Abstract
Research on the mechanisms of emesis has implicated multiple neurotransmitters via both central (dorsal vagal complex) and peripheral (enteric neurons and enterochromaffin cells) anatomical substrates. Taking advantage of advances in receptor-specific agonists, and utilizing Fos expression as a functional activity marker, this study demonstrates a strong, but incomplete, overlap in anatomical substrates for a variety of emetogens. We used cisplatin and specific agonists to 5-HT3 serotonergic, D2/D3 dopaminergic, and NK1 tachykininergic receptors to induce vomiting in the least shrew (Cryptotis parva), and quantified the resulting Fos expression. The least shrew is a small mammal whose responses to emetic challenges are very similar to its human counterparts. In all cases, the enteric nervous system, nucleus of the solitary tract, and dorsal motor nucleus of the vagus demonstrated significantly increased Fos immunoreactivity (Fos-IR). However, Fos-IR induction was notably absent from the area postrema following the dopaminergic and NK1 receptor-specific agents. Two brain nuclei not usually discussed regarding emesis, the dorsal raphe nucleus and paraventricular thalamic nucleus, also demonstrated increased emesis-related Fos-IR. Taken together, these data suggest the dorsal vagal complex is part of a common pathway for a variety of distinct emetogens, but there are central emetic substrates, both medullary and diencephalic, that can be accessed without directly stimulating the area postrema.
Keywords: cisplatin, dopamine, dorsal vagal complex, serotonin, substance P, vomiting, paraventricular thalamic nucleus, dorsal raphe nucleus, myenteric plexus, submucosal plexus
1. Introduction
Chemotherapy-induced vomiting (CIV), as with emesis in general, is an anatomically complex reflex requiring extensive sensory integration and the timed coordination of several different motor outputs. In fact, CIV is expressed in humans as a biphasic behavior, with an acute vomiting phase (set of vomiting bouts) shortly after cisplatin administration, a quiescent intermediate phase with little or no emesis, and finally a delayed vomiting phase which can occur as many as seven days after cisplatin administration. The pharmacology of CIV is as complex as its anatomy and expression, in that serotonin (5-HT), dopamine (DA), Substance P (SP), and certain prostanoids are proemetic (Cubeddu et al., 1992; Darmani et al., 2009; Darmani et al., 2008; Darmani et al., 1999; Hesketh et al., 2003; Kan et al., 2002; Tanihata et al., 2004), whereas exogenous cannabinoids can be potent antiemetics (Darmani and Johnson, 2004; Simoneau et al., 2001; Van Sickle et al., 2003). CIV is initiated by a substantial release of 5-HT in the gastrointestinal (GI) tract and brain (Darmani et al., 2009), acting on vagal afferents containing 5-HT3 receptors (Miller and Nonaka, 1992). These activate specific nuclei in the medulla (Endo et al., 2000; Hesketh et al., 2003; Higa et al., 2006), and the resulting output is an intestinal retroperistaltic wave, relaxation of the lower esophageal sphincter, and powerful contractions of the stomach and abdominal/intracostal musculature (Travagli et al., 2006; Veyrat-Follet et al., 1997), finally expelling the toxic GI contents from the stomach. Although cisplatin and other emetogenic conditions, such as exposure to non-chemical stimuli (e.g., motion) or enteric bacterial toxins, demonstrate overlapping activation patterns (Bureau et al., 2006; Ito et al., 2002; Miller and Ruggiero, 1994), cisplatin and its related chemotherapeutic agents are some of the most potent emetogens known (du Bois et al., 1997).
In the central nervous system, the heart of the emetic reflex is the medullary dorsal vagal complex (DVC), a multifunctional cluster of three nuclei. The area postrema (AP), a circumventricular organ, allows bloodborne emetogens access to the brain. The dorsal motor nucleus of the vagus (DMNX) sends efferents to the GI tract to modulate retroperistaltic activity, and to a central pattern generator (CPG) defined initially by Fukuda et al. (Fukuda and Koga, 1992), which mediates thoracic muscle activity and emetic prodromal responses (Fukuda et al., 1999; Koga et al., 1998; Onishi et al., 2007). The medial nucleus of the solitary tract (NTS) is critical for integrating the diverse vagal (nodose ganglionic) and central (many nuclei) emesis-related afferents (Hornby, 2001; Koga and Fukuda, 1992; Thor and Helke, 1987; Van Sickle et al., 2003). However, there is evidence for the necessity of 1) peripheral and central components besides those above (Darmani et al., 2009; Darmani et al., 2008; Hawthorn et al., 1988); 2) overlapping multiple rather than individual neurotransmitter activities (Darmani et al., 2009; Hesketh et al., 2003; Higa et al., 2006); and 3) non-traditional ligand-receptor interactions (Darmani, 2002; Darmani et al., 2005; Sharkey et al., 2007; Xiong et al., 2007), for proper expression of emesis.
Despite the pharmacological and anatomical complexity of this reflex, modern CIV research is dominated by the very simplified hypothesis that each phase of vomiting (acute or delayed) caused by chemotherapy is expressed by a single transmitter system (5-HT or SP, respectively) acting within a single anatomical compartment (enteric or central nervous system, respectively). This hypothesis appears to describe only part of the process as it was developed from incomplete neurotransmitter results obtained from the early ferret and human studies (reviewed by Darmani and Ray, 2009). Moreover, the accepted CIV doctrine has been further supported using animal models in which significant variations exist from the human biphasic response – the house musk shrew (Suncus murinus), for example, does not respond to cisplatin with two clearly differentiated phases of vomiting, even at lethal doses of the drug (Sam et al., 2003). Recent work in the least shrew, a small mammal whose responses to emetic challenges are very similar to its human counterparts (Darmani, 2005, 1998; Darmani et al., 2005), suggests that while 5-HT and SP are indeed key components of the mechanism of CIV, other transmitters previously described (e.g., dopamine) and both central and peripheral anatomical compartments are also involved in both phases of vomiting (Darmani et al., 2009).
Because of the questions regarding the hypothetical mechanism of CIV, and because the least shrew has been shown to produce human-like responses to emetogens, this study sought to use Fos immunoreactivity (Fos-IR) in the least shrew to define common anatomical substrates mediating vomiting and CIV. Indeed, while published Fos-IR studies have only focused on cisplatin or other nonspecific emetogens (Boissonade et al., 1994; Horn et al., 2006; Szelenyi et al., 1994; Van Sickle et al., 2003), the current comparative study tested cisplatin against well known representatives of different classes of receptor-selective emetic drugs (e.g. 2-methylserotonin) targeted to specific emesis-modulating receptors (e.g. 5-HT3 receptors) to identify Fos-immunoreactive nuclei in specific emetic loci common to all classes of tested emetogens. Furthermore, when possible, the neurochemistry of activated neurons was studied through colocalization of Fos with immunochemical markers specific for various neurochemical phenotypes.
2. Methods
2.1 Animals and experimental treatment protocol
Adult female least shrews (C. parva, N = 28) from the Western University Animal Facilities colony were housed in groups on a 14:10 light:dark cycle and fed and watered ad libitum. The shrews were 45-60 days old and weighed 4-6g. All experiments were performed between 11:00 and 17:30 hours, and in accordance with NIH guidelines and Western University IACUC standards. On the day of experimentation, groups of 5-8 shrews (one group per experimental condition) were brought from the animal facility and separated to individual cages, and allowed to adapt to the new conditions for 2-3 hours minimum to reduce potential novelty-induced Fos immunoreactivity. During this time period food was restricted, but not water.
After adaptation, shrews were given four mealworms (Tenebrio sp.) each. Thirty minutes after the mealworms, shrews were injected with the given dose of one of the emetogens listed in Table 1. All drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA) except GR73632 (Tocris Bioscience, Ellisville, MO, USA), and dissolved in distilled water. Doses were based on those used in our previous studies (Darmani and Crim, 2005; Darmani and Johnson, 2004; Darmani et al., 2008). After emetogen injection, shrews were observed for vomiting behavior for 30 minutes. For these studies, a shrew was transcardially perfused 90 min after the first vomiting occurred, typically 95-105 min after emetogen injection. Although the period from first vomit to perfusion was kept stable, the post-injection time could vary depending on the period between injection and the first bout of vomiting. The injection-to-first-vomit period was generally between 5 and 15 minutes for all tested emetogens. Vehicle-injected shrews were perfused 110 min after injection. Thus, perfusions were timed to match the peak period of Fos expression, 60-120 min post-stimulus (Dragunow and Faull, 1989).
Table 1. Emetogens Used in This Study to Induce Fos-IR and Their Putative Activities.
All injections were intraperitoneal and a constant 50 μl volume. Percent (%) Vomited represents the percentage of shrews injected with the given dose of emetogen (or vehicle) which vomited within the 30 minute observation period described in Materials and Methods. Abbreviations: 5-HT3 – serotonin 5-HT3 receptor; D2, D3 – dopamine receptor subtypes; N/A – Not applicable; NK1R – Neurokinin-1 (Substance P) receptor.
| Emetogen | Dose (mg/kg) | % Vomited | Activity |
|---|---|---|---|
| Cisplatin | 10 | 100% | ** |
| 2-Methylserotonin | 5 | 75% | 5-HT3 agonist |
| Quinpirole | 2 | 100% | D2-preferring agonist |
| Quinelorane | 2 | 75% | D2/D3-preferring agonist |
| GR73632 | 5 | 100% | NK1R agonist |
| Vehicle (saline) | N/A | 0% | N/A |
Cisplatin has multiple activities but, at the minimum, it induces a potent release of serotonin and SP in the GI tract (see Introduction).
After the appropriate time period had elapsed, shrews were anesthetized with a lethal dose of pentobarbital (100 mg/kg) and perfused transcardially via blunted needle with a peristaltic pump. The shrew was perfused with ice cold 4% paraformaldehyde and 5% picric acid in pH 7.4, 0.1M phosphate buffer (PB) for 10 min. Brains were removed and stored in 30% sucrose in 0.1M PB overnight, then embedded in blocks of 12% gelatin in 30% sucrose/PB. An intestinal segment approximately 1 cm long, and with the rostralmost end about 1 cm from the antrum (typically representative of jejunum), was also removed and embedded as described for the brain blocks. The blocks were postfixed for 3 h in 2% paraformaldehyde/PB, then rinsed and immersed in 30% sucrose/PB until they sank (usually 1-2 h). The brain block was cut sagittally on a freezing benchtop microtome (Leica, Bannockburn, IL, USA) at 30 m into 5 series, and stored in PB with 0.03% sodium azide. The intestinal block was cut longitudinally into 30 m sections, resulting in mostly obliquely oriented slices containing most or all layers of the intestinal wall and enteric nervous system (ENS), with various widths of each layer exposed and unobscured by the other layers.
2.2 Fos and neurotransmitter immunohistochemistry
Immunolabeling was accomplished by blocking a free-floating series with 10% normal horse serum (NHS) and 3% hydrogen peroxide in PB with 0.3% Triton X-100 (TX) for 30 min. After rinsing in PB, tissue was put in rabbit anti-Fos polyclonal antibody (CalBiochem, San Diego, CA, USA; Cat #PC38; 1:10 000 dilution), with 5% NHS and 0.3% TX in PB, and incubated for about 42 h at room temperature (RT) with gentle shaking. One series of both brain and gut sections was immersed in the diluent above, but without primary antibody. Only commercially available, previously characterized (with bibliographical information on the characterization provided by the supplier) antibodies were used. For each lot of primary antibody, a 3-4 point dilution series was performed using the same secondary/tertiary and visualization steps described below for non-fluorescent labeling. After rinsing in PB, the tissue was placed in biotinylated donkey anti-rabbit IgG secondary antibody (Jackson Immunoresearch, West Grove, PA, USA; Cat# 711-065-152; 1:600) diluted in the same diluent described for the primary antibody, and the tissue incubated at RT with shaking for 75 min. Tissue was then rinsed and incubated for 60 min in HRP-conjugated avidin-biotin complex (Vector Labs, Burlingame, CA, USA; Vectastain kit diluted 1:2) in PB. Tissue was then rinsed twice in PB, then once in imidazole-acetate buffer (0.1M, pH 7.4), then reacted for 6 min in 0.0006% hydrogen peroxide with 2% nickel ammonium sulfate-enhanced diaminobenzidine (DAB, 0.05% in 0.1M imidazole-acetate buffer). All reagents for DAB processing were purchased from Sigma-Aldrich. Other series were processed for immunofluorescence using monoclonal rat anti-Substance P (Millipore/Chemicon, Temecula, CA, USA; Cat# MAB356; 1:1000) or polyclonal rabbit anti-serotonin (Invitrogen/Zymed, Carlsbad, CA, USA, Cat# 18-0077; 1:400). Tissue was blocked, and primary antibodies reacted overnight after rinsing as described for the anti-Fos primary. Control series substituting normal serum for the primary antibody were also run for all secondary antibodies used with the multi-label fluorescent tyramide amplification described below. For the anti-5-HT primary, tissue was incubated for 90 min in monovalent goat anti-rabbit FAb fragments (Jackson; Cat # 111-007-003; 1:500) in primary antibody diluent, and washed three times in PB prior to exposure to the second rabbit primary antibody. Secondary antibodies were HRP-conjugated donkey IgG (Jackson) raised against the appropriate species (rat, Cat# 712-035-150; or rabbit, Cat# 711-035-152), diluted 1:800 in the same diluent described above. Series were reacted for 90 min, then rinsed 3 times in PB, then reacted in the dark with Pacific Blue, Alexa488, or Alexa594 conjugated tyramide produced in lab using reactive AlexaFluor™ dyes from Invitrogen and tyramine from Sigma-Aldrich. Tissue was reacted with the purified tyramide conjugate (1:200 dilution) for 20 min with 0.006% hydrogen peroxide in PB. Finally, control series were run as described above using either DAB or fluorescent (Alexa488) visualization steps, with normal serum substituted for the primary antibodies.
After reacting, tissue was rinsed thoroughly in PB and mounted onto gel-subbed slides out of PB. After air-drying, slides were dehydrated through a series of ascending ethanols (50%-75%-90%-100%), then cleared in xylene. Cleared slides were coverslipped with DEPEX (Electron Microscopy Sciences, Hatfield, PA, USA).
2.3 Fos counting and image analysis
Initially, the few animals which did not vomit following drug injection (but not vehicle injection) were removed from the counting pool. Then immunohistochemically processed sections covering the entire brain were surveyed for Fos-IR. Areas demonstrating Fos-IR were marked as regions of interest prior to being counted by a different observer (blinded to condition). Photomicrographs of regions of interest were taken at 1600×1200 px digital resolution with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) attached to a PC running version 4.0 of the SPOT software and mounted to a Nikon Eclipse E600 microscope. Images were exported to Adobe Photoshop 7, and relevant structures identified using an atlas produced in lab (Ray and Darmani, 2007). A solid black outline was drawn as an overlay layer, to identify the region(s) of interest in a given image. The image was then passed through a high-pass threshold filter set to 75% black, which turns any gray level below the threshold to white and any above to black. This filter eliminates variations in background, as well as potential false positives created by nonspecific, weak to moderate background labeling, including marginally-labeled nuclei whose cells were not clearly activated. Fos+ nuclei for a given region of interest were then counted by an observer blind to the animal's treatment condition. A nucleus was only counted as positive if it retained its ovoid shape after high-pass filtering and was fully within the defined region of interest. For each region, the same number of sections were counted per animal: 3 sections each through AP/NTS/DRN, 2 sections each through all other brain nuclei, and 2 images each from 2 sections of intestine (enteric nervous system). No attempt was made to extrapolate the number of positive nuclei based on the structure's volume. Non-stereological nucleus counts for each structure and each emetogen were individually averaged. A two-way analysis of variance, followed by Student's t-test for independent samples and unequal sample sizes, was used to compare numbers of Fos-IR+ nuclei between emetogen-induced vomiting and vehicle-injected, non-vomiting controls. Immunofluorescent series were examined qualitatively for each primary antibody using a Nikon (Melville, NY, USA) D-eclipse C1 three-laser scanning confocal microscope, noting the relative density of the various markers in the DVC, and noting terminals or other structures (e.g. DRN) which were co-labeled for multiple antigens. Region-scale images (e.g., whole DVC sections) were taken with a 20x Nikon Plan Apo objective (N/A 0.75) in a single optical plane measuring ~ 2.4 m3/voxel, and cellular-scale images (e.g., terminal fields) were taken with a 60x Nikon Plan Apo VC objective (N/A 0.95) in a single optical plane measuring ~ 0.6 m3/voxel.
3. Results
3.1 Fos immunolabeling and neurotransmitter colocalization
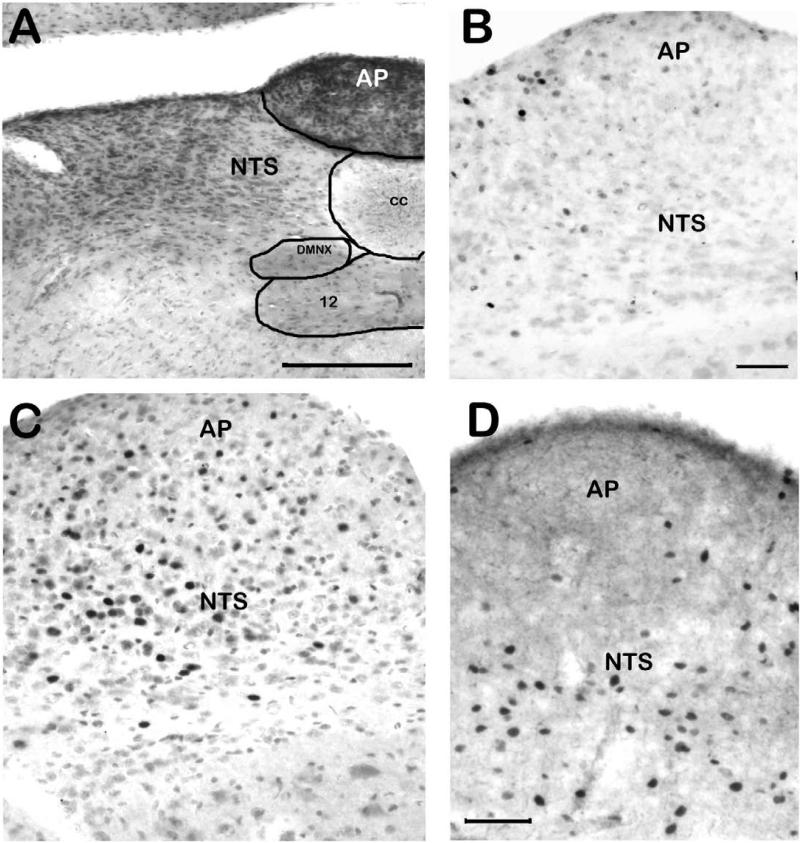
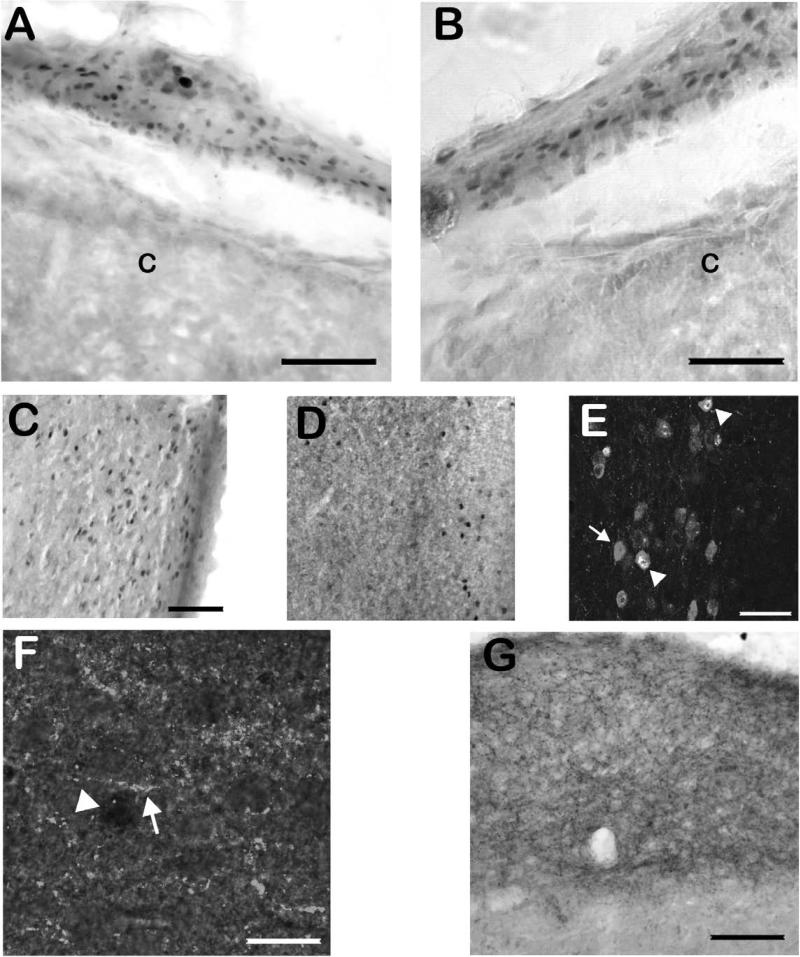
Upon the initial survey (see section 2.3), the majority of the brain demonstrated at least some Fos-IR. Areas which showed no Fos-IR, and areas which showed dense Fos-IR under all conditions (all different drug conditions plus vehicle control conditions), are not reported here. Figure 1 demonstrates immunolabeling for Fos in the brainstem of the least shrew. A section stained with cresyl violet (fig. 1A) is provided to demonstrate the cytoarchitectonic differences in the AP, NTS, and DMNX. Although vehicle injection or normal behavioral activity resulted in some Fos-IR in all analyzed regions (figure 1B), Fos-IR+ nuclei resulting from cisplatin-induced vomiting (figure 1C) were found extensively throughout the AP, NTS and DMNX. As shown in figure 1D, Fos+ nuclei were found primarily in the NTS and DMNX, but not the AP, following quinpirole-induced vomiting. Although variations in perfusion made background variable across animals, the filtering-counting paradigm described in section 2.3 eliminated this potential interference. Fos was found in areas besides the brainstem. Numerous Fos-IR nuclei were also found in many neurons in the ENS (figure 2A/B), although the small size and minimal physical separation of the nerve plexi in the shrew intestine made visually separating the submucosal and myenteric plexi unreliable. Thus, counts of ENS-localized Fos-IR did not distinguish between nerve plexi. An unexpected result was significant Fos-IR increases in the paraventricular thalamic nucleus (PVT) and dorsal raphe nucleus (DRN) related to emesis (figure 2C/D). Neurons of the DRN which were Fos-IR+ were sometimes co-labeled with serotonin-like immunoreactivity, and fibers which appeared to terminate in the DRN on Fos-IR neurons were SP-immunoreactive (figure 2E/F). Neither 5-HT nor SP colocalized with Fos-IR neurons in the DVC, although dense fiber and bouton-like immunoreactivity for each of the neurotransmitter-related antigens was found there (figure 2G).
Figure 1. Examples of Fos immunoreactivity in the brainstem related to emetogen administration.
In all Fos-IR panels, immunolabeling was performed on free-floating tissue sections using avidin-biotin-peroxidase amplification and nickel-enhanced DAB visualization. A) Coronal section through the DVC stained with cresyl violet. Note the different cytoarchitectonic details such as cell size and packing, which differentiate each subnucleus within the DVC. B) Sagittal section demonstrating Fos-IR in the DVC of a vehicle-injected shrew. Relatively few Fos-IR nuclei are found. C) The cisplatin-injected shrew DVC had numerous Fos-IR nuclei. Weakly labeled nuclei were not quantified. D) In a quinpirole-injected shrew, Fos-IR+ nuclei (the dark, filled ovals) were found through the NTS, but only infrequently in the AP. Abbreviations: 12 – hypoglossal nucleus; AP – area postrema; cc – central canal; DMNX – dorsal motor nucleus of the vagus; DVC – dorsal vagal complex; NTS – nucleus of the solitary tract. Scale bars: A, 200 μm; B-C, 100 m; D, 100 μm.
Figure 2. Examples of Fos and other immunohistochemical labeling related to emetogen administration.
A-B) Examples of Fos-IR in the ENS following cisplatin administration. The thin layers of the nerve plexi prevented quantification of ENS labeling specific to submucosal versus myenteric labeling, so results are expressed for Fos-IR in both nerve plexi. C) Fos-IR in the PVT following cisplatin injection. The image is a sagittal section, with the ventricle to the right of the image. D) Sagittal section demonstrating Fos-IR in the DRN following cisplatin injection. In all of the above panels, immunolabeling was performed on free-floating tissue sections using avidin-biotin-peroxidase amplification and nickel-enhanced DAB visualization. E) Fos-IR in the DRN colocalized with 5-HT immunoreactivity. Grey 5-HT+ somata (arrow) sometimes had bright white, Fos-IR+ nuclei (arrowheads). In the upper left corner, a white nucleus with no 5-HT colocalization (a non-serotonergic raphe neuron) can be seen. F) Immunofluorescent labeling for SP fibers in the DRN overlaid onto a brightfield image of nickel-DAB-visualized Fos-IR from the same cisplatin-injected shrew demonstrates frequent fibers (arrow) coursing between, and making putative contacts with, Fos-IR neurons (arrowhead). SP immunolabeling indicated a dense fiber plexus in the DRN. For E and F, immunostaining was performed as above, but substituted fluorochrome-conjugated tyramide for nickel-enhanced DAB. Images were taken as fluorescent color images and converted to greyscale. G) SP-IR in the DVC of the least shrew visualized using nickel-enhanced DAB. The sagittal section demonstrates extremely dense fibers and putative terminals throughout the NTS, and to a lesser extent, in the AP. Abbreviations: 5-HT – serotonin; AP – area postrema; C – intestinal crypts; DRN – dorsal raphe nucleus; DVC – dorsal vagal complex; ENS – enteric nervous system; NTS – nucleus of the solitary tract; PVT – paraventricular thalamic nucleus; SP – substance P. Scale bars: A-D, 150 μm; E, 25 μm; F, 10 μm; G, 150 μm.
3.2 Quantification of Fos immunoreactivity
The number of Fos-IR positive nuclei for each emetogen and vehicle is delineated in Table 2 in terms of brain region (or ENS). The described regions represent the areas of the brain or enteric system which are known to be involved in the emetic reflex (e.g. the DVC nuclei), or which demonstrated Fos-IR following emesis induced by all the tested emetogens. No shrews vomited when injected with vehicle instead of emetogen. In the DVC, the nucleus of the solitary tract and dorsal motor nucleus of the vagus showed significantly higher Fos-IR after vomiting induced by all tested emetogens, when compared to non-vomiting vehicle controls. The smaller sample size of DMNX neurons was due to the relatively large size of the DMNX somata, which restricted the number of neurons per section. Compared to vehicle injection, Fos-IR in the AP was significantly higher following 2-methylserotonin and cisplatin, but not following quinpirole, quinelorane, or GR73632-induced vomiting. The dorsal raphe nucleus (DRN), paraventricular thalamic nucleus (PVT), and intestinal ENS all demonstrated significantly higher Fos-IR (relative to vehicle injection) following emesis induced by all the tested emetogens.
Table 2. Number of Fos-Immunoreactive Nuclei per Region after Administration of Various Emetogens.
Values are given as the mean total number of Fos-IR nuclei per region of interest ± SEM, and boldface values are significantly higher than the corresponding value in vehicle-injected shrews (t-test; p ≤ 0.05). Abbreviations: 2Me5HT – 2-Methyl-Serotonin; AP – Area Postrema; CIS – Cisplatin; CPG – Central Pattern Generator; DMNX – Dorsal Motor Nucleus of the Vagus; DRN – Dorsal Raphe Nucleus; ENS – Enteric Nervous System; NTS – Nucleus of the Solitary Tract; PVT – Paraventricular Thalamic Nucleus
| Region | Emetogen | |||||
|---|---|---|---|---|---|---|
| Vehicle |
CIS |
2Me5HT |
Quinpirole |
Quinelorane |
GR73632 |
|
| AP | 7.3 ± 2.1 | 35 ± 5.3 | 18.3 ± 4.4 | 12 ± 1.9 | 12.3 ± 5 | 7.5 ± 1.9 |
| NTS | 12.5 ± 3.3 | 81.3 ± 7.4 | 62.3 ± 6.5 | 55.3 ± 6.5 | 53 ± 9.6 | 75.5 ± 6.4 |
| DMNX | 4 ± 0.2 | 11.5 ± 0.8 | 10 ± 1.1 | 10 ± 2 | 8.7 ± 1.4 | 11.3 ± 2.2 |
| DRN | 28 ± 8.4 | 83.3 ± 4.4 | 58.7 ± 8.4 | 76.5 ± 5.6 | 64.7 ± 8.1 | 81.8 ± 7.7 |
| PVT | 3.3 ± 0.1 | 12 ± 0.2 | 9 ± 1.7 | 11.3 ± 1.2 | 9 ± 0.8 | 10 ± 0.1 |
| ENS | 4.5 ± 1.2 | 10.8 ± 0.5 | 13.3 ± 1 | 9 ± 0.6 | 10.3 ± 1.1 | 12.3 ± 0.2 |
| CPG | − | ++ | + | + | + | ++ |
The one area which could not be fully quantified was the CPG. The area has been primarily defined physiologically, and in larger species (Fukuda et al., 1999; Koga et al., 1998; Onishi et al., 2007). Although somewhat localized to the ventrolateral medulla near the nucleus ambiguus and retrofacial nuclei, the region has no clear morphological features to define it, especially in the least shrew. While more neurons in this area did appear to demonstrate Fos-IR following vomiting, it was impossible within the confines of this study (i.e. without electrophysiological recording) to define the region well enough to reliably quantify the immunoreactivity across shrews. Therefore, the CPG is described semiquantitatively based on empirical evidence, and not analyzed statistically. The observers who performed the counting, blind to the condition of the shrew, determined that relative to vehicle-injected shrews, there was at least a modest increase in Fos-IR in the area of the CPG when vomiting was induced by any emetogen, but subjectively greater increases followed cisplatin- and GR73632-induced emesis.
4. Discussion
Fos IHC is frequently used for identifying neuronal activation related to a particular behavior (Dragunow and Robertson, 1988; Hunt et al., 1987; Johnson et al., 1992; Sagar et al., 1988). Studies examining emesis-related Fos-IR across different species or various nonspecific emetogens such as radiation and cisplatin, found certain consistently activated areas, including the DVC nuclei (Bureau et al., 2006; Horn et al., 2006; Ito et al., 2003; Miller et al., 1994; Yip and Chahl, 1999). Previous studies have utilized Fos with nonspecific emetogens (Miller et al., 1994; Watson et al., 1995), or with receptor-specific compounds in non-emetic animals (Yip and Chahl, 1999). We believe that the pharmacological data regarding the least shrew as a reliable human analogue is reflected by the limited Fos-IR data currently available. In fact, previous Fos-IR data related to cannabinoid blockade of CIV and produced in the least shrew (Ray et al., 2009) very closely mimicked their counterparts in the frequently used ferret model (Van Sickle et al., 2003). Thus, we report the first use of a battery of receptor-specific and nonspecific emetogens in combination with Fos-IR in an emesis-capable animal. Our data support the findings of the DVC as a key central component of emesis (Ito et al., 2003; Miller et al., 1994; Van Sickle et al., 2003), and the enteric nervous system as a key peripheral component (Berthoud et al., 2001; Darmani et al., 2008; Kirchgessner et al., 1992). Furthermore, Fos-IR increased in the NTS for all tested agonists (see Table 1), further evidence for its role as a critical site for integration of diverse emetic signals. In the few cases where drugs did not produce 100% vomiting in a group, the emesis-free animals were not quantified. These are rare events, and would have required the use of an inordinate number of animals to reach a statistically significant group number. Although these cases could have provided further clarification of the source of Fos-IR (see below), they could also have been representative of a specific underlying genetic variation, or possibly could be inadvertent injection into an internal organ such as the liver rather than the peritoneal cavity.
Neither the dopaminergic agonists nor the NK1 agonist caused significant activation of the AP, although D2 and D3 receptors are present (Hyde et al., 1996; Yoshikawa et al., 1996), and apomorphine, 7-OH-DPAT, and quinpirole-induced vomiting are partially mediated there (Wang and Borison, 1952; Yoshikawa et al., 1996). One potential reason for this discrepancy may be that AP dopaminergic receptors appear localized primarily to NTS dendrites which project into the AP. Fos, a nuclear antigen, is expressed in the nuclei of the NTS neurons after stimulation of these dendrites, but not in AP neuronal nuclei. This Fos-IR pattern has been noted previously in the ferret following loperamide-induced vomiting (Bhandari et al., 1992; Zaman et al., 2000). These dopaminergic agents are reasonably permeable to the brain and could induce vomiting through direct actions on the NTS or DMNX, a possibility supported by the observed significant increases in NTS/DMNX Fos-IR. Indeed, in human brainstem, D2/D3 receptor subtypes are found throughout the DVC, not just in the AP (Hyde et al., 1996). Also, while apomorphine, quinpirole, and 7-OH-DPAT are effective emetogens in the least shrew (Darmani and Crim, 2005; Darmani et al., 1999), there are no anatomical (e.g., lesion) data as yet to support localization of the effect to the AP in this species. The NK1 receptor is expressed in the AP of the guinea pig (Yip and Chahl, 2001), but autoradiographic binding of the specific NK1 antagonist CP99,994 in the ferret demonstrated reduced binding in the AP relative to the NTS (Watson et al., 1995), suggesting there may be species differences in the level of NK1 expression in the AP. In one example, loperamide-induced vomiting produced Fos-IR in the AP, and CP99,994 administration had no effect on its expression, despite successfully blocking vomiting with the same injection (Zaman et al., 2000). Taken together these data suggest one or both of two mechanisms: 1) NK1-containing dendrites of NTS neurons in the AP, as described for the dopamine receptors above; and 2) species differences in NK1 expression in the AP.
Unlike the above emetogens, the nonspecific emetogen cisplatin and the 5-HT3 receptor agonist 2-methylserotonin (2-Me-5-HT) both stimulated all studied nuclei. Some overlap in these compounds’ emetic mechanisms is likely, in that CIV involves significant release of 5-HT from enteroendocrine cells (Fukui et al., 1993; Minami et al., 2003), and significantly increased plasma serotonin or metabolites which correlate with the acute phase of CIV (Cubeddu et al., 1992; Hesketh et al., 2003; Higa et al., 2006). Furthermore, 5-HT3 receptors are found in the AP in high concentrations (Higgins et al., 1989). AP lesions can abolish the emetic response to loperamide (Bhandari et al., 1992), and systemically or AP-applied 5-HT3 antagonists will reduce vomiting (Bhandari et al., 1992; Higgins et al., 1989; Reynolds et al., 1991). However, cisplatin-induced increases in Fos-IR in the AP were found to be unaffected by 5-HT3 antagonist pretreatment (Reynolds et al., 1991). Fos-IR in the AP is unaffected possibly because antagonism of 5-HT3 receptors at NTS-localized vagal afferent terminals blocks the behavior of vomiting, while peripheral 5-HT in the bloodstream can still stimulate the exposed neurons in the AP. Finally, our data also support the involvement of peripheral communication, likely via vagal afferent stimulation (Andrews, 1992; Hornby, 2001), or secondarily by stimulating GI tract activity. Although Fos-IR in the ENS has been noted less frequently (Darmani et al., 2008; Kirchgessner et al., 1992), it is a logical consequence of activation of ENS neurons which coordinate intestinal smooth muscle activity under both normal and pathophysiological conditions.
Functional activation of the CPG appeared ambiguous. Statistical analysis for the CPG was not possible because without electrophysiological identification, the CPG is poorly defined, especially in the tiny least shrew brainstem. While there appeared to be a few more Fos-IR nuclei in the area corresponding to the CPG in the least shrew based on empirical observation, there was no rigorous method available to quantify this. Thus, clearly defining the CPG region in the least shrew will require the development of markers which can differentiate the region from its surrounding fiber tracts and other cell groups. In addition, other studies have reported the central nucleus of the amygdala as being activated following administration of known emetogens (Bureau et al., 2006; Horn et al., 2006). In the shrew, Fos-IR in the amygdala was extensive in both experimental and control conditions, but the cytoarchitectonic subdivisions of the amygdala were not as clear as those of the DVC (see figure 1A). Because the central subnucleus was not reliably distinguishable from the other subnuclei, we did not attempt to quantify amygdaloid labeling.
Notably increased Fos-IR in the PVT and DRN was also found. In limited studies, the PVT has been suggested as a viscerosensory relay nucleus with heavy limbic cortical (Bubser and Deutch, 1998; Moga et al., 1995; Thompson and Robertson, 1987) and hypothalamic (Arluison and Derer, 1993; Moga et al., 1995) connectivity. In fact, the NTS and other brainstem nuclei also have direct connections with the PVT (Ricardo and Koh, 1978). Thus, Fos-IR in the PVT is likely related to thalamocortical gating of the negative sensations associated with vomiting. Indeed, previous studies have linked emesis-related Fos-IR in several diencephalic nuclei including the PVT and central nucleus of the amygdala to emotional state and memory (Bureau et al., 2006; Horn et al., 2006). Fos-IR in serotonergic DRN neurons might, at first glance, imply the stimulation of central 5-HT nuclei concomitantly with the emesis-related peripheral surge of 5-HT (Andrews, 1992; Darmani et al., 2009). However, DRN neurons express 5-HT1A autoreceptors, and NK1 receptors are found on both serotonergic and non-serotonergic DRN neurons and dendrites. Stimulation of DRN NK1 receptors has a biphasic effect by exciting raphe interneurons (Commons and Valentino, 2002) and serotonergic neurons (Valentino et al., 2003). This increases local serotonin release very briefly, until the released serotonin activates the 5-HT1A autoreceptors (Guiard et al., 2007), ultimately suppressing serotonin release (Conley et al., 2002; Valentino et al., 2003). Given the release of both 5-HT and SP in vomiting (Darmani et al., 2009; Hesketh et al., 2003; Higa et al., 2006), the finding of Fos-IR in verified serotonergic DRN neurons (figure 1F) would seem paradoxical. One possible explanation is that stimulation of non-serotonergic raphe neurons, or the co-release of other neurotransmitters with 5-HT prior to inhibition of serotonergic output, could induce stimulation sufficient for Fos expression. Indeed, there is evidence that SP acts preferentially on DRN non-serotonergic dendrites (Commons and Valentino, 2002) and stimulates glutamatergic raphe neurotransmission (Commons et al., 2003; Commons and Valentino, 2002; Valentino et al., 2003). This combination of stimulated local activity and concomitant inhibition of “distant” 5-HT release by DRN efferents could modulate motivation or emotional state (Commons et al., 2003; Guiard et al., 2007). Thus, these forebrain-projecting nuclei are well-positioned to coordinate the core emetic reflex arc with the accompanying visceral discomfort and negative emotional state.
There are several potential confounds in interpreting Fos-IR data, such as their poor resolution of non-quantitative (firing pattern) changes (Dragunow and Faull, 1989; Morgan and Curran, 1989), which can create false negatives. Interpretation is especially difficult when attempting to assess causation, as Fos-IR can be the result of other behaviors occurring at the same time as the behavior to be studied (behavioral interference). Not only can this interference occur from other behaviors, the behavior being studied can have both sensory input, neural processing, and motor output “subdivisions” which may or may not be responsible for the increased Fos-IR. In this study, Fos-IR could be the result of cisplatin or the other drugs acting directly on receptors, or it could be the result of vomiting due to the drugs’ activities. In addition, a few areas such as the prefrontal and visual cortices were highly Fos-immunoreactive across all conditions (data not shown), and likely represent areas where nonspecific activation (interference) was prominent. However, the drugs studied in these experiments all have significant receptor- or drug-specific effects aside from inducing vomiting in least shrews, such as ear-scratches produced by GR73632 (Darmani et al., 2008), head-twitches and ear-scratches induced by 2-methyl serotonin (Darmani and Johnson, 2004) and locomotor activity caused by the dopaminergic agents (Darmani and Crim, 2005). Although still correlative, much of the Fos-IR data presented here can be more strongly associated with vomiting when they were not unique to a particular agent or class of agents (e.g., the increased Fos-IR in the NTS under all conditions). Furthermore, the exact role of the various nuclei cannot be resolved without electrophysiological or other evidence, a few inferences based on the collected data regarding vomiting can be made. In emesis, the NTS is a major integrative site (Davis et al., 2004; Onishi et al., 2007), but does not directly generate motor activity, and no evidence exists suggesting there is no quantitative change in firing rate. Thus, our NTS data are more likely to be related to induction rather than expression (motor output) of vomiting. The DMNX, however, has both motor output and local circuit neurons (Boissonade et al., 1996; Hornby and Abrahams, 2000; Krowicki and Hornby, 2000), and could be related to induction and/or expression.
The CPG is also a potential quandary. Vomiting-related CPG neurons are interspersed with neurons which subsume other autonomic functions, especially respiration (Fukuda et al., 1999; Koga et al., 1998). Thus, not all these neurons will express vomiting-related Fos-IR. This problem is exacerbated in the least shrew, in which the number of neurons responsive to a particular stimulus will be at a premium. Additionally, because prodromal signs of vomiting include other major autonomic activations (e.g. salivation) (Fukuda et al., 1999; Onishi et al., 2007), neurons in the CPG could demonstrate increased Fos-IR correlated with, but not directly mediating, vomiting. Thus, the empirically noted minimal difference could be a false negative, or it could be related to 1) prodromal signs of vomiting, or 2) direct mediation of the emetic reflex by neurons being partially masked by their small numbers and ambiguous localization in the least shrew. The apparently greater effect of cisplatin and GR73632-induced vomiting on the CPG could be the result of activating NK1 receptors via SP release (cisplatin) (Hesketh et al., 2003) or direct agonism (GR73632). Indeed, the CPG is densely innervated by, and highly responsive to, SP-containing fibers (Darmani et al., 2009; Fukuda et al., 1999; Onishi et al., 2007).
In conclusion, this study confirms previous work demonstrating that in the brain, the DVC mediates the expression of vomiting caused by nonspecific emetogens with diverse activity profiles, as well as emetic signaling generated by a variety of specific neurotransmitter systems. Furthermore, we found significant activation in the ENS related to emesis, supporting the involvement of GI-related peripheral mechanisms. Most interestingly, PVT and DRN neurons were also consistently Fos-positive following emesis, suggesting these nuclei are involved in communicating information on visceral activation to higher brain regions.
Acknowledgements
We would like to thank Lisa Griggs for her technical assistance. This project was funded through NIH grant #R01CA115331 to Dr. Darmani.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews PL. Physiology of nausea and vomiting. Br J Anaesth. 1992;69:2S–19S. doi: 10.1093/bja/69.supplement_1.2s. [DOI] [PubMed] [Google Scholar]
- Arluison M, Derer P. Forebrain connections of the rat paraventricular thalamic nucleus as demonstrated using the carbocyanide dye DiI. Neurobiology (Bp) 1993;1:337–50. [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Zheng H. Vagal-enteric interface: vagal activation-induced expression of c-Fos and p-CREB in neurons of the upper gastrointestinal tract and pancreas. Anat Rec. 2001;262:29–40. doi: 10.1002/1097-0185(20010101)262:1<29::AID-AR1008>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Bingham S, Andrews PL. The neuropharmacology of loperamide-induced emesis in the ferret: the role of the area postrema, vagus, opiate and 5-HT3 receptors. Neuropharmacology. 1992;31:735–42. doi: 10.1016/0028-3908(92)90034-m. [DOI] [PubMed] [Google Scholar]
- Boissonade FM, Davison JS, Egizii R, Lucier GE, Sharkey KA. The dorsal vagal complex of the ferret: anatomical and immunohistochemical studies. Neurogastroenterol Motil. 1996;8:255–72. doi: 10.1111/j.1365-2982.1996.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Boissonade FM, Sharkey KA, Davison JS. Fos expression in ferret dorsal vagal complex after peripheral emetic stimuli. Am J Physiol. 1994;266:R1118–26. doi: 10.1152/ajpregu.1994.266.4.R1118. [DOI] [PubMed] [Google Scholar]
- Bubser M, Deutch AY. Thalamic paraventricular nucleus neurons collateralize to innervate the prefrontal cortex and nucleus accumbens. Brain Res. 1998;787:304–10. doi: 10.1016/s0006-8993(97)01373-5. [DOI] [PubMed] [Google Scholar]
- Bureau Y, Handa M, Zhu Y, Laliberte F, Moore CS, Liu S, Huang Z, MacDonald D, Xu DG, Robertson GS. Neuroanatomical and pharmacological assessment of Fos expression induced in the rat brain by the phosphodiesterase-4 inhibitor 6-(4-pyridylmethyl)-8-(3-nitrophenyl) quinoline. Neuropharmacology. 2006;51:974–85. doi: 10.1016/j.neuropharm.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–15. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Commons KG, Valentino RJ. Cellular basis for the effects of substance P in the periaqueductal gray and dorsal raphe nucleus. J Comp Neurol. 2002;447:82–97. doi: 10.1002/cne.10228. [DOI] [PubMed] [Google Scholar]
- Conley RK, Cumberbatch MJ, Mason GS, Williamson DJ, Harrison T, Locker K, Swain C, Maubach K, O'Donnell R, Rigby M, Hewson L, Smith D, Rupniak NM. Substance P (neurokinin 1) receptor antagonists enhance dorsal raphe neuronal activity. J Neurosci. 2002;22:7730–6. doi: 10.1523/JNEUROSCI.22-17-07730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu LX, Hoffmann IS, Fuenmayor NT, Malave JJ. Changes in serotonin metabolism in cancer patients: its relationship to nausea and vomiting induced by chemotherapeutic drugs. Br J Cancer. 1992;66:198–203. doi: 10.1038/bjc.1992.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA. Endocannabinoids and gastrointestinal function. In: Onaivi ES, Sugiura T, Di Marzo V, editors. The Brain and Body's Marijuana and Beyond. CRC; Boca Raton: 2005. pp. 393–418. [Google Scholar]
- Darmani NA. The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta(9)-tetrahydrocannabinol and other cannnabinoids. J Pharmacol Exp Ther. 2002;300:34–42. doi: 10.1124/jpet.300.1.34. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Serotonin 5-HT3 receptor antagonists prevent cisplatin-induced emesis in Cryptotis parva: a new experimental model of emesis. J Neural Transm. 1998;105:1143–54. doi: 10.1007/s007020050118. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Crim JL. Delta-9-tetrahydrocannabinol differentially suppresses emesis versus enhanced locomotor activity produced by chemically diverse dopamine D2/D3 receptor agonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav. 2005;80:35–44. doi: 10.1016/j.pbb.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Crim JL, Janoyan JJ, Abad J, Ramirez J. A re-evaluation of the neurotransmitter basis of chemotherapy-induced immediate and delayed vomiting: evidence from the least shrew. Brain Res. 2009;1248:40–58. doi: 10.1016/j.brainres.2008.10.063. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Johnson JC. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol. 2004;488:201–12. doi: 10.1016/j.ejphar.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Darmani NA, McClanahan BA, Trinh C, Petrosino S, Valenti M, Di Marzo V. Cisplatin increases brain 2-arachidonoylglycerol (2-AG) and concomitantly reduces intestinal 2-AG and anandamide levels in the least shrew. Neuropharmacology. 2005;49:502–13. doi: 10.1016/j.neuropharm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Ray AP. Evidence for a re-evaluation of the neurochemical and anatomical bases of chemotherapy-induced vomiting. Chem Rev. 2009;109:3158–3199. doi: 10.1021/cr900117p. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Wang Y, Abad J, Ray AP, Thrush GR, Ramirez J. Utilization of the least shrew as a rapid and selective screening model for the antiemetic potential and brain penetration of substance P and NK(1) receptor antagonists. Brain Res. 2008;1214:58–72. doi: 10.1016/j.brainres.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Zhao W, Ahmad B. The role of D2 and D3 dopamine receptors in the mediation of emesis in Cryptotis parva (the least shrew). J Neural Transm. 1999;106:1045–61. doi: 10.1007/s007020050222. [DOI] [PubMed] [Google Scholar]
- Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004;1017:208–17. doi: 10.1016/j.brainres.2004.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–5. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson HA. Localization and induction of c-fos protein-like immunoreactive material in the nuclei of adult mammalian neurons. Brain Res. 1988;440:252–60. doi: 10.1016/0006-8993(88)90993-6. [DOI] [PubMed] [Google Scholar]
- du Bois A, Vach W, Siebert C, Holy R, Ledergerber M, Wechsel U, Kriesinger-Schroeder H. The relationship between parameters of serotonin metabolism and emetogenic potential of platinum-based chemotherapy regimens. Support Care Cancer. 1997;5:212–8. doi: 10.1007/s005200050062. [DOI] [PubMed] [Google Scholar]
- Endo T, Minami M, Hirafuji M, Ogawa T, Akita K, Nemoto M, Saito H, Yoshioka M, Parvez SH. Neurochemistry and neuropharmacology of emesis - the role of serotonin. Toxicology. 2000;153:189–201. doi: 10.1016/s0300-483x(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T. Non-respiratory neurons in the Botzinger complex exhibiting appropriate firing patterns to generate the emetic act in dogs. Neurosci Res. 1992;14:180–94. doi: 10.1016/0168-0102(92)90079-r. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T, Furukawa N, Nakamura E, Shiroshita Y. The tachykinin NK1 receptor antagonist GR205171 abolishes the retching activity of neurons comprising the central pattern generator for vomiting in dogs. Neurosci Res. 1999;33:25–32. doi: 10.1016/s0168-0102(98)00106-0. [DOI] [PubMed] [Google Scholar]
- Fukui H, Yamamoto M, Ando T, Sasaki S, Sato S. Increase in serotonin levels in the dog ileum and blood by cisplatin as measured by microdialysis. Neuropharmacology. 1993;32:959–68. doi: 10.1016/0028-3908(93)90060-g. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Guilloux JP, Reperant C, Hunt SP, Toth M, Gardier AM. Substance P neurokinin 1 receptor activation within the dorsal raphe nucleus controls serotonin release in the mouse frontal cortex. Mol Pharmacol. 2007;72:1411–8. doi: 10.1124/mol.107.040113. [DOI] [PubMed] [Google Scholar]
- Hawthorn J, Ostler KJ, Andrews PL. The role of the abdominal visceral innervation and 5-hydroxytryptamine M-receptors in vomiting induced by the cytotoxic drugs cyclophosphamide and cis-platin in the ferret. Q J Exp Physiol. 1988;73:7–21. doi: 10.1113/expphysiol.1988.sp003124. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Van Belle S, Aapro M, Tattersall FD, Naylor RJ, Hargreaves R, Carides AD, Evans JK, Horgan KJ. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer. 2003;39:1074–80. doi: 10.1016/s0959-8049(02)00674-3. [DOI] [PubMed] [Google Scholar]
- Higa GM, Auber ML, Altaha R, Piktel D, Kurian S, Hobbs G, Landreth K. 5-Hydroxyindoleacetic acid and substance P profiles in patients receiving emetogenic chemotherapy. J Oncol Pharm Pract. 2006;12:201–09. doi: 10.1177/1078155206072080. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Kilpatrick GJ, Bunce KT, Jones BJ, Tyers MB. 5-HT3 receptor antagonists injected into the area postrema inhibit cisplatin-induced emesis in the ferret. Br J Pharmacol. 1989;97:247–55. doi: 10.1111/j.1476-5381.1989.tb11948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: Neural pathways for acute and delayed visceral sickness. Auton Neurosci. 2006 doi: 10.1016/j.autneu.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111(Suppl 8A):106S–12S. doi: 10.1016/s0002-9343(01)00849-x. [DOI] [PubMed] [Google Scholar]
- Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108(Suppl 4a):90S–98S. doi: 10.1016/s0002-9343(99)00345-9. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–4. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Knable MB, Murray AM. Distribution of dopamine D1-D4 receptor subtypes in human dorsal vagal complex. Synapse. 1996;24:224–32. doi: 10.1002/(SICI)1098-2396(199611)24:3<224::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S. Immunohistochemical demonstration of c-fos protein in neurons of the medulla oblongata of the musk shrew (Suncus murinus) after veratrine administration. Exp Anim. 2002;51:19–25. doi: 10.1538/expanim.51.19. [DOI] [PubMed] [Google Scholar]
- Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S. Induction of Fos protein in neurons in the medulla oblongata after motion- and X-irradiation-induced emesis in musk shrews (Suncus murinus). Auton Neurosci. 2003;107:1–8. doi: 10.1016/S1566-0702(03)00026-2. [DOI] [PubMed] [Google Scholar]
- Johnson KB, Criswell HE, Jensen KF, Simson PE, Mueller RA, Breese GR. Comparison of the D1-dopamine agonists SKF-38393 and A-68930 in neonatal 6-hydroxydopamine-lesioned rats: behavioral effects and induction of c-fos-like immunoreactivity. J Pharmacol Exp Ther. 1992;262:855–65. [PubMed] [Google Scholar]
- Kan KK, Jones RL, Ngan MP, Rudd JA. Actions of prostanoids to induce emesis and defecation in the ferret. Eur J Pharmacol. 2002;453:299–308. doi: 10.1016/s0014-2999(02)02424-x. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992;12:235–48. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Fukuda H. Neurons in the nucleus of the solitary tract mediating inputs from emetic vagal afferents and the area postrema to the pattern generator for the emetic act in dogs. Neurosci Res. 1992;14:166–79. doi: 10.1016/0168-0102(92)90078-q. [DOI] [PubMed] [Google Scholar]
- Koga T, Qu R, Fukuda H. The central pattern generator for vomiting may exist in the reticular area dorsomedial to the retrofacial nucleus in dogs. Exp Brain Res. 1998;118:139–47. doi: 10.1007/s002210050265. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Hornby PJ. Substance P in the Dorsal Motor Nucleus of the Vagus Evokes Gastric Motor Inhibition via Neurokinin 1 Receptor in Rat. J Pharmacol Exp Ther. 2000;293:214–21. [PubMed] [Google Scholar]
- Miller AD, Nonaka S. Mechanisms of vomiting induced by serotonin-3 receptor agonists in the cat: effect of vagotomy, splanchnicectomy or area postrema lesion. J Pharmacol Exp Ther. 1992;260:509–17. [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res. 1994;647:255–64. doi: 10.1016/0006-8993(94)91325-0. [DOI] [PubMed] [Google Scholar]
- Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci. 1994;14:871–88. doi: 10.1523/JNEUROSCI.14-02-00871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Endo T, Hirafuji M, Hamaue N, Liu Y, Hiroshige T, Nemoto M, Saito H, Yoshioka M. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther. 2003;99:149–65. doi: 10.1016/s0163-7258(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–38. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Calcium and proto-oncogene involvement in the immediate-early response in the nervous system. Ann N Y Acad Sci. 1989;568:283–90. doi: 10.1111/j.1749-6632.1989.tb12518.x. [DOI] [PubMed] [Google Scholar]
- Onishi T, Mori T, Yanagihara M, Furukawa N, Fukuda H. Similarities of the neuronal circuit for the induction of fictive vomiting between ferrets and dogs. Auton Neurosci. 2007;136:20–30. doi: 10.1016/j.autneu.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Ray AP, Darmani NA. A histologically derived stereotaxic atlas and substance P immunohistochemistry in the brain of the least shrew (Cryptotis parva) support its role as a model organism for behavioral and pharmacological research. Brain Res. 2007;1156:99–111. doi: 10.1016/j.brainres.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray AP, Griggs L, Darmani NA. Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew. Behav Brain Res. 2009;196:30–6. doi: 10.1016/j.bbr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694). Brain Res. 1991;565:231–6. doi: 10.1016/0006-8993(91)91654-j. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–31. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sam TS, Cheng JT, Johnston KD, Kan KK, Ngan MP, Rudd JA, Wai MK, Yeung JH. Action of 5-HT3 antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew). Eur J Pharmacol. 2003:135–45. doi: 10.1016/s0014-2999(03)01863-6. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, Guglielmotti V, Davison JS, Di Marzo V. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci. 2007;25:2773–82. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- Simoneau II, Hamza MS, Mata HP, Siegel EM, Vanderah TW, Porreca F, Makriyannis A, Malan TP., Jr The cannabinoid agonist WIN55,212-2 suppresses opioid-induced emesis in ferrets. Anesthesiology. 2001;94:882–7. doi: 10.1097/00000542-200105000-00029. [DOI] [PubMed] [Google Scholar]
- Szelenyi I, Herold H, Gothert M. Emesis induced in domestic pigs: a new experimental tool for detection of antiemetic drugs and for evaluation of emetogenic potential of new anticancer agents. J Pharmacol Toxicol Methods. 1994;32:109–16. doi: 10.1016/1056-8719(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Tanihata S, Oda S, Nakai S, Uchiyama T. Antiemetic effect of dexamethasone on cisplatin-induced early and delayed emesis in the pigeon. Eur J Pharmacol. 2004;484:311–21. doi: 10.1016/j.ejphar.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Robertson RT. Organization of subcortical pathways for sensory projections to the limbic cortex. I. Subcortical projections to the medial limbic cortex in the rat. J Comp Neurol. 1987;265:175–88. doi: 10.1002/cne.902650203. [DOI] [PubMed] [Google Scholar]
- Thor KB, Helke CJ. Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol. 1987;265:275–93. doi: 10.1002/cne.902650210. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Bey V, Pernar L, Commons KG. Substance P Acts through local circuits within the rat dorsal raphe nucleus to alter serotonergic neuronal activity. J Neurosci. 2003;23:7155–9. doi: 10.1523/JNEUROSCI.23-18-07155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–76. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- Veyrat-Follet C, Farinotti R, Palmer JL. Physiology of chemotherapy-induced emesis and antiemetic therapy. Predictive models for evaluation of new compounds. Drugs. 1997;53:206–34. doi: 10.2165/00003495-199753020-00003. [DOI] [PubMed] [Google Scholar]
- Wang SC, Borison HL. A new concept of organization of the central emetic mechanism: recent studies on the sites of action of apomorphine, copper sulfate and cardiac glycosides. Gastroenterology. 1952;22:1–12. [PubMed] [Google Scholar]
- Watson JW, Gonsalves SF, Fossa AA, McLean S, Seeger T, Obach S, Andrews PL. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol. 1995;115:84–94. doi: 10.1111/j.1476-5381.1995.tb16324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Hosoi M, Koo B, Zhang L. Anandamide inhibition of 5-HT3A receptors varies with receptor density and desensitization. Mol Pharmacol. 2007 doi: 10.1124/mol.107.039149. [DOI] [PubMed] [Google Scholar]
- Yip J, Chahl LA. Distribution of Fos-like immunoreactivity in guinea-pig brain following administration of the neurokinin-1 receptor agonist, [SAR9,MET(O2)11]substance P. Neuroscience. 1999;94:663–73. doi: 10.1016/s0306-4522(99)00283-3. [DOI] [PubMed] [Google Scholar]
- Yip J, Chahl LA. Localization of NK1 and NK3 receptors in guinea-pig brain. Regul Pept. 2001;98:55–62. doi: 10.1016/s0167-0115(00)00228-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Yoshida N, Hosoki K. Involvement of dopamine D3 receptors in the area postrema in R(+)-7-OH-DPAT-induced emesis in the ferret. Eur J Pharmacol. 1996;301:143–9. doi: 10.1016/0014-2999(96)00061-1. [DOI] [PubMed] [Google Scholar]
- Zaman S, Woods AJ, Watson JW, Reynolds DJ, Andrews PL. The effect of the NK1 receptor antagonist CP-99,994 on emesis and c-fos protein induction by loperamide in the ferret. Neuropharmacology. 2000;39:316–23. doi: 10.1016/s0028-3908(99)00113-6. [DOI] [PubMed] [Google Scholar]