In the March 1 issue of Int J Cancer an editorial concludes that “Therefore, this population-based study has provided evidence that molecular screening and genetic testing all colorectal tumors diagnosed before age 50 years will identify most Lynch syndrome cases”.1 The study referred to was published in the same issue of the journal.2 We strongly disagree with the conclusion that patients over age 50 should not be screened for Lynch syndrome. Because the detection of Lynch syndrome in an individual followed by intensified clinical surveillance and prophylactic interventions can save many lives,3, 4 this proposal1 is potentially harmful to public health. Detecting Lynch syndrome in people over age 50 is not only important for the probands in whom the mismatch repair gene mutation is first detected, but notably for family members who are found to be mutation carriers after genetic counseling and testing.5
Below is a brief account of our reasons for rejecting the proposed age limit for screening.
Even though the study was said to be population-based, it excluded patients aged 60 or over2. All conclusions about the older group of patients are extrapolations.
After screening for microsatellite instability (MSI) in archival material 98 patients were considered high risk candidates for Lynch syndrome (“red flag”). Among these, 25 had a previously known mutation, and 35 were studied for mutation revealing 11 further cases. Thus, no mutational information was available for 38 patients (including the four patients with results pending). The number of carriers of Lynch syndrome in these was extrapolated and the authors thereby arrived at an estimate of 0.83% as the overall frequency of Lynch syndrome in colorectal cancer patients in Western Australia. Recent data from a large cohort in which all 4 mismatch repair genes were studied suggest a frequency of 2.8% in the population of Central Ohio.5, 6 We propose that significant numbers of Lynch syndrome cases went undetected in the Australian study (see 3 and 4 below).
The authors refer to two other studies in which the prevalence of Lynch syndrome was low. In one of them the prevalence of Lynch syndrome was 0.86% whereas MSI was present in 16% of tumors.7 MSI was studied using a battery of 12 markers but only the coding regions of MSH2 and MLH1 were sequenced in search of mutations.7 In the other study the prevalence of Lynch syndrome was 0.9% and MSI was present in only 6.7% of the tumors.8 Of note, in this study only BAT26 was used to screen for MSI.8 We propose that MSI cases (and Lynch syndrome cases) were overlooked in the Australian study as well as the two other large studies. In our experience a common reason for false negative MSI results is that the proportion of tumor cells is low in the material from which DNA is extracted. This is especially true when no microdissection is practiced as was the case here.2 In addition, two of these studies only evaluated one microsatellite marker (BAT26). A recent study summarizing the clinical sensitivity of MSI testing to detect Lynch syndrome found that studies using three or more mononucleotide markers had consistently higher clinical sensitivities (91% vs. 80% for MLH1, 87% vs. 84% for MSH2 and 77% vs. 55% for MSH6) than those using less.9
Mutation detection was by MLPA followed by direct sequencing of the coding region of the four main mismatch repair genes.2 In this way promoter region mutations (rare) and intronic mutations affecting splicing (common) cannot be found. We propose that this resulted in mutations being missed. In support of this assumption we note that there were no intronic mutations among the 11 probands found in the study itself. In contrast, there were at least 7 (possibly 10) intronic mutations among the 25 probands whose mutations had been diagnosed previously or who belonged to already known mutation-positive families.
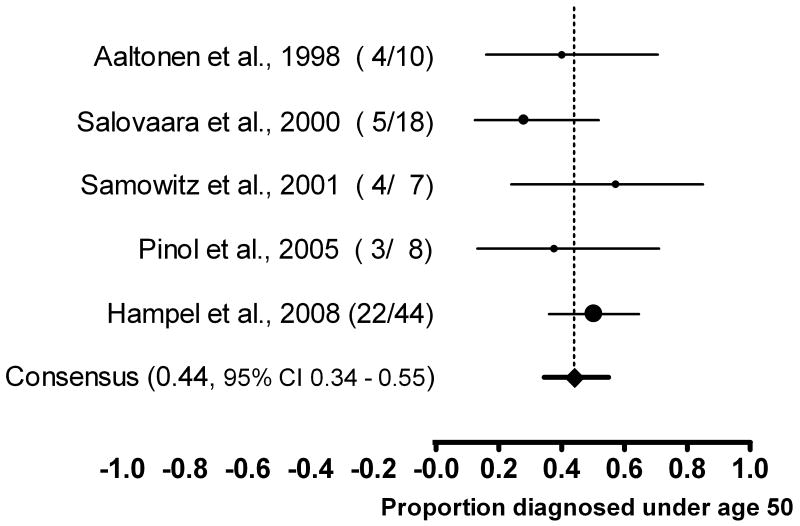
Perhaps most importantly, in the recent (and so far only) comprehensive population-based study in which every CRC tumor was prescreened for MSI and mutation analysis performed on all MSI positive cases, 44/1566 (2.8%) patients had a Lynch syndrome mutation.10 Among these, exactly half were 50 years or older. Five publications report on a total of 87 individuals identified with Lynch syndrome drawn from the general population of newly diagnosed colorectal cancer.5, 7, 8, 11, 12 Using a random effects model (Figure 1), the consensus estimate for the proportion of Lynch syndrome identified among colorectal cancer cases diagnosed prior to age 50 is 44% (95% CI 34% to 55%). The point estimates ranged from a low of 28% to a high of 57%. A formal test indicated that the results between studies were homogeneous (Q=3.2, df=4, p=0.5, I2=0).
FIGURE 1.
Consensus estimates for the proportion of Lynch syndrome identified prior to age 50. Data derived from five major studies
Based on the points made above and the evidence in the literature, we conclude that screening only patients under the age of 50 will miss at least 50% of Lynch syndrome cases and the recommendation made to restrict routine testing to younger colorectal cancer cases is not reasonable. We note that the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group has recently adopted the following recommendation9:
“There is sufficient evidence to recommend offering genetic testing for Lynch syndrome to individuals with newly diagnosed colorectal cancer to reduce morbidity and mortality in relatives.”
Acknowledgments
Albert de la Chapelle and Heather Hampel are supported by grant CA16058 from the National Cancer Institute, USA. Glenn Palomaki is partially supported as a consultant to the EGAPP initiative, funded by the Centers for Disease Control and Prevention through a contract with McKing Consulting Corporation
References
- 1.Jenkins MA, Dowty JG, Hopper JL, Southey MC. Molecular screening of all colorectal tumors diagnosed before age 50 years followed by genetic testing efficiently identifies Lynch syndrome cases. Int J Cancer. 2009;124:x–i. doi: 10.1002/ijc.24173. [DOI] [PubMed] [Google Scholar]
- 2.Schofield L, Watson N, Grieu F, Li WQ, Zeps N, Harvey J, Stewart C, Abdo M, Goldblatt J, Iacopetta B. Population-based detection of Lynch syndrome in young colorectal cancer patients using microsatellite instability as the initial test. Int J Cancer. 2009;124:1097–102. doi: 10.1002/ijc.23863. [DOI] [PubMed] [Google Scholar]
- 3.Jarvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomaki P, De La Chapelle A, Mecklin JP. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–34. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 4.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, Daniels MS, White KG, Boyd-Rogers SG, Conrad PG, Yang KY, Rubin MM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–9. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 5.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA, Panescu J, Fix D, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–8. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 7.Samowitz WS, Curtin K, Lin HH, Robertson MA, Schaffer D, Nichols M, Gruenthal K, Leppert MF, Slattery ML. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology. 2001;121:830–8. doi: 10.1053/gast.2001.27996. [DOI] [PubMed] [Google Scholar]
- 8.Pinol V, Castells A, Andreu M, Castellvi-Bel S, Alenda C, Llor X, Xicola RM, Rodriguez-Moranta F, Paya A, Jover R, Bessa X. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. Jama. 2005;293:1986–94. doi: 10.1001/jama.293.16.1986. [DOI] [PubMed] [Google Scholar]
- 9.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Clendenning M, Sotamaa K, Prior T, Westman JA, Panescu J, Fix D, et al. Feasibility of Screening for Lynch Syndrome Among Patients With Colorectal Cancer. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomaki P, Chadwick RB, Kaariainen H, Eskelinen M, Jarvinen H, Mecklin JP, de la Chapelle A. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–7. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 12.Salovaara R, Loukola A, Kristo P, Kaariainen H, Ahtola H, Eskelinen M, Harkonen N, Julkunen R, Kangas E, Ojala S, Tulikoura J, Valkamo E, et al. Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2000;18:2193–200. doi: 10.1200/JCO.2000.18.11.2193. [DOI] [PubMed] [Google Scholar]