines are of variable immunogenicity, yet generally protect against challenge with either homologous or heterologous H7 viruses [38–41]. Several inactivated H7 virus vaccines are currently being evaluated in Phase I clinical trials.
The H7N3 BC 2004/AA ca vaccine was generally well tolerated by our subjects. The majority of symptoms were unlikely to be related to the vaccine: 6 of the 8 subjects who had upper respiratory tract symptoms after the first dose had rhinovirus detected by rRT-PCR, but only 1 of the 8 was shedding vaccine virus at the time. The laboratory abnormalities are also unlikely to be related to the vaccine. One subject had an episode of transient neutropenia during the study that resolved by the next day. He had a history of lower neutrophil counts, both before and long after vaccination, and likely has benign ethnic neutropenia, which is common and well documented in those of African or Mediterranean descent [46, 47]. Four subjects had 5 episodes of elevated ALT during the study. Only 2 of these had vaccine virus detected by rRT-PCR after dose 1. This elevation of serum transaminases is not likely to be due to vaccine virus, as this was not a phenomenon seen with wild-type H7 influenza infection. Rather, this is most likely due to alterations in diet and activity commonly seen in Phase I inpatient studies [48, 49].
Similar to the other avian LAIVs tested, including H9N2 G9/AA ca[27], H5N1 VN 2004/AA ca, and H5N1 HK 2003/AA ca (Karron et al., manuscript submitted for publication), the H7N3 BC 2004/AA ca vaccine influenza A virus was more restricted in replication than the H1N1/AA ca and H3N2/AA ca influenza A viruses contained in the intranasal seasonal LAIV in seronegative subjects. The reason for this higher restriction is not completely understood, but may be related to decreased affinity of the avian HAs for receptors in the upper airways of humans. Human influenza virus HAs preferentially bind to receptors terminating in N-acetylneuraminic acid α2,6 galactose (α2,6Gal), which are abundant on non-ciliated epithelial cells in the upper and lower human respiratory tract. Many avian influenza viruses, on the other hand, preferentially bind to receptors terminating in α2,3Gal [34], which are found in type II pneumocytes in humans but in limited numbers of epithelial cells in the upper respiratory tract [50, 51]. However, this interpretation is offered with the caveat that human parainfluenza viruses that bind α2,3Gal replicate to high titer in the upper respiratory tract of humans [52–54]. Alternatively, the constellation of the avian influenza HA and NA genes combined with internal protein gene segments from a human influenza virus may have resulted in a vaccine with a host-range restricted phenotype for humans. It is possible that an avian virus that emerges as a pandemic virus (i.e., acquires genetic mutations that allow it to transmit readily among the human population) will have HA or NA alterations that control infectivity and spread in humans. Such HA or NA genes, when incorporated into LAIV may be more infectious and immunogenic in humans than the LAIV vaccines containing the avian HA and NA genes of the present report.
Despite this restriction, the H7N3 vaccine was detected more frequently by viral culture than either the H9N2 or H5N1 LAIVs. This may be because contemporary North American H7 HAs, such as the one included in this vaccine, possess an increased affinity for α2,6Gal receptors when compared to Eurasian H7 HAs [55]. Since H9N2 G9 HK 1997 influenza A viruses can also bind to α2,6Gal receptors, this cannot be the only explanation for the observed differences in replication between vaccine strains; rather, it may be a contributing factor. In addition, the H7N3 vaccine has an NA antigen that is not contained in circulating human strains of influenza so individuals are unlikely to have preexisting immunity to the NA that could limit viral replication.
A surprising finding in our study was the difference in the rate of vaccine virus detection by rRT-PCR following the first and second dose of vaccine. Seventeen of 21 subjects had virus detected by rRT-PCR on day 1 after the first vaccination, 13 of whom did not have any virus detected on subsequent days. In contrast, none of the subjects had vaccine virus detected by rRT-PCR following the second dose of vaccine. In previous studies, the detection of vaccine virus on day 1 only was attributed to detection of input virus[27]. However, the findings from this study suggest that low-level replication of vaccine virus occurs after the first dose of vaccine, but that replication following the second dose is inhibited, perhaps by the adaptive immune response. The pattern of ASC responses (discussed below) supports this hypothesis. While we chose not to include individuals with nasal wash specimens that were positive by rRT-PCR on day 1 only in our calculations of percentages of infected vaccinees, these data suggest that perhaps the H7N3 BC 2004/AA ca influenza A virus was more infectious than we have estimated.
Although the co-circulating rhinovirus had the potential to confound our data, we do not believe that it affected either vaccine virus replication or the immune response to the vaccine. Of those who shed rhinovirus, 4/6 (67%) were positive for vaccine virus by rRT-PCR on day 1. One of these subjects (17%) also had vaccine virus detectable by culture. Of those who were rhinovirus negative, 4/15 (27%) had vaccine virus detectable by culture, and 13/15 (87%) had positive rRT-PCR for vaccine virus (Table 1). Those who shed rhinovirus were neither more nor less likely to have a serological response to the vaccine (Table 3).
One of the difficulties in evaluating potential vaccines for avian influenza lies in determining the correlates of vaccine-induced immunity [3]. For inactivated seasonal influenza vaccines, the correlate of protection accepted by regulatory agencies has been defined as an HI titer of ≧1:40, measured in a standard assay using 4U of HA [56, 57]. However, seasonal LAIV has been shown to protect against natural infection or wt challenge even when HI titers ≧1:40 were not achieved, presumably through the induction of local mucosal antibody and cellular immunity [21, 58]. Moreover, this may not be the best correlate of protection against infection with H5 and H7 avian influenza viruses, since individuals infected with these viruses often fail to develop HI responses of this magnitude [14, 35]. For this reason, alternative assays, such as the microneutralization assay, ELISA [35] and, in the case of H7, a modified version of the HI assay using horse RBC and 2U of HA have been developed [14].
To assess the antibody response to the H7N3 BC 2004/AA ca vaccine, we used several assays in addition to the standard HI and microneutralization assays, in order to further understanding of the immune response to this novel antigen. We tested sera in an HI assay using 2 HAU of antigen and horse erythrocytes, since a reanalysis of serum from the H7N7 virus outbreak in The Netherlands showed that this was the most sensitive and specific method for detecting infection. In that study, the modified HI assay using 2 HAU was positive in 85% of patients with culture-proven H7N7 infection. However, most individuals had only modest increases in HI titer, such that only 6% had titers of 1:40 [14]. Using 2 HAU of antigen, we found that 62% of volunteers had a 4-fold or greater rise in HI titers after either 1 or both doses of vaccine. The mean post-vaccination titer at 2 months was 1:8 in all subjects and 1:14 in the vaccinees who had a 4-fold or greater rise in titer after any dose (Table 1). Titers of 1:32 were achieved in 14% of vaccinees, comparable to what was observed following natural infection.
We also measured neutralizing antibody, H7-specific IgG and IgA ELISA antibody[59], and production of ASCs against the vaccine virus, AA ca, and rH7 HA antigen. Interestingly, the most sensitive measure of response was the serum IgA assay, despite the fact that a heterologous H7 was used (derived from the Eurasian H7N7 A/Netherlands/219/03 virus). The Archetti-Horsfall value of antigenic relatedness for these two HA antigens, tested with post-infection mouse sera, is 33%, which represents a 4 to 8-fold difference in cross-neutralizing titer [60]. Eleven of 21 volunteers had a 4-fold or greater rise in serum IgA titer after the first dose, and 5 of 17 responded after the second dose. Serum IgA has been used as a marker for response to parainfluenza and respiratory syncytial virus vaccines in infants who may have circulating maternal IgG [61, 62]. The biological significance of this response remains to be determined. Serum IgA may be a surrogate marker for a mucosal immune response that is triggered by the intranasal vaccine. Clements and Murphy found that there was a significant association between serum and nasal wash IgA and IgG after either live or inactivated H1N1 or H3N2 influenza vaccine [63]. Although we also measured nasal wash H7-specific IgA, our assay may not be sensitive enough to detect a mucosal response in most vaccinees.
Measurement of vaccine-specific ASC showed a greater response to the first dose than to the second dose of vaccine (Figure 1). As discussed above, this might reflect the decreased infectivity of the second dose of vaccine. The magnitude of the vaccine-specific IgG ASC response observed in H7-naïve individuals after the first dose of vaccine was comparable to that previously observed in older children administered the intranasal seasonal LAIV [36]. Although serum IgA was the most sensitive measure of antibody response, this was not reflected in the IgA ASC data. This may be due to timing: the ASC were measured on day 7 based on work by Sasaki et al [36], and the ELISA for IgA was performed on day 28 sera. The kinetics of the ASC response to AI viruses may be different than to the human influenza viruses.
In our study, the immunological response to the vaccine in a few individuals increased gradually, such that 2-fold rises in titer were observed following each dose of vaccine. Moreover, delayed rises in HI and/or neutralizing antibody titers were observed in 3 of 4 individuals who received only 1 dose of vaccine, with higher titers achieved at the 2 month than at the 1 month time-point. These data, combined with the rRT-PCR data discussed above, suggest that although the best responses occurred in those who received two doses, perhaps a single dose of vaccine would be sufficient to induce an immune response, which would be ideal in the event of a pandemic. This is supported by preclinical studies of this vaccine, in which mice that received only 1 dose of the H7N3 BC 2004/AA ca vaccine had increasing neutralizing antibody titers between days 28 and 56, rising to nearly the same level as those that had received 2 doses [26]. The immune response of humans to a single dose of the H7N3 BC 2004/AA ca vaccine should be explored further.
In summary, we have shown that 2 doses of a live attenuated cold adapted H7N3 BC 2004/AA ca vaccine are generally well tolerated and immunogenic when given to healthy adults. Replication of the vaccine virus was restricted, especially after the second dose. The detection of vaccine virus by rRT-PCR on day 1 after the first vaccination may reflect a low level of replication not detected by culture. We found that serum IgA antibody to rH7 HA antigen was the most sensitive measure of the immune response to this vaccine. Our data suggest that H7N3 BC 2004/AA ca should be investigated further and that it might be useful to assess the immunogenicity of a single dose of this vaccine.
Acknowledgments
We wish to thank Julie McArthur, Philana Liang, Bridget McMahon, Jasmine Jennings, Ruval Comendador and Paula Williams-Soro for expert clinical and administrative assistance, Bhagvanji Thumar for expert technical assistance, Wilbert van Panhuis for statistical analysis and Romeo Paredes for expert data management. We would also like to thank Edith Matsuyama, Silas Nwagwu, Steve Thompson, Weidong Cui, Chin-Fen Yang, Bin Lu, Lynne Fitch, Alfred Pan, and Meredith Uebersax of Medimmune for vaccine manufacture and testing. This research was supported by the Intramural Research Program of the NIAID, NIH. This research was performed as a Cooperative Research and Development Agreement (CRADA No: AI-0155) between the Laboratory of Infectious Diseases, NIAID and MedImmune.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003 Nov 22;362(9397):1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004 Feb 3;101(5):1356–61. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol. 2007 Apr;7(4):267–78. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLay PD, Casey HL, Tubiash HS. Comparative study of fowl plague virus and a virus isolated from man. Public Hlth Rep. 1967 Jul;82(7):615–20. [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell CH, Webster RG, Breese SS., Jr Fowl plague virus from man. J Infect Dis. 1970 Dec;122(6):513–6. doi: 10.1093/infdis/122.6.513. [DOI] [PubMed] [Google Scholar]
- 6.Taylor HR, Turner AJ. A case report of fowl plague keratoconjunctivitis. Br J Ophthalmol. 1977 Feb;61(2):86–8. doi: 10.1136/bjo.61.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster RG, Geraci J, Petursson G, Skirnisson K. Conjunctivitis in human beings caused by influenza A virus of seals. N Engl J Med. 1981 Apr 9;304(15):911. doi: 10.1056/NEJM198104093041515. [DOI] [PubMed] [Google Scholar]
- 8.Shortridge KF. Pandemic influenza: a zoonosis? Semin Respir Infect. 1992 Mar;7(1):11–25. [PubMed] [Google Scholar]
- 9.Kurtz J, Manvell RJ, Banks J. Avian influenza virus isolated from a woman with conjunctivitis. Lancet. 1996 Sep 28;348(9031):901–2. doi: 10.1016/S0140-6736(05)64783-6. [DOI] [PubMed] [Google Scholar]
- 10.Puzelli S, Di Trani L, Fabiani C, Campitelli L, De Marco MA, Capua I, et al. Serological analysis of serum samples from humans exposed to avian H7 influenza viruses in Italy between 1999 and 2003. J Infect Dis. 2005 Oct 15;192(8):1318–22. doi: 10.1086/444390. [DOI] [PubMed] [Google Scholar]
- 11.Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004 Feb 21;363(9409):587–93. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 12.Tweed SA, Skowronski DM, David ST, Larder A, Petric M, Lees W, et al. Human illness from avian influenza H7N3, British Columbia. Emerg Infect Dis. 2004 Dec;10(12):2196–9. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen-Van-Tam JS, Nair P, Acheson P, Baker A, Barker M, Bracebridge S, et al. Outbreak of low pathogenicity H7N3 avian influenza in UK, including associated case of human conjunctivitis. Euro Surveill. 2006;11(5):E060504 2. doi: 10.2807/esw.11.18.02952-en. [DOI] [PubMed] [Google Scholar]
- 14.Meijer A, Bosman A, van de Kamp EE, Wilbrink B, van Beest Holle Mdu R, Koopmans M. Measurement of antibodies to avian influenza virus A(H7N7) in humans by hemagglutination inhibition test. J Virol Methods. 2006 Mar;132(1–2):113–20. doi: 10.1016/j.jviromet.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Myers KP, Setterquist SF, Capuano AW, Gray GC. Infection due to 3 avian influenza subtypes in United States veterinarians. Clin Infect Dis. 2007 Jul 1;45(1):4–9. doi: 10.1086/518579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luke CJ, Subbarao K. Vaccines for pandemic influenza. Emerg Infect Dis. 2006 Jan;12(1):66–72. doi: 10.3201/eid1201.051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007 Feb 15;356(7):685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 18.Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006 Oct;25(10):860–9. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 19.Ashkenazi S, Vertruyen A, Aristegui J, Esposito S, McKeith DD, Klemola T, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006 Oct;25(10):870–9. doi: 10.1097/01.inf.0000237829.66310.85. [DOI] [PubMed] [Google Scholar]
- 20.Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999 Jul 14;282(2):137–44. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 21.Belshe R, Lee MS, Walker RE, Stoddard J, Mendelman PM. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev Vaccines. 2004 Dec;3(6):643–54. doi: 10.1586/14760584.3.6.643. [DOI] [PubMed] [Google Scholar]
- 22.Mendelman PM, Rappaport R, Cho I, Block S, Gruber W, August M, et al. Live attenuated influenza vaccine induces cross-reactive antibody responses in children against an A/Fujian/411/2002-like H3N2 antigenic variant strain. Pediatr Infect Dis J. 2004 Nov;23(11):1053–5. doi: 10.1097/01.inf.0000143643.44463.b1. [DOI] [PubMed] [Google Scholar]
- 23.Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998 May 14;338(20):1405–12. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Matsuoka Y, Swayne D, Chen Q, Cox NJ, Murphy BR, et al. Generation and characterization of a cold-adapted influenza A H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine. 2003 Oct 1;21(27–30):4430–6. doi: 10.1016/s0264-410x(03)00430-4. [DOI] [PubMed] [Google Scholar]
- 25.Suguitan AL, Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006 Sep;3(9):e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph T, McAuliffe J, Lu B, Vogel L, Swayne D, Jin H, et al. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology. 2008 Jun 26; doi: 10.1016/j.virol.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karron RA, Callahan K, Luke C, Thumar B, McAuliffe J, Schappell E, et al. A Live Attenuated H9N2 Influenza Vaccine Is Well Tolerated and Immunogenic in Healthy Adults. J Infect Dis. 2009 Mar 1;199(5):711–6. doi: 10.1086/596558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Tables on the Clinical trials of pandemic influenza prototype vaccines. 2008 3/07/2008 [cited; Available from: http://www.who.int/vaccine_research/diseases/influenza/flu_trials_tables/en/index.html.
- 29.Maassab HF. Adaptation and growth characteristics of influenza virus at 25 degrees c. Nature. 1967 Feb 11;213(5076):612–4. doi: 10.1038/213612a0. [DOI] [PubMed] [Google Scholar]
- 30.Clements ML, O'Donnel S, Levine MM, Chanock RM, Murphy BR. Dose response of A/Alaska/6/77 (H3N2) cold adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983;40:1044. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karron RA, Wright PF, Crowe JE, Jr, Clements ML, Thompson J, Makhene M, et al. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants and children. J Infect Dis. 1997;176:1428–36. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 32.Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen, Hadisoedarsuno W, Purba W, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006 Nov 23;355(21):2186–94. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
- 33.Murphy BR, Tierney EL, Barbour BA, Yolken RH, Alling DW, Holley HP, Jr, et al. Use of the enzyme-linked immunosorbent assay to detect serum antibody responses of volunteers who received attenuated influenza A virus vaccines. Infect Immun. 1980 Aug;29(2):342–7. doi: 10.1128/iai.29.2.342-347.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephenson I, Wood JM, Nicholson KG, Zambon MC. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J Med Virol. 2003 Jul;70(3):391–8. doi: 10.1002/jmv.10408. [DOI] [PubMed] [Google Scholar]
- 35.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999 Apr;37(4):937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007 Jan;81(1):215–28. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkan M, Joshi SB, Joshi YB, Hodinka RL. Real-time PCR for detection of human rhinoviruses in children. 23rd Annual Clinical Virology Symposium and Annual Meeting of Pan American Society for Clinical Virology; Clearwater, FL.. 2007. p. TP57. [Google Scholar]
- 38.Jadhao SJ, Achenbach J, Swayne DE, Donis R, Cox N, Matsuoka Y. Development of Eurasian H7N7/PR8 high growth reassortant virus for clinical evaluation as an inactivated pandemic influenza vaccine. Vaccine. 2008 Mar 25;26(14):1742–50. doi: 10.1016/j.vaccine.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Pappas C, Matsuoka Y, Swayne DE, Donis RO. Development and evaluation of an Influenza virus subtype H7N2 vaccine candidate for pandemic preparedness. Clin Vaccine Immunol. 2007 Nov;14(11):1425–32. doi: 10.1128/CVI.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteley A, Major D, Legastelois I, Campitelli L, Donatelli I, Thompson CI, et al. Generation of candidate human influenza vaccine strains in cell culture - rehearsing the European response to an H7N1 pandemic threat. Influenza and Other Respiratory Viruses. 2007 July 2007;1(4):157–66. doi: 10.1111/j.1750-2659.2007.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Wit E, Munster VJ, Spronken MI, Bestebroer TM, Baas C, Beyer WE, et al. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J Virol. 2005 Oct;79(19):12401–7. doi: 10.1128/JVI.79.19.12401-12407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakabe S, Sakoda Y, Haraguchi Y, Isoda N, Soda K, Takakuwa H, et al. A vaccine prepared from a non-pathogenic H7N7 virus isolated from natural reservoir conferred protective immunity against the challenge with lethal dose of highly pathogenic avian influenza virus in chickens. Vaccine. 2008 Apr 16;26(17):2127–34. doi: 10.1016/j.vaccine.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Le Gall-Recule G, Cherbonnel M, Pelotte N, Blanchard P, Morin Y, Jestin V. Importance of a prime-boost DNA/protein vaccination to protect chickens against low-pathogenic H7 avian influenza infection. Avian Dis. 2007 Mar;51(1 Suppl):490–4. doi: 10.1637/7592-040206R.1. [DOI] [PubMed] [Google Scholar]
- 44.Capua I, Marangon S. The use of vaccination to combat multiple introductions of Notifiable Avian Influenza viruses of the H5 and H7 subtypes between 2000 and 2006 in Italy. Vaccine. 2007 Jun 28;25(27):4987–95. doi: 10.1016/j.vaccine.2007.01.113. [DOI] [PubMed] [Google Scholar]
- 45.Toro H, Tang DC, Suarez DL, Zhang J, Shi Z. Protection of chickens against avian influenza with non-replicating adenovirus-vectored vaccine. Vaccine. 2008 May 19;26(21):2640–6. doi: 10.1016/j.vaccine.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med. 1999 Jan;133(1):15–22. doi: 10.1053/lc.1999.v133.a94931. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007 Apr 3;146(7):486–92. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 48.Rosenzweig P, Miget N, Brohier S. Transaminase elevation on placebo during phase I trials: prevalence and significance. Br J Clin Pharmacol. 1999 Jul;48(1):19–23. doi: 10.1046/j.1365-2125.1999.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purkins L, Love ER, Eve MD, Wooldridge CL, Cowan C, Smart TS, et al. The influence of diet upon liver function tests and serum lipids in healthy male volunteers resident in a Phase I unit. Br J Clin Pharmacol. 2004 Feb;57(2):199–208. doi: 10.1046/j.1365-2125.2003.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: Influenza virus receptors in the human airway. Nature. 2006;440(7083):435–6. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 51.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RAM, Osterhaus ADME, et al. H5N1 virus attachment to lower respiratory tract. Science. 2006 April 21;312(5772):399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 52.Bartlett EJ, Hennessey M, Skiadopoulos MH, Schmidt AC, Collins PL, Murphy BR, et al. Role of interferon in the replication of human parainfluenza virus type 1 wild type and mutant viruses in human ciliated airway epithelium. J Virol. 2008 Aug;82(16):8059–70. doi: 10.1128/JVI.02263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bateman AC, Busch MG, Karasin AI, Bovin N, Olsen CW. Amino acid 226 in the hemagglutinin of H4N6 influenza virus determines binding affinity for alpha2,6-linked sialic acid and infectivity levels in primary swine and human respiratory epithelial cells. J Virol. 2008 Aug;82(16):8204–9. doi: 10.1128/JVI.00718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy BR, Richman DD, Chalhub EG, Uhlendorf CP, Baron S, Chanock RM. Failure of attenuated temperature-sensitive influenza A (H3N2) virus to induce heterologous interference in humans to parainfluenza type 1 virus. Infect Immun. 1975;12(1):62–8. doi: 10.1128/iai.12.1.62-68.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, et al. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7558–63. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008 Aug 1;47(3):401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 57.Eichelberger M, Golding H, Hess M, Weir J, Subbarao K, Luke CJ, et al. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10–11, 2007. Vaccine. 2008 Aug 12;26(34):4299–303. doi: 10.1016/j.vaccine.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000 Mar;181(3):1133–7. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 59.Murphy BR, Rennels MB, Douglas RG, Jr, Betts RF, Couch RB, Cate TR, Jr, et al. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect Immun. 1980;29(2):348–55. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Archetti I, Horsfall FL., Jr Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J Exp Med. 1950 Nov 1;92(5):441–62. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karron RA, Belshe RB, Wright PF, Thumar B, Burns B, Newman F, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr Infect Dis J. 2003 May;22(5):394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- 62.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Jr, Boyce TG, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–42. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 63.Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986 Jan;23(1):66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]