Preface
Incoming sensory information is sent to the brain along modality-specific channels corresponding to the five senses. Each of these channels further parses the incoming signals into parallel streams to provide a compact, efficient input to the brain. Ultimately, these parallel input signals must be elaborated upon and integrated within the cortex to provide a unified and coherent percept. Recent studies in the primate visual cortex have greatly contributed to our understanding of how this goal is accomplished. Multiple strategies including retinal tiling, hierarchical and parallel processing and modularity, defined spatially and by cell type-specific connectivity, are all used by the visual system to recover the rich detail of our visual surroundings.
Introduction
Our richest life experiences and longest-lasting memories are formed through our interactions with the sensory world around us and have a profound impact on our personality and sense of self. Each of these sensations is transmitted to our brains through distinct biological machinery such as that found in the nose or under the skin. Yet, rather than perceiving the world as a disjointed collection of attributes, we most often experience a single unified percept. Parallel processing of sensory information is a commonly used strategy in the mammalian brain, not only between sensory modalities but across features of a single sense as well. Gasser and Erlanger1 first demonstrated that the sensations of pain and temperature are transmitted through axons of different caliber than those transmitting touch. This work was shortly followed up by Bishop2 who proposed that the three different classes of axons he found in the optic nerve process different sensory qualities related to vision. These early discoveries laid the foundation for our current understanding of the nervous system’s parallel processing strategies3–8.
The need for parallel processing in the visual system is immediately appreciated when one considers the multitude of qualities that are present in the visual environment and the physical limitations of the way this information is initially encoded and signaled to the brain (Box 1). Colour, depth, shape and motion are just a few of the many dimensions by which we interpret our visual environment and generate appropriate behaviour9. Remarkably, this complexity in our visual surroundings is first encoded as a pattern of light on a two dimensional array of photoreceptors, with little direct resemblance to the original input or the ultimate percept. Within just a few hundred microns of retinal thickness, this initial signal encoded by our photoreceptors must be transformed into an adequate representation of the entire visual scene. This representation, however, must also be sufficiently condensed so that the axons carrying this information can pass through the optic nerve, which forms an anatomical bottleneck along the route from the eye to the brain. Probably owing to these constraints, incoming visual signals are processed by at least 80 anatomically and physiologically distinct neural cell populations and 20 separate circuits within the retina. These circuits comprise at least a dozen parallel pathways that project to the brain for further processing10. The visual cortex has the job of extracting the relevant information from this reduced signal and to further elaborate and integrate the information into a unified and coherent perceptual experience.
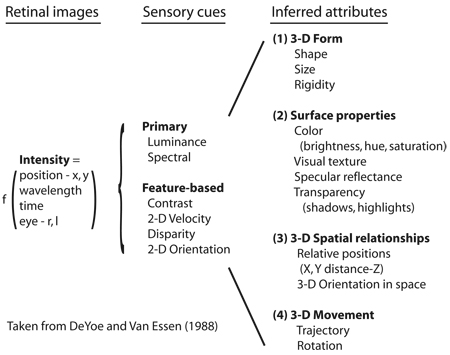
Box 1 From retinal input to cortical processing and perception
Visual input is initially encoded in the retina as a two dimensional distribution of light intensity, expressed as a function of position, wavelength and time in each of the two eyes. This retinal image is transferred to the visual cortex where primary sensory cues and, later, inferred attributes, are eventually computed (see figure). Parallel processing strategies are employed from the outset to overcome the constraints of the individual ganglion cell's limited bandwidth and the anatomical bottleneck of the optic nerve. Adapted from DeYoe and Van Essen (1988).
Many years of research have uncovered important details concerning the anatomy and functional organization of the primate visual system. This Review will focus on recent advances in our understanding of how primary visual cortex integrates parallel inputs, and constructs new, parallel outputs. These findings have been particularly helpful in elucidating the complex relationship between early parallel pathways of the retina and the processing streams in visual cortex. Instead of attempting to provide a comprehensive analysis at each level of the visual system, we will highlight key principles, such as retinal tiling, hierarchical and parallel processing and modularity (defined spatially and by cell type-specific connectivity), with the aim of providing a unified and coherent understanding of parallel processing strategies in the visual system. We hope that this will inform not only our understanding of visual perception, but of sensory processing in general.
Parallel Pathways from Retina to Cortex
The first steps in seeing begin in the retina, where a dense array of photoreceptors convert the incoming pattern of light into an electrochemical signal11. The photoreceptor mosaic encodes the intensity of light as a function of position (two dimensions), wavelength and time (Box 1). Much information is lost from the outset, such as the exact spectral composition of the image. Nevertheless, computations carried out within our visual system are supplied with enough information to support highly precise hue discriminations and other perceptual abilities that inform our everyday behaviour. Remarkably, many of these computations are carried out within the retina, before visual signals even leave the eye. Specialized circuits extract basic sensory cues, such as spatial contrast and temporal frequency from the initial intensity distribution, and encode these properties across approximately 1.5 million ganglion cells which form the optic nerve that connects the eye to the brain.
Due to the anatomical bottleneck of the optic nerve, retinal output must be efficiently condensed. The strategy used by the mammalian visual system is to reduce the representation of the visual scene to a limited number of specialized, parallel output channels. Rather than send visual signals from the eye to the brain along a homogeneous population of ganglion cells, in the primate, at least 17 distinct ganglion cell types exist in the retina and at least 13 of these project in parallel to the lateral geniculate nucleus (LGN) of the thalamus and on to visual cortex10 (Figure 1). Each ganglion cell type is thought to tile the retina, providing a complete representation across the entire visual field of the primary sensory cues it conveys to the brain12. These cues include different spatial and temporal frequencies and luminance and colour contrasts in the image. At any given point in the visual field, multiple ganglion cell types convey different aspects of the visual input simultaneously and in parallel to the brain.
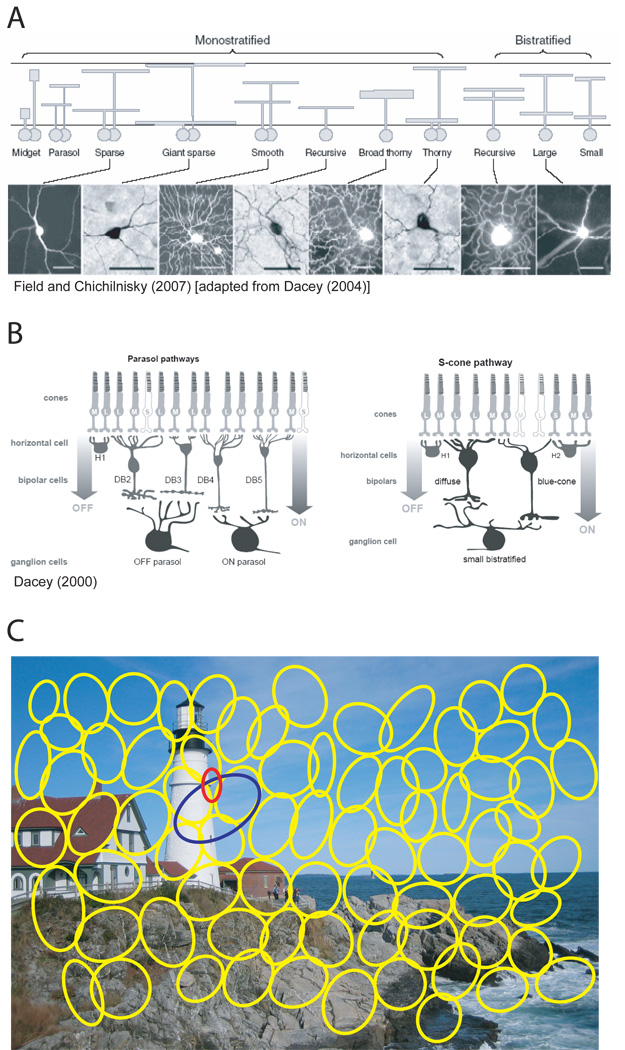
Figure 1. Parallel processing in the retina.
(A) Over a dozen different ganglion cell types exist in the retina, each with their own distinct set of morphological features including soma size, dendritic-field size and density, and level of stratification within the inner plexiform layer (IPL). Top panel shows schematic cross-sectional representation of morphologically distinct retinal ganglion cell types exhibiting either monostratified or bistratified dendritic arborization in the IPL; the boundaries of the IPL are indicated schematically by horizontal lines. The vertical position of each schematic dendritic arbor indicates its characteristic stratification in the IPL. Lower panel shows top views of filled cells obtained using retrograde photostaining from rhodamine dextran injections in the LGN and superior colliculus; scale bar is 50µm. (B) Each ganglion cell type, such as parasol (left) or small bistratified (right) have a unique set of inputs from photoreceptor cells, horizontal cells, bipolar and amacrine cells. These retinal sub-circuits confer specialized physiological response properties to the ganglion cells14. (C) The receptive field mosaic of an actual population of ON-parasol ganglion cells (yellow; from Field and Chichilnisky (2007)) is overlayed on an example visual scene, not drawn to scale. Missing cells in the mosaic are likely to reflect experimental undersampling rather than gaps in the retinal representation. Each ganglion cell type tiles the retina so that at any given point in the visual field, multiple ganglion cell types (red, blue, and yellow ellipses) are present and signal complementary visual information simultaneously and in parallel to the brain. (A) Obtained from Field and Chichilnisky (2007), which was originally adapted from Dacey (2004).
Each ganglion cell type has its own distinct set of morphological features, such as soma size and dendritic field size and density13. Many of these features vary substantially as a function of retinal eccentricity, but at any given eccentricity allow for nearly unambiguous cell type classification. Each ganglion cell type also has a distinct pattern of dendritic stratification within the inner plexiform layer (IPL), allowing for highly specific patterns of synaptic connectivity with functionally and/or anatomically defined bipolar and amacrine cell types8 (Figures 1A, B). These bipolar and amacrine cell types, in turn, have their own unique connections with photoreceptors and horizontal cells in the outer plexiform layer (OPL), forming distinct anatomical circuits from photoreceptors to ganglion cells (Figure 1B). Finally, the soma of each ganglion cell type appears to be regularly spaced across the retina so that, collectively, their dendritic fields cover the retina uniformly and with constant overlap (Figure 1C). This highly ordered mosaic architecture holds up only among ganglion cells of the same type: subsets of a type do not cover the entire visual field and different types display no systematic spatial relationship12. Together, these structural features suggest that each ganglion cell type is a fundamental unit of the retina and underlies a unique channel of visual information.
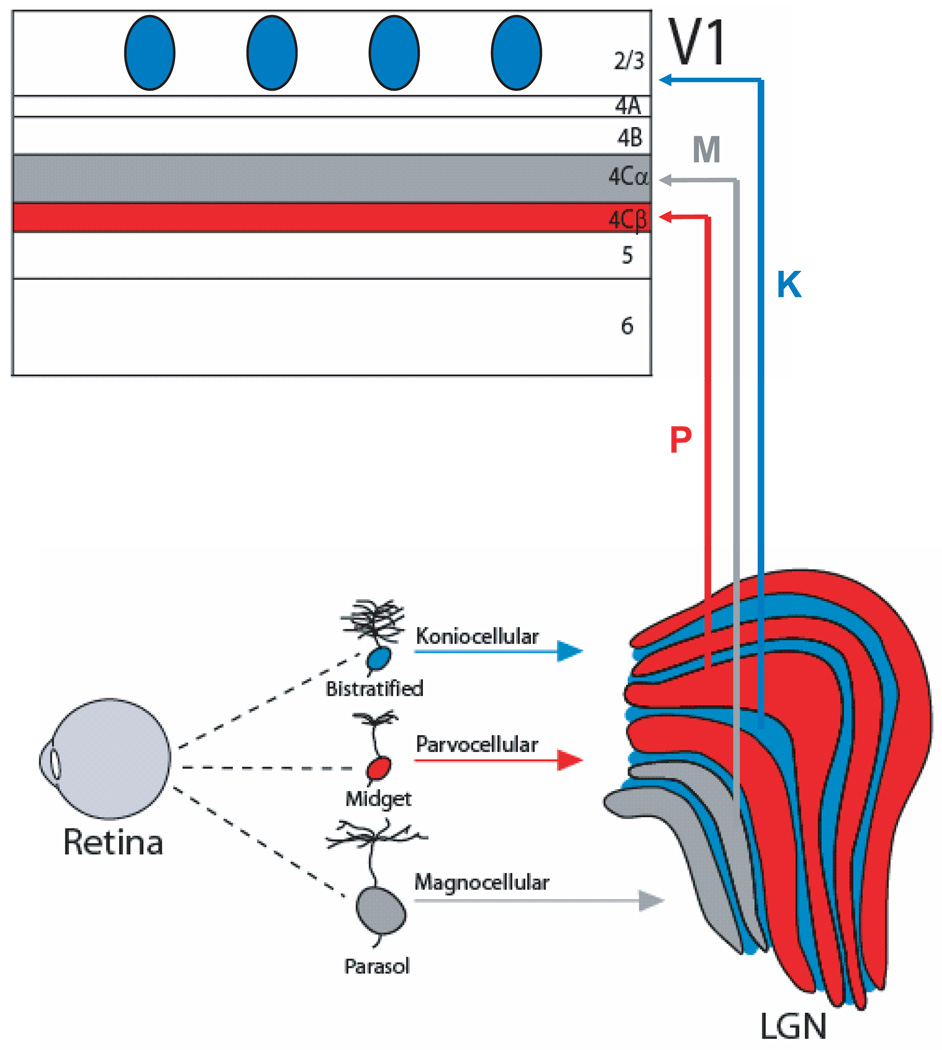
Three retinal ganglion cell types are particularly well characterized and have been linked to parallel pathways that remain anatomically segregated through the LGN and into the input layers and compartments of primary visual cortex (V1)5, 14 (Figure 2). Midget, parasol and bistratified ganglion cells comprise approximately 90% of all ganglion cells found in the primate retina and, together, they functionally complement each other to extend the range of vision in the wavelength and spatio-temporal frequency domains15. Midget ganglion cells are considered to be the origin of the parvocellular (P) pathway and comprise approximately 70% of the total population of cells projecting to the LGN14. These cells convey a red/green colour-opponent signal to the P layers of the LGN, which in turn project to layers 4Cβ and 6 of V114, 16–19. Cells in this pathway typically have small receptive fields, low contrast sensitivity, slow axonal conduction velocities and sensitivity to high spatial and low temporal frequencies. Parasol ganglion cells are considered to be the origin of the magnocellular (M) pathway and comprise approximately 10% of the total population of cells projecting to the LGN14. These cells convey a broadband, achromatic signal to the M layers of the LGN and on to layers 4Cα and 6 of V116–19. Cells in this pathway generally have large receptive fields, high contrast sensitivity, fast axonal conduction velocities and sensitivity to high temporal and low spatial frequencies. Finally, small and large bistratified ganglion cells make up at least part of the koniocellular (K) pathway and together comprise approximately 8% of the total population of cells projecting to the LGN14. These cells convey a Blue-ON/Yellow-OFF colour-opponent signal20, 21 to K layers 3 and 4 of the LGN, which in turn project to layer 1 and to the cytochrome oxidase (CO) blobs (described below) of layer 2/3 in V15, 17, 20, 22, 23. K cells in the LGN are defined as cells that express αCAM kinase and/or calbindin22 and are found not only in the intercalated K layers but also scattered throughout the M and P layers. This has made it difficult to characterize the full range of physiological response properties that are carried by the K pathway. For example, layer 4A of V1 receives a Blue-OFF/Yellow-ON colour-opponent input from the LGN17, but neither the locations of these LGN cells nor the type of retinal ganglion cell providing their input has been determined with certainty.
Figure 2. Parallel pathways from retina to cortex.
Midget, parasol and bistratified ganglion cells are well characterized and have been linked to parallel pathways that remain anatomically separate and distinct through the lateral geniculate nucleus (LGN) and into primary visual cortex (V1). Parasol ganglion cells project to magnocellular (M) layers of the LGN and on to layer 4Cα of V1 (gray). Midget ganglion cells project to parvocellular (P) layers of the LGN and on to layer 4Cβ of V1 (red). Small and large bistratified ganglion cells project to koniocellular (K) layers of the LGN and on to the cytochrome oxidase expressing patches (or blobs) of layer 2/3 (blue). Although these ganglion cell types are numerically dominant within the retina, many more types are known to exist and are likely to subserve important parallel pathways that are yet to be identified.
It is likely that many more parallel pathways exist between the retina and V1. The remaining retinal ganglion cells in this pathway are less numerous (12% of the total), but they consist of more types that are likely to provide diverse information about the visual scene. In fact, assuming that each ganglion cell type uniformly tiles the retina, most of the variation in numbers of ganglion cells for each cell type might be explained simply by dendritic field size: the larger the cell type’s dendritic field size, the fewer of those cells will be required to cover the entire retina12. Whether these additional cell types maintain strict segregation in their connections through the LGN and into V1 remains unclear. Nevertheless, many studies have indicated functional and anatomical heterogeneity within the LGN that might be explained by additional, unidentified parallel pathways. Two prevalent types of visual response properties have been reported in the M layers of the LGN and three in the P layers24, 25. Furthermore, there is some indication that the two dorsal, central and ventral K layers of the LGN each have their own characteristic response properties and projection targets within V15. Finally, the rod-and cone-mediated responses of intrinsically photosensitive, melanopsin-expressing retinal ganglion cells have recently been characterized and suggest that this cell population may constitute a separate projection pathway to the visual cortex that conveys luminance and colour-opponent information26.
The sensory cues that are carried by the parallel pathways bear little resemblance to our perceptual experience, and the functional role of each pathway is still poorly understood. Visual attributes such as motion, shape and colour must be computed from these sensory cues and integrated within cortex to create a unified and coherent percept. Lesion studies have shown that computing these attributes does not rely on one pathway alone. M lesions, for example, result in a large decrease in luminance contrast sensitivity for stimuli of high temporal and low spatial frequencies27, and have little effect on colour contrast sensitivity27, 28 or speed and direction of motion discriminations. By contrast, P (and possibly K) lesions cause an almost complete loss of colour vision28–30, reduce luminance contrast sensitivity for stimuli of higher spatial and lower temporal frequencies27, 28, 31, and have little effect on shape discriminations30, 32. These findings are consistent with the visual response properties of cells within each pathway, and highlight the fact that no single pathway appears to have a monopoly over any particular set of computations, such as those related to motion or shape perception. Ultimately, the key to uncovering the role of the early parallel pathways may require a better understanding of the relationship between these pathways and processing streams within the visual cortex.
Primary Visual Cortex and Parallel Processing Strategies
Once the condensed and parallel signals from the retina and LGN arrive in visual cortex, the original components of the visual scene must be extracted, elaborated upon and integrated into a unified percept. The visual cortex uses both hierarchical and modular processing in order to accomplish these goals33. Although the basic tuning properties of cells do not differ substantially between the retina and LGN, within the first cortical synapses of V1 new and more complex information is extracted such as orientation, direction and colour selectivity6. These ‘inferred attributes’9 (Box 1) are organized into overlapping functional maps, with a columnar organization for orientation tuning, ocular dominance and visual space34, 35. As visual information passes through V1 and on to extrastriate cortex, the response properties found within each subsequent area tend to increase in complexity and selectivity. New computations are carried out along the way, eventually leading to highly specialized areas concerned with object recognition and sensorimotor integration36. Information flow within this hierarchical network is not unidirectional and dense feedback connections provide a substrate for recurrent processing37. However, the functional details of this feedback system remain largely unknown and are beyond the scope of this review.
Most visual information from the LGN passes through V1 before being processed further in extrastriate visual cortex. Different strategies might be used within V1 to transfer parallel input signals into multiple output streams (Figure 3A). One possibility is that the parallel inputs stay separate within cortex and continue on past V1. A second possibility is that the parallel inputs converge indiscriminately within V1 and lack any organization or relationship with processing streams in extrastriate cortex. A third possibility, which current evidence favours, is that the parallel inputs converge within V1, but do so in an organized and specific way so that new parallel streams of information are systematically conveyed to the rest of visual cortex.
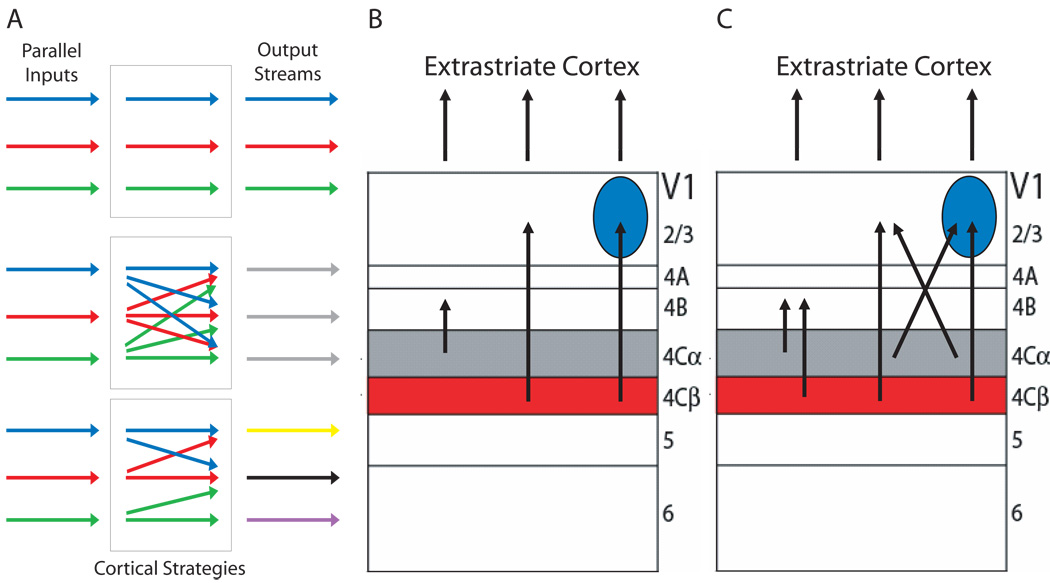
Figure 3. Cortical processing strategies.
(A) Multiple strategies might be used by a visual cortical area (rectangles) in order to transform parallel inputs (arrows to the left of rectangles) into multiple outputs (arrows to the right of rectangles). One possibility (top) is that segregation of inputs is maintained (arrows within top rectangle) and passed directly on to the outputs. A second possibility (middle) is that these inputs mix indiscriminately (arrows within middle rectangle) and bear no systematic relationship to the outputs. A third possibility (bottom) is that the parallel inputs converge in an organized and specific way (arrows within bottom rectangle) so as to form the basis for specialized outputs. (B) Early models of V1 proposed that M and P pathway inputs remained segregated within V1 as they passed through layers 4B and 2/3 respectively and on to extrastriate cortex. (C) Recent studies have provided evidence for extensive intermixing and convergence of M, P and K pathway inputs, suggesting that V1 outputs bear little or no systematic relationship to its parallel inputs. Cytochrome oxidase-expressing blobs are shown as blue circles.
Originally, it was thought that the early parallel pathways might maintain strict segregation within V1 and underlie separate processing streams in extrastriate cortex6, 38 (Figure 3B). The distinctive laminar organization of V1 and the alternating light and dark staining for the mitochondrial enzyme cytochrome oxidase (CO) in the superficial layers39, which is correlated with the pattern of direct thalamic input to V1 due to the higher levels of metabolic activity in these regions, suggested a high degree of processing modularity and the possibility that parallel inputs to V1 remain well segregated. Furthermore, the functional parcellation of extrastriate cortex into separate processing streams (discussed further below), which suggested M- and P-like segregation of function well beyond V1, led Livingstone and Hubel to propose that the P pathway formed the basis for colour and form processing within the CO blobs (dark staining) and interblobs (light staining) in layer 2/3, and that the M pathway formed the basis for motion and depth-related processing in layer 4B6. This scheme was attractive in its simplicity and was supported by early studies suggesting a clean anatomical segregation of M and P inputs to layers 4B and 2/3 respectively40, as well as a functional segregation of fast, achromatic responses in layer 4B and colour-opponent or orientation-selective responses in blobs and interblobs respectively41–43. More recent studies, however, have called into question such a strict segregation44.
Although the concept of modular function within V1 has stood the test of time, it is now clear that rather than maintain strict segregation, the early parallel pathways converge significantly within the first few synapses in V1 (Figure 3C). This is most evident in the connections from layer 4C to the CO blobs and interblobs of layer 2/3, which provide ample opportunity for mixing of early parallel pathways. The blobs and interblobs of layer 2/3 receive convergent input from M and P pathways, with the blobs receiving additional direct input from the K layers of the LGN22, 45–48. Importantly, the K pathway was discovered after Livingstone and Hubel’s theory was first proposed, leaving its contributions to colour, form and motion/depth processing largely unaccounted for. Functionally, the intermixing of M, P and K pathways in V1 has been confirmed by showing that lesions of either M or P layers of the LGN can strongly affect the response properties of cells in both CO blobs and interblobs of V149, 50. It is therefore unsurprising that recent studies have often failed to find a clear relationship between the CO-stained compartments and form or colour processing51–53, with several indicating that orientation and colour tuning are commonly found in the same V1 cells and across almost all layers52, 54, 55. It is tempting to conclude from these data that early parallel pathways converge indiscriminately within V1 and lack any systematic influence on the computations that form outputs to the rest of the brain. Nevertheless, a closer inspection of V1 modularity at the level of specific cell types and their connections tells a different story.
Recent evidence suggests that functional modules in visual cortex are defined not only by laminar and spatial compartmentalization, but also by specialized connectivity (Figure 4). For example, in layer 4B of V1 (Figure 4A), cells from the M-recipient layer 4Cα form dense axonal branches, whereas cells from the P-recipient layer 4Cβ pass through layer 4B without branching. Likewise, pyramidal cells in the K-recipient layer 2/3 blobs have only sparse axonal branches in layer 4B45, 56, suggesting that layer 4B is a segregated conduit of information from the M pathway, with little or no intermixing from the P or K pathways. However, layer 4B contains two types of morphologically distinct projection neurons: spiny stellates and pyramids. While the dendrites of spiny stellates are almost entirely confined to layers 4B and 4Cα, pyramids have an apical dendrite that extends through layer 2/3 and into layer 1. This places the apical dendrites of pyramids in a position to receive input from both P and K pathways in the superficial layers. Photostimulation studies in monkey V1 slices have confirmed that while spiny stellates receive input only from M-recipient layer 4Cα, pyramids receive substantial, though less dominant input from P-recipient 4Cβ as well57 (Figure 4A). Analysis at the laminar level turned out to be misleading, as the majority cell type in layer 4B (pyramids) receives mixed M and P inputs and possibly K inputs as well.
Figure 4. Spatial and cell type-specific connectivity in V1.
(A) Layer 4B of V1 contains two excitatory cell types known as pyramidal (black, left) and stellate (black, right). Both of these cell types receive direct input from cells in M-dominated layer 4Cα (red), but only pyramids have apical dendrites that pass above layer 4B and into layer 2/3. The apical dendrites of these pyramids are in a position to receive inputs from P-dominated layer 4Cβ projections into layer 2/3 (blue). Mixed M and P inputs onto pyramids and M only inputs onto stellates has been confirmed in photostimulation studies on macaque monkey V1 slices57. (B) Layer 3B contains pyramidal cells that project out of V1 (projecting pyramid) and those that remain within V1 (local pyramid). Projecting pyramids (left) receive input only from M-dominated layer 4Cα (gray arrow), whereas local pyramids receive mixed input from both M-dominated layer 4Cα and P-dominated layer 4Cβ (red arrow). Red X denotes lack of input from layer 4Cβ. (C) Outputs from V1 to V2 were originally thought to maintain strict segregation, with layer 4B of V1 projecting to the cytochrome oxidase (CO)-stained thick stripes of V2, and the CO blobs and interblobs of layer 2/3 projecting to the thin and pale stripes of V2, respectively (arrows). This spatial modularity of outputs has recently been called into question with evidence that layer 2/3 blobs and interblobs in V1 provide substantial input to the thin stripes in V2, and that all projection layers underneath interblobs, including layer 4B, project to both thick and/or pale stripes (dashed arrows with question marks). (D) Specialized and distinct populations of cells project from layer 4B of V1 to area MT (middle temporal, also known as visual area V5) or V2. Area MT receives input from a population of cells with large cell bodies and dense dendritic trees. The majority of these cells are stellates (80%), but a smaller number of pyramids (20%) also project to MT and are positioned preferentially underneath CO blobs where their apical dendrites can receive M inputs from layer 4Cα (red circles). V2 receives input from a population of cells with smaller cell bodies and sparse dendritic trees, the majority of which are pyramidal. Together, these anatomical specializations are consistent with layer 4B of V1 relaying a quick, M-dominated signal to MT and a more mixed M and P signal to V273.
Modularity based on cell types and connectivity has been supported by subsequent studies. Photostimulation data has shown that local neurons within layer 3 receive convergent input from both M-recipient layer 4Cα and P-recipient 4Cβ, but projection neurons within that layer receive direct input only from layer 4Cα (Figure 4B). These layer 3 projection neurons have densely tufted apical dendrites that distinguish them morphologically from neighboring local neurons58. Many different cell types exist in layer 6 of V1 and each receives a unique combination of inputs from the other layers of V159. It appears that cell type-specific connectivity allows for systematic combinations of early parallel pathway inputs despite the misleading appearance of indiscriminate intermixing at the laminar or compartmental level (Figure 3, Figure 4). It remains unclear how these different connection patterns might underlie different visual response properties. Eventually, highly specialized functional properties found within V1, including the modularity of function proposed by Livingstone and Hubel, may be mapped onto the different cell types discussed above.
The same spatial and cell type-specific modules that allow for specialized intermixing of early parallel pathway input, also form the substrates for multiple output streams to extrastriate cortex (Figures 4C, D). Outputs from V1 to the second visual area (V2) have been particularly well studied. In V2, there is a repeating pattern of CO-stained compartments known as thick, pale and thin stripes, each with their own characteristic functional properties and afferent/efferent connection patterns60–63. It was originally thought that layer 4B of V1 projected to the thick stripes of V2 and that the CO blobs and interblobs projected to the thin and pale stripes, respectively64, 65 (Figure 4C). However, recent studies using intrinsic optical imaging and/or improved anatomical tracers have shown that such a clean correspondence between compartments in V1 and V2 is unlikely. One of these studies has suggested that all projection layers underneath interblobs, including layer 4B, project to both thick and/or pale stripes in V266, while another study has proposed that layer 2/3 blobs and interblobs provide substantial input to thin stripes67. Despite this apparent breakdown in the spatial segregation of outputs from V1 to V2, it is likely that at a submodular level, segregated outputs do indeed exist. There are increasing indications that each of the V2 stripe compartments can be further broken down into functionally specialized submodules, such as hue-selective maps in the thin stripes or disparity-selective clustering in the thick stripes68, 69. Although the cell-type-specific connection patterns in V2 remain unknown, it is likely that these smaller functional compartments, or even specific cell types within them, receive segregated input patterns from V1. Inspection of V1 at the level of specific cell types and their connection patterns may also help us understand the degree of segregation in the outputs from V1 to V2, and the rest of visual cortex.
Output modularity based on cell type-specific connectivity has been demonstrated in the connections between layer 4B of V1 and extrastriate cortex (Figure 4D). Cells projecting to V2, the third visual area (V3) or MT (middle temporal, also known as visual area V5) are intermixed within layer 4B70–72. It was unclear whether these different output streams were anatomically and/or functionally distinct at a sublaminar level. Early studies suggested that a majority of layer 4B cells projecting to MT are spiny stellate and, as such, anatomically distinct from other output cells within this layer of V171 and that layer 4B cells projecting to MT are a separate population from those projecting to V2 72. A recent study using a modified rabies virus that expresses green fluorescent protein (GFP) confirmed earlier indications that layer 4B cells projecting to MT are primarily spiny stellate and have larger cell bodies than layer 4B cells projecting to V2, but it also uncovered other important anatomical differences73. Layer 4B cells projecting to MT are large cells with dense dendritic trees that lie close to the bottom of the layer. The majority of these cells are spiny stellate, but a substantial minority are pyramidal cells located preferentially underneath CO blobs. Layer 4B cells projecting to V2 are smaller with sparse dendritic trees situated throughout the layer and are mostly pyramidal cells located preferentially underneath CO interblobs. These anatomical specializations are consistent with these two cell populations conveying quick74, 75, M-dominated signals to MT and more mixed M and P signals to V2. Therefore, it seems that early parallel pathways of the retina and LGN are recombined within V1 into both spatial and cell type-specific modules, forming multiple output channels that project to specific areas of extrastriate cortex.
Processing Strategies in Extrastriate Cortex and Beyond
The outputs from V1 and V2 to MT and visual area 4 (V4), represent the beginning of a more pronounced anatomical and functional segregation of signals known as the dorsal and ventral streams76 (Figure 5). MT is specialized for processing motion and depth, whereas V4 is specialized for processing form and possibly colour36, 77–79. These two areas reflect earlier segregation within the output modules of V1 and V2, as cells in layer 4B of V1 and the thick stripes of V2 project to MT, whereas the pale and thin stripes of V2 project to V460, 62, 70, 71, 80. Functional evidence provides additional support, as layer 4B of V1 and the thick stripes of V2 have a high proportion of direction- and disparity-selective neurons, whereas layer 2/3 of V1 and the pale and thin stripes of V2 consist mainly of neurons that are selective for orientation and colour. It would seem, therefore, that one of the goals of the systematic integration of parallel inputs that occurs within V1 and V2 is to construct new output channels that can support at least two new parallel streams of information flow.
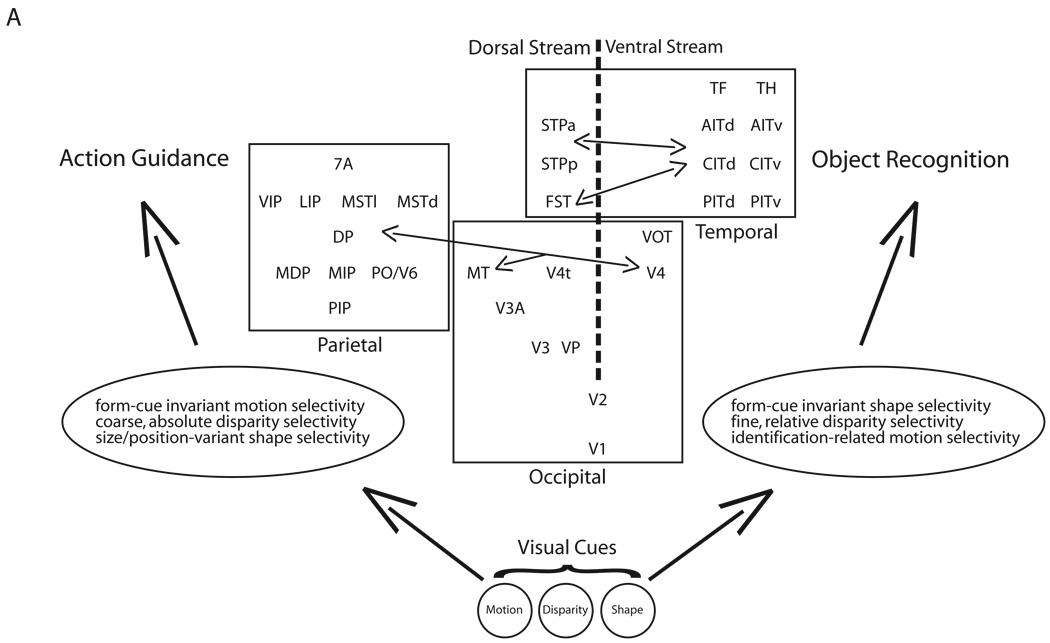
Figure 5. Parallel processing streams of extrastriate cortex.
Dorsal (left of vertical, dotted line) and ventral (right of vertical, dotted line) streams constitute separate but interconnected pathways through occipital, parietal and temporal extrastriate visual cortex (rectangular boundaries). Vertical position indicates the hierarchical relationship between areas. Interconnections between streams can be found at essentially every level of the hierarchy. For simplicity, not all areas have been included and only those cross-stream connections mentioned in the text have been drawn. Dorsal and ventral processing streams subserve different behavioural goals, with the dorsal stream aimed at the visual control of skilled actions and the ventral stream aimed at object recognition. The very same sensory cues, such as motion, disparity and shape are processed along both processing streams, but within each stream distinct computations are performed on these same cues in order to support different behavioural goals. V1,Visual area 1; V2, visual area 2; V3, visual area 3; VP, ventral posterior; V3A, visual area 3A; MT, middle temporal; V4, visual area 4; V4t, visual area 4 transitional; VOT, ventral occipitotemporal; FST, fundus of superior temporal; PITd, posterior inferotemporal (dorsal); PITv, posterior inferotemporal (ventral); CITd, central inferotemporal (dorsal); CITv, central inferotemporal (ventral); AITd, anterior inferotemporal (dorsal); AITv, anterior inferotemporal (ventral); STPp, superior temporal polysensory (posterior); STPa, superior temporal polysensory (anterior); TF, temporal area F; TH, temporal area H; MSTd, medial superior temporal (dorsal); MSTl, medial superior temporal (lateral); PO, parieto-occipital; V6, visual area 6; PIP, posterior intraparietal; VIP, ventral intraparietal; LIP, lateral intraparietal; MIP, medial intraparietal; MDP, medial dorsal parietal; DP, dorsal prelunate; 7a, visual area 7a.
Anatomical studies have established that extrastriate cortex is indeed composed of at least two segregated but interacting parallel processing streams (Figure 5). The dorsal pathway consists of a large number of interconnected extrastriate cortical areas in the parietal cortex downstream of MT, including medial superior temporal (MST), fundus of the superior temporal (FST), superior temporal polysensory (STP), ventral intraparietal (VIP), lateral intraparietal (LIP) and 7a to name just a few81–85. The apparent absence of substantial crosstalk between a dorsal-dorsal pathway through visual area 6 (V6) and the superior parietal lobule (SPL) and a ventral-dorsal pathway through MT and the inferior parietal lobule (IPL) indicates that the dorsal stream may actually consist of two relatively segregated subcircuits86. The ventral pathway also consists of a large number of interconnected extrastriate cortical areas in the temporal cortex downstream of V4 including temporal areas F (TF) and H (TH) and the various subdivisions of infero-temporal (IT) cortex87–89. It has been suggested that these parallel dorsal and ventral pathways maintain segregation all the way into motor-related frontal cortical areas such as the frontal eye field (FEF)90. Likewise, within the dorsal stream, segregated inputs from the SPL to dorsal premotor area (PMd) and the IPL to ventral premotor area (PMv) have been shown to exist91. While dorsal and ventral streams clearly make up two relatively separate circuits, the anatomical segregation between the two streams is by no means absolute. There is clear evidence of crosstalk between streams, such as the reported connections between V4 and areas MT and LIP81, 82, 85, as well as between anterior inferotemporal (AIT) cortex and areas FST, VIP and STP83, 84, 92. New subdivisions of parietal and IT cortex continue to be established93–95, and the specific connection patterns of these areas have, for the most part, reinforced the notion of segregated but interacting dorsal and ventral processing streams96, 97.
Functional evidence indicates that the dorsal and ventral processing streams operate relatively independently as well. Neuronal processing along the dorsal stream is best characterized by direction of motion and binocular disparity selectivity in MT98, 99, more complex motion analysis related to locomotion and pursuit/tracking in areas downstream from MT in the STS (MST, FST, and STP)100, 101, and computations informing target selection for arm and eye movements, object manipulation and visuospatial attention in areas of the IPL (LIP, VIP, and V6). By contrast, neuronal processing along the ventral stream is best characterized by colour and contour selectivity in V477, 78, 102, more complex combinations of colours, patterns, and/or shapes in posterior inferotemporal (PIT) cortex103, 104, and invariant representations of 2-D and 3-D shapes and objects in AIT. Lesion studies have further corroborated the observed physiological differences along the two processing streams, with dorsal stream lesions affecting smooth pursuit eye movements, speed discriminations, complex motion perception and the accurate encoding of visual space105–109 and ventral stream lesions affecting orientation and complex shape discriminations, perceptual invariance and attention110–112.
Although the existence of relatively separate and independent dorsal and ventral streams is now firmly established, our understanding of these two processing streams has undergone important revision in recent years. Ungerleider and Mishkin7 first proposed that dorsal and ventral streams mediated spatial and object related visual capacities, suggesting that each stream might process different visual attributes. In more recent years, however, a slightly different perspective has emphasized distinctions in the processing goals of each stream. In this view, the dorsal stream’s goal is to mediate navigation and the visual control of skilled actions directed at objects in the visual world, whereas the ventral stream’s goal is to transform visual inputs into representations that embody the enduring characteristics of objects and their spatial relationships113. Building on this conceptualization, Rizzolatti et al.86 proposed that the two anatomically segregated subcircuits of the dorsal stream might mediate different behavioural goals as well: the dorsal-dorsal pathway concerned with the control of action ‘on-line’ (while the action is ongoing) and the ventral-dorsal pathway for space perception and ‘action understanding’ (the recognition of actions made by others). This framework implies that the visual attributes that are processed along parallel streams in extrastriate cortex might actually be similar, and that the important differences between the streams can be found in the manner by which these attributes are used to inform their respective goals. This would be consistent with the systematic intermixing of parallel inputs that occurs within V1, as the resulting outputs formed there, which subserve the dorsal and ventral streams, do not maintain exclusive rights over the attributes conveyed along any single input channel. Instead, these attributes are integrated in varying combinations and to differing degrees along each output channel, the details of which might be particularly relevant to the computations and behavioural goals of the dorsal and ventral streams.
Indeed, it has become clear that both the dorsal and ventral streams are likely to process the same set of visual attributes, but for different behavioural goals (Figure 5). Possibly, the best example of a visual feature that is processed along both dorsal and ventral streams is binocular disparity. Binocular disparity information is found in dorsal areas MT and MST114, 115, as well as in ventral areas V4 and IT116–118. In recent years, however, critical differences have been shown to exist in the way the two streams encode disparity and the types of behaviour such encoding schemes might inform. Sensitivity along the dorsal stream to coarse and absolute disparities, and to anti-correlated random dot stereograms (RDS), suggest that such processing may be used to spatially orient and guide actions119, 120. By contrast, sensitivity along the ventral stream to fine and relative disparities, and reduced or absent responses to anti-correlated RDS, suggest that such processing might be closely linked to 3-D shape perception121, 122. Disparity is not the only visual attribute that is processed in both dorsal and ventral streams, both shape and motion-related computations can be found throughout extrastriate cortex as well123–127. Again, recent studies have shown important differences in the way these attributes are encoded and the behavior or perception they likely inform128–130. Finally, recent studies have also shown that many extrastriate areas can become selective to stimulus properties that are not typically encoded by neurons in that area, such as the shape selectivity found in area MT following associative learning between directions of motion and static shapes131. Such findings challenge the idea that each visual cortical area provides a stable representation of the stimulus features it encodes and suggests that plasticity within cortex might allow behavioural context and the statistics of the visual environment to dynamically modify such representations.
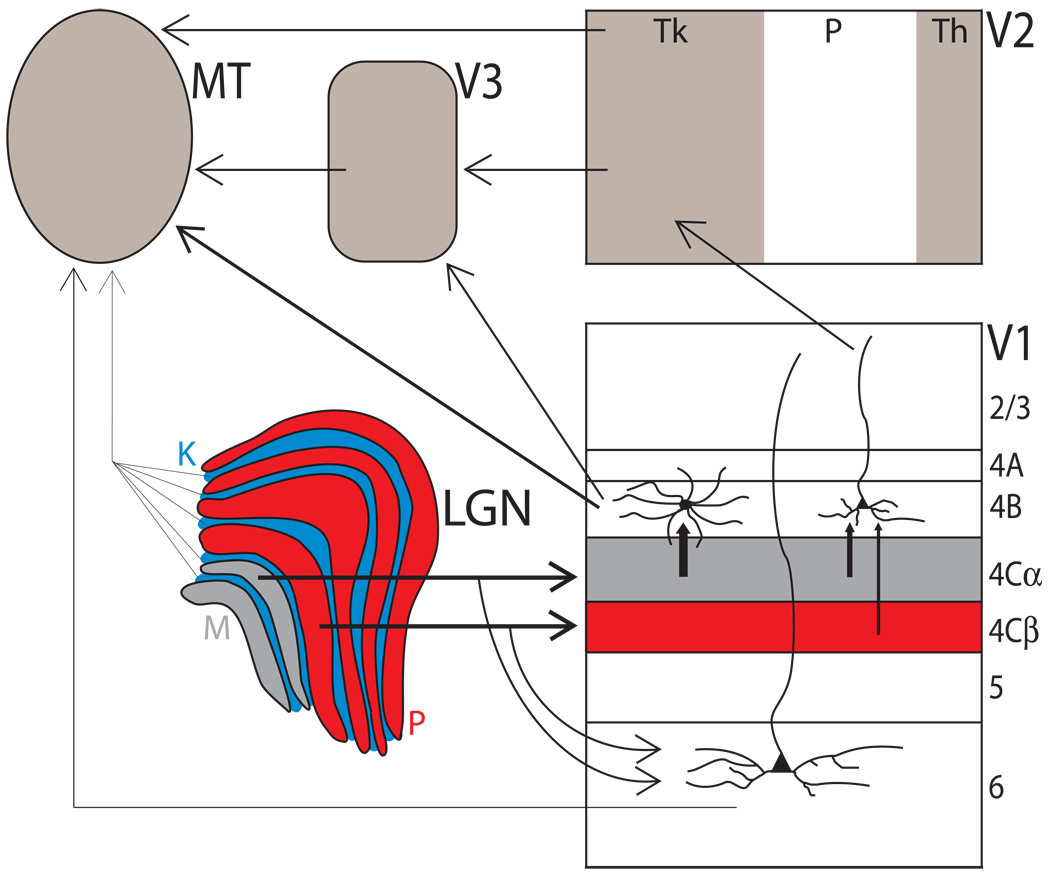
As with V1, each extrastriate cortical area receives input from multiple areas upstream and sends outputs to multiple areas downstream. MT provides a good example of such an extrastriate cortical area, as it receives input from many sources in parallel (Figure 6). While the bulk of visual information to MT passes through layer 4C of V1, it has recently been discovered that MT receives at least two separate inputs streams that bypass this main route altogether. Sparsely scattered K cells in the LGN provide a direct, monosynaptic input to MT132 and a denser population of M and P cells provides a disynaptic input to MT via layer 6 Meynert cells in V1133. The main ascending input through layer 4B of V1 both directly to MT and indirectly through V3 is dominated by 4Cα and the M pathway. Nevertheless, MT eventually receives P input through layer 4Cβ, likely via indirect input through V2134. These data, along with numerous lesion studies27, suggest that, MT and the dorsal stream rely heavily, but not exclusively, on visual information provided by the M pathway. Each of the input pathways to MT provides for varying degrees of M, P and K pathway convergence, probably providing visual information that is uniquely suited for specific computations and visual tasks.
Figure 6. Multiple input streams to area MT.
Multiple input streams exist from the lateral geniculate nucleus (LGN) to the middle temporal (MT) area. The major ascending input to MT passes through M layers of the LGN, and layers 4Cα and 4B of V1. V2 and V3 provide indirect inputs from layers 4Cα and 4B of V1, with V2 probably providing inputs from layer 4Cβ after a small number of additional synapses134. Bypassing layer 4C altogether, a sparse monosynaptic projection from K cells in the LGN to MT and a disynaptic projection from M and P layers of the LGN through layer 6 Meynert cells in V1 to MT have both been identified132, 133. Area MT is likely to use similar strategies to those found in V1 in order to process these parallel inputs and transform their signals into multiple output streams. The thickness of each arrow represents the strength of the connection. Tk, thick; Th, thin; P, pale.
What type of information might each of these parallel input pathways provide to MT? Few studies have been able to directly assess the function of an identified cell population or pathway within primate visual cortex. Movshon and Newsome135 used antidromic stimulation to identify and functionally characterize neurons in V1 that project directly to MT. In a technically challenging set of experiments, the authors were able to identify and functionally characterize only 9 MT-projecting cells out of the 745 total cells recorded. Nevertheless, they reported a highly specialized and homogeneous population of direction-selective cells that are distinct from the general population in V1. Whether this direct input from V1 provides different information to MT than indirect inputs through V2 or V3 was investigated in awake, fixating monkeys in which the indirect pathway to MT through V2/V3 was inactivated by cooling the lunate sulcus136. MT disparity selectivity was severely disrupted even as direction and speed selectivity remained largely intact. These results suggest that the direct pathway from V1 to MT provides speed and direction of motion information, whereas the indirect pathway provides disparity information. Although we know much less about other input pathways to MT and pathways that provide input to other extrastriate cortical areas, segregation of function along parallel inputs is likely to be similar in V4 and the rest of extrastriate cortex.
Ultimately, we must understand how each of these inputs is processed and integrated to form outputs to areas downstream. As the problem faced in extrastriate cortex is so similar to that faced in V1 (parallel inputs and multiple outputs), we might expect to find similar properties, such as modularity defined spatially and by cell-type specific connections. Indeed, within many extrastriate cortical areas there are clear examples of spatial modularity that are similar to those seen in V1 and V2. MT contains direction and disparity columns and clustered speed-tuning modules137–139, and V3 contains modules for disparity and orientation140, 141. There is also evidence that V4 may contain separate modules142–144, possibly related to segregated processing of colour77. Few studies have directly investigated the vertical organization of extrastriate cortical areas, but those that have find evidence for distinct computations performed within different cortical layers145, 146. There is also evidence to suggest that these functional modules serve as a substrate for the formation of a new set of segregated outputs to be sent downstream147. We have few details on these modules of extrastriate cortex, but there is strong evidence that they are ubiquitous and probably contain submodular organization and specialized connectivity similar to that seen in the compartments of V1 and V2.
Conclusions and Future Directions
Over the past fifty years, we have gained great insight into the ways by which the brain processes incoming visual inputs and recovers the information necessary to adequately inform our perception and everyday behaviour. It is clear that whether in the retina, V1, or beyond, parallel processing is a ubiquitous feature of the visual system. Several key principles have been elucidated regarding parallel processing strategies that will help us in understanding visual perception and sensory processing in general (Box 2). In the retina, over a dozen distinct ganglion cell types parse the incoming visual signals into functionally and anatomically specialized channels that project in parallel to the LGN and on to V1. Each of these ganglion cell types tiles the retina and provides a complete representation across the entire visual field of the attributes it conveys to the brain. Once in V1, these parallel input channels are recombined within modules that are defined spatially and by local connectivity, thus forming new parallel channels of information that can be sent on to the rest of the brain. The outputs formed in V1 and V2 lead to segregated, but interacting dorsal and ventral processing streams in extrastriate cortex. Both streams make use of a similar set of visual attributes, but perform different computations in order to mediate non-overlapping behavioural goals. Within each stream, however, each extrastriate cortical area probably uses the same strategies found in V1 to recombine and integrate multiple inputs and form new outputs to send downstream.
Box 2 Parallel processing in other sensory systems
Parallel processing is not only a ubiquitous property of the visual system, but it is found across the brain in all other sensory modalities as well. The multiple touch receptors underneath our skin and the different olfactory receptors in our nose are the beginnings of a similar process by which incoming sensory information is parsed into separate channels of information and eventually recombined to form a unified and coherent percept. Some of the properties discussed in this review may be unique to the visual system, but many are likely to be shared with other sensory systems. For instance, the mosaic tiling found along the body wall in the periphery of the somatosensory system is similarly structured to the tiling that occurs in the retina153–155. There is also evidence for mosaic organization in the periphery of the olfactory epithelium in the olfactory system156, but these mosaics do not show the same degree of spatial order as that found in the retina, probably due to the fact that, in some cases, they process essentially non-spatial modalities. The modularity that exists in visual cortex appears to be typical of sensory cortical areas in general157–159, and in the few cases for which it has been studied, cell-type specific connectivity appears common as well160–163. Finally, there is substantial support for the existence of hierarchically organized, parallel processing streams in each of the other sensory cortices164–168.
Although much has been uncovered regarding parallel processing strategies in the visual system, many questions still remain. How many more parallel pathways exist between the retina and V1, and what are their anatomical and functional properties? How many different output pathways are formed in V1 and what is the functional contribution of each pathway to processing in the dorsal and ventral streams of extrastriate cortex? In order to answer some of these questions, methods that can directly relate detailed cell morphology and connection patterns to visual response properties and perception will be necessary. Some recently developed methods are already helping us on this endevour73, 148–152 (Box 3). Two photon microscopy, viral tracing and reversible inactivation or activation methods can all play a useful role in taking the next step in understanding parallel processing strategies in the visual system. Further research is necessary to fully understand how parallel processing strategies are used within and between each sensory modality so that we can enjoy our seamless perception of the world around us.
Box 3 Modern techniques to investigate parallel processing in the visual system
Numerous techniques have been developed to gain further insight into the mechanisms underlying parallel processing in the primate visual system73, 133, 134, 148–152. Two photon calcium imaging. Using femtosecond pulsed laser light in combination with genetically-encoded or bulk-loaded fluorescent calcium indicators, it is possible to image the morphology and visual response properties of neurons deep within the intact brain. This technique offers the possibility of studying modularity of visual cortical areas at single-cell resolution, as well as correlating function with specific cell types and circuits148.
Viral tracing. Genetically-modified viruses have become increasingly useful for tracing mono-and multisynaptic connections, and for introducing foreign genes into neurons of the primate brain. Rabies virus, in particular, has proven to be a powerful tool for uncovering multisynaptic connections of the primate visual system with the hope of elucidating the complex circuitry within and between different visual areas73, 133, 134, 150.
Reversible Inactivation/Activation. Only recently, the possibility of activating and inactivating specific cell types and circuits has become a reality. By introducing and activating non-mammalian receptors in specific neuron populations of the primate visual system, these neurons are hyperpolarized or depolarized. For example, activation of the allatostatin receptor by the application of its ligand opens inwardly rectifying potassium channels and hyperpolarizes the neurons so that they are no longer visually responsive149. Alternatively, the receptors Channelrhodopsin and Halorhodopsin can be activated by light and depolarize or hyperpolarize the neurons respectively151, 152
Online Summary
Multiple parallel processing strategies, involving over a dozen retinal ganglion cell types, can be found in the retina. Each ganglion cell type tiles the retina to provide a complete representation across the entire visual field of the visual attributes it conveys to the brain.
Three retinal ganglion cell types have been particularly well characterized both anatomically and physiologically and project in parallel from the retina, through the lateral geniculate nucleus of the thalamus to the primary visual cortex.
Primary visual cortex receives parallel inputs from the thalamus and uses modularity, defined spatially and by cell-type specific connectivity, to recombine these inputs into new parallel outputs.
Beyond primary visual cortex, separate but interacting dorsal and ventral streams perform distinct computations on similar visual information to support distinct behavioural goals.
Less is known about the parallel processing strategies used in extrastriate visual cortex. However, there are strong indications that these areas employ many of the same strategies found in primary visual cortex.
Many of the parallel processing strategies found in the primate visual system are found in the other sensory processing systems of the mammalian brain.
Acknowledgements
We thank R. Born and J. Maunsell for helpful comments and the National Institutes of Health for their support (RO1-EY010742 and F32-EY018982).
Glossary
- Percept
the perception that arises internally in the mind, based on an external stimulus, such as a visual stimulus
- Photoreceptor
a specialized cell in the retina, which detects light and responds with a change in membrane potential and a change in neurotransmitter release
- Tiling
relatively uniform and complete coverage of space
- Hierarchical Processing
processing in serial order with more sophisticated properties emerging at higher levels by building up from simpler properties at lower levels
- Parallel Processing
simultaneous processing of information through independent circuits
- Modularity
Repeating modules are used to conduct similar operations. Typically, in the visual cortex, each module will perform an operation related to visual information from a portion of visual space. All of the modules together cover the space so that the operation would be conducted over the entire visual scene
- Eccentricity
distance from the center. Typically used to describe the distance of a visual receptive field from the center of gaze and expressed as an angle, in degrees
- Colour-opponent
a visual receptive field having the property of excitation in response to one colour and inhibition to another colour
- Receptive Field
the location in visual space where a change in light can cause a change in neuronal activity
- Broadband
typically used to describe visual receptive fields that are not colour opponent. Whether a cell is excited or inhibited by a stimulus at a particular part of its receptive field is relatively independent of the wavelength of light
- Random Dot Stereogram
pair of random dot images that generate the sensation of depth when the eyes are positioned so that they focus at a location in front of or behind the images
- Disynaptic
traversing two synaptic contacts. If a transsynaptic anatomical tracer were to spread from one neuron across synaptic contacts to a second neuron, the spread would be monosynaptic. If the tracer continued to spread from the second neuron, across more synaptic contacts, to a third neuron, then the spread would be disynaptic
- Meynert Cell
a large projection neurons in the deep layers of the visual cortex
- Antidromic Stimulation
used to determine whether a neuron recorded in one location projects to another distant location. Antidromic stimulation at the distant location generates action potentials that propagate back to, and are detected at, the recorded neuron
- Fixation
maintenance of eye position at a particular location
Biographies
Jonathan Nassi is a postdoctoral fellow in the Department of Neurobiology at Harvard Medical School, working in the laboratories of Richard Born and Clay Reid. He received his B.Sc. in Cognitive Science from the University of California, Los Angeles. He received his Ph.D. in Neuroscience from the University of California, San Diego, where he investigated multi-synaptic connections in the visual cortex of non-human primates in the laboratory of Ed Callaway at the Salk Institute for Biological Studies. His current research is focused on relating identified anatomical circuits in the visual cortex to their response properties and behavioural function.
Ed Callaway is a Professor at the Salk Institute for Biological Studies in La Jolla, California. After graduate work studying neuromuscular development with David Van Essen at Caltech, he moved to the Rockefeller University, New York, where he did postdoctoral work with Larry Katz, focusing on development of the visual cortex. Present research in the Callaway lab is aimed at understanding cortical organization and function, particularly in the visual system. The lab also uses molecular biology and virology to develop novel tools for investigation of the structure and function of the nervous system.
References
- 1.Gasser HS, Erlanger J. The role of fiber size in the establishment of a nerve block by pressure or cocaine. Am. J. Physiol. 1929;88:581–591. [Google Scholar]
- 2.Bishop GH. Fiber groups in the optic nerves. Am. J. Physiol. 1933;106:460–470. [Google Scholar]
- 3.Callaway EM. Structure and function of parallel pathways in the primate early visual system. J Physiol. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 5.Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- 6.Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 7.Ungerleider LG, Mishkin M. The Analysis of Visual Behavior. In: Ingle DJ, Mansfield RJW, Goodale MS, editors. Cambridge: MIT Press; 1982. pp. 549–586. [Google Scholar]
- 8.Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 9.DeYoe EA, Van Essen DC. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 1988;11:219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- 10.Dacey DM. The Cognitive Neurosciences. In: Gazzaniga MS, editor. Cambridge: MIT Press; 2004. pp. 281–301. [Google Scholar]
- 11.Rodieck RW. The first steps in seeing (Sinauer Associates, 1998) [Google Scholar]
- 12.Field GD, Chichilnisky EJ. Information processing in the primate retina: circuitry and coding. Annu Rev Neurosci. 2007;30:1–30. doi: 10.1146/annurev.neuro.30.051606.094252. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, Rodieck RW. Parasol and midget ganglion cells of the primate retina. J Comp Neurol. 1989;289:434–454. doi: 10.1002/cne.902890308. [DOI] [PubMed] [Google Scholar]
- 14.Dacey DM. Parallel pathways for spectral coding in primate retina. Annu Rev Neurosci. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- 15.Schiller PH, Logothetis NK. The color-opponent and broad-band channels of the primate visual system. Trends Neurosci. 1990;13:392–398. doi: 10.1016/0166-2236(90)90117-s. [DOI] [PubMed] [Google Scholar]
- 16.Blasdel GG, Lund JS. Termination of afferent axons in macaque striate cortex. J Neurosci. 1983;3:1389–1413. doi: 10.1523/JNEUROSCI.03-07-01389.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S, Callaway EM. Parallel colour-opponent pathways to primary visual cortex. Nature. 2003;426:668–671. doi: 10.1038/nature02167.This paper shows that colour-opponent LGN neurons with red/green, blue-On, or blue-Off receptive fields, project in parallel to the primate V1 and terminate in anatomically separate compartments.
- 18.Hendrickson AE, Wilson JR, Ogren MP. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol. 1978;182:123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- 19.Michael CR. Retinal afferent arborization patterns, dendritic field orientations, and the segregation of function in the lateral geniculate nucleus of the monkey. Proc. Natl. Acad. Sci U.S.A. 1988;85:4914–4918. doi: 10.1073/pnas.85.13.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 21.Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1.This paper shows that there are at least a dozen anatomically distinct cell types that project in parallel from the retina to the primate LGN.
- 22.Hendry SH, Yoshioka T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science. 1994;264:575–577. doi: 10.1126/science.8160015.This paper demonstrates that staining for calbindin or alpha-Cam kinase reveals a population of neurons in the primate LGN which are distinct from the magnocellular and parvocellular neurons. These are termed koniocellular.
- 23.Livingstone MS, Hubel DH. Thalamic inputs to cytochrome oxidase-rich regions in monkey visual cortex. Proc Natl Acad Sci U S A. 1982;79:6098–6101. doi: 10.1073/pnas.79.19.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan E, Shapley RM. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, et al. A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus) J Physiol. 2001;531:203–218. doi: 10.1111/j.1469-7793.2001.0203j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 27.Merigan WH, Byrne CE, Maunsell JH. Does primate motion perception depend on the magnocellular pathway? J Neurosci. 1991;11:3422–3429. doi: 10.1523/JNEUROSCI.11-11-03422.1991.This paper demonstrates that motion perception can persist following inactivation of the magnocellular LGN. However, there was some impairment in motion perception at high temporal frequencies and low spatial frequencies.
- 28.Merigan WH, Katz LM, Maunsell JH. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. J Neurosci. 1991;11:994–1001. doi: 10.1523/JNEUROSCI.11-04-00994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merigan WH. Chromatic and achromatic vision of macaques: role of the P pathway. J Neurosci. 1989;9:776–783. doi: 10.1523/JNEUROSCI.09-03-00776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiller PH, Logothetis NK, Charles ER. Role of the color-opponent and broad-band channels in vision. Vis Neurosci. 1990;5:321–346. doi: 10.1017/s0952523800000420. [DOI] [PubMed] [Google Scholar]
- 31.Merigan WH, Eskin TA. Spatio-temporal vision of macaques with severe loss of P beta retinal ganglion cells. Vision Res. 1986;26:1751–1761. doi: 10.1016/0042-6989(86)90125-2. [DOI] [PubMed] [Google Scholar]
- 32.Schiller PH, Logothetis NK, Charles ER. Functions of the colour-opponent and broad-band channels of the visual system. Nature. 1990;343:68–70. doi: 10.1038/343068a0. [DOI] [PubMed] [Google Scholar]
- 33.Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988;335:311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]
- 34.Bartfeld E, Grinvald A. Relationships between orientation-preference pinwheels, cytochrome oxidase blobs, and ocular-dominance columns in primate striate cortex. Proc Natl Acad Sci U S A. 1992;89:11905–11909. doi: 10.1073/pnas.89.24.11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obermayer K, Blasdel GG. Geometry of orientation and ocular dominance columns in monkey striate cortex. J Neurosci. 1993;13:4114–4129. doi: 10.1523/JNEUROSCI.13-10-04114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orban GA. Higher order visual processing in macaque extrastriate cortex. Physiol Rev. 2008;88:59–89. doi: 10.1152/physrev.00008.2007. [DOI] [PubMed] [Google Scholar]
- 37.Bullier J, Hupe JM, James AC, Girard P. The role of feedback connections in shaping the responses of visual cortical neurons. Prog Brain Res. 2001;134:193–204. doi: 10.1016/s0079-6123(01)34014-1. [DOI] [PubMed] [Google Scholar]
- 38.Maunsell JHR. Matters of Intelligence. In: Vaina LM, editor. Dordrecht, Holland: Reidel Press; 1987. pp. 59–87. [Google Scholar]
- 39.Horton JC, Hubel DH. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981;292:762–764. doi: 10.1038/292762a0.This paper demonstrates a modular anatomical feature in the primate V1 that led the way for later studies correlating function and/or connectivity with V1 modularity.
- 40.Fitzpatrick D, Lund JS, Blasdel GG. Intrinsic connections of macaque striate cortex: afferent and efferent connections of lamina 4C. J Neurosci. 1985;5:3329–3349. doi: 10.1523/JNEUROSCI.05-12-03329.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawken MJ, Parker AJ, Lund JS. Laminar organization and contrast sensitivity of direction-selective cells in the striate cortex of the Old World monkey. J Neurosci. 1988;8:3541–3548. doi: 10.1523/JNEUROSCI.08-10-03541.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984.This paper demonstrates the functional specialization of cytochrome oxidase patches in V1.
- 43.Ts’o DY, Gilbert CD. The organization of chromatic and spatial interactions in the primate striate cortex. J Neurosci. 1988;8:1712–1727. doi: 10.1523/JNEUROSCI.08-05-01712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sincich LC, Horton JC. The Circuitry of V1 and V2: Integration of Color, Form, and Motion. Annu Rev Neurosci. 2004 doi: 10.1146/annurev.neuro.28.061604.135731. [DOI] [PubMed] [Google Scholar]
- 45.Callaway EM, Wiser AK. Contributions of individual layer 2–5 spiny neurons to local circuits in macaque primary visual cortex. Vis Neurosci. 1996;13:907–922. doi: 10.1017/s0952523800009159. [DOI] [PubMed] [Google Scholar]
- 46.Lachica EA, Beck PD, Casagrande VA. Parallel pathways in macaque monkey striate cortex: anatomically defined columns in layer III. Proc Natl Acad Sci U S A. 1992;89:3566–3570. doi: 10.1073/pnas.89.8.3566.This paper shows that neurons in the cytochrome oxidase patches of V1 receive convergent input from the magnocellular and parvocellular recipient layers of V1.
- 47.Yabuta NH, Callaway EM. Functional streams and local connections of layer 4C neurons in primary visual cortex of the macaque monkey. J Neurosci. 1998;18:9489–9499. doi: 10.1523/JNEUROSCI.18-22-09489.1998.This paper describes anatomical substrates for the convergence of information from both magnocellular and parvocellular pathways within both cytochrome oxidase patches and interpatches of V1
- 48.Yoshioka T, Levitt JB, Lund JS. Independence and merger of thalamocortical channels within macaque monkey primary visual cortex: anatomy of interlaminar projections. Vis Neurosci. 1994;11:467–489. doi: 10.1017/s0952523800002406. [DOI] [PubMed] [Google Scholar]
- 49.Malpeli JG, Schiller PH, Colby CL. Response properties of single cells in monkey striate cortex during reversible inactivation of individual lateral geniculate laminae. J Neurophysiol. 1981;46:1102–1119. doi: 10.1152/jn.1981.46.5.1102. [DOI] [PubMed] [Google Scholar]
- 50.Nealey TA, Maunsell JH. Magnocellular and parvocellular contributions to the responses of neurons in macaque striate cortex. J Neurosci. 1994;14:2069–2079. doi: 10.1523/JNEUROSCI.14-04-02069.1994.This paper demonstrates that following inactivation of either the magnocellular or parvocellular pathway, visual responsivity can persist for neurons in both cytochrome oxidase patches and interpatches of V1
- 51.Edwards DP, Purpura KP, Kaplan E. Contrast sensitivity and spatial frequency response of primate cortical neurons in and around the cytochrome oxidase blobs. Vision Res. 1995;35:1501–1523. doi: 10.1016/0042-6989(94)00253-i. [DOI] [PubMed] [Google Scholar]
- 52.Leventhal AG, Thompson KG, Liu D, Zhou Y, Ault SJ. Concomitant sensitivity to orientation, direction, and color of cells in layers 2, 3, and 4 of monkey striate cortex. J Neurosci. 1995;15:1808–1818. doi: 10.1523/JNEUROSCI.15-03-01808.1995.This paper challenges the tripartite scheme introduced in ref. 42. It demonstrated that there is not a strict segregation of V1 neurons selective for orientation, direction and colour.
- 53.O’Keefe LP, Levitt JB, Kiper DC, Shapley RM, Movshon JA. Functional organization of owl monkey lateral geniculate nucleus and visual cortex. J Neurophysiol. 1998;80:594–609. doi: 10.1152/jn.1998.80.2.594. [DOI] [PubMed] [Google Scholar]
- 54.Johnson EN, Hawken MJ, Shapley R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nat Neurosci. 2001;4:409–416. doi: 10.1038/86061. [DOI] [PubMed] [Google Scholar]
- 55.Lennie P, Krauskopf J, Sclar G. Chromatic mechanisms in striate cortex of macaque. J Neurosci. 1990;10:649–669. doi: 10.1523/JNEUROSCI.10-02-00649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson JC, Martin KA, Whitteridge D. Form, function, and intracortical projections of neurons in the striate cortex of the monkey Macacus nemestrinus. Cereb Cortex. 1993;3:412–420. doi: 10.1093/cercor/3.5.412. [DOI] [PubMed] [Google Scholar]
- 57.Yabuta NH, Sawatari A, Callaway EM. Two functional channels from primary visual cortex to dorsal visual cortical areas. Science. 2001;292:297–300. doi: 10.1126/science.1057916.This paper demonstrates that local inputs to layer 4B neurons in V1 depend on cell type. Spiny stellate neurons receive input only from the magnocellular-recipient layer 4Cα, while pyramidal neurons receive input from both layer 4Cα and the parvocellular-recipient layer 4Cβ.
- 58.Sawatari A, Callaway EM. Diversity and cell type specificity of local excitatory connections to neurons in layer 3B of monkey primary visual cortex. Neuron. 2000;25:459–471. doi: 10.1016/s0896-6273(00)80908-3. [DOI] [PubMed] [Google Scholar]
- 59.Briggs F, Callaway EM. Layer-specific input to distinct cell types in layer 6 of monkey primary visual cortex. J Neurosci. 2001;21:3600–3608. doi: 10.1523/JNEUROSCI.21-10-03600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeYoe EA, Van Essen DC. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985;317:58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- 61.Roe AW, Ts’o DY. Visual topography in primate V2: multiple representation across functional stripes. J Neurosci. 1995;15:3689–3715. doi: 10.1523/JNEUROSCI.15-05-03689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shipp S, Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985;315:322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- 63.Shipp S, Zeki S. The functional organization of area V2, I: specialization across stripes and layers. Vis Neurosci. 2002;19:187–210. doi: 10.1017/s0952523802191164. [DOI] [PubMed] [Google Scholar]
- 64.Livingstone MS, Hubel DH. Specificity of cortico-cortical connections in monkey visual system. Nature. 1983;304:531–534. doi: 10.1038/304531a0. [DOI] [PubMed] [Google Scholar]
- 65.Livingstone MS, Hubel DH. Connections between layer 4B of area 17 and the thick cytochrome oxidase stripes of area 18 in the squirrel monkey. J Neurosci. 1987;7:3371–3377. doi: 10.1523/JNEUROSCI.07-11-03371.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sincich LC, Horton JC. Divided by cytochrome oxidase: a map of the projections from V1 to V2 in macaques. Science. 2002;295:1734–1737. doi: 10.1126/science.1067902.This paper demonstrates the connectivity between cytochrome oxidase-defined modules in primate area V1 and those in area V2
- 67.Xiao Y, Felleman DJ. Projections from primary visual cortex to cytochrome oxidase thin stripes and interstripes of macaque visual area 2. Proc Natl Acad Sci U S A. 2004;101:7147–7151. doi: 10.1073/pnas.0402052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen G, Lu HD, Roe AW. A map for horizontal disparity in monkey V2. Neuron. 2008;58:442–450. doi: 10.1016/j.neuron.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao Y, Wang Y, Felleman DJ. A spatially organized representation of colour in macaque cortical area V2. Nature. 2003;421:535–539. doi: 10.1038/nature01372. [DOI] [PubMed] [Google Scholar]
- 70.Burkhalter A, Felleman DJ, Newsome WT, Van Essen DC. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vision Res. 1986;26:63–80. doi: 10.1016/0042-6989(86)90071-4. [DOI] [PubMed] [Google Scholar]
- 71.Shipp S, Zeki S. The Organization of Connections between Areas V5 and V1 in Macaque Monkey Visual Cortex. Eur J Neurosci. 1989;1:309–332. doi: 10.1111/j.1460-9568.1989.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 72.Sincich LC, Horton JC. Independent projection streams from macaque striate cortex to the second visual area and middle temporal area. J Neurosci. 2003;23:5684–5692. doi: 10.1523/JNEUROSCI.23-13-05684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nassi JJ, Callaway EM. Specialized circuits from primary visual cortex to V2 and area MT. Neuron. 2007;55:799–808. doi: 10.1016/j.neuron.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raiguel SE, Lagae L, Gulyas B, Orban GA. Response latencies of visual cells in macaque areas V1, V2 and V5. Brain Res. 1989;493:155–159. doi: 10.1016/0006-8993(89)91010-x. [DOI] [PubMed] [Google Scholar]
- 75.Schmolesky MT, et al. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- 76.Maunsell JH, Newsome WT. Visual processing in monkey extrastriate cortex. Annu Rev Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- 77.Conway BR, Moeller S, Tsao DY. Specialized color modules in macaque extrastriate cortex. Neuron. 2007;56:560–573. doi: 10.1016/j.neuron.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pasupathy A, Connor CE. Responses to contour features in macaque area V4. J Neurophysiol. 1999;82:2490–2502. doi: 10.1152/jn.1999.82.5.2490. [DOI] [PubMed] [Google Scholar]
- 79.Schein SJ, Desimone R. Spectral properties of V4 neurons in the macaque. J Neurosci. 1990;10:3369–3389. doi: 10.1523/JNEUROSCI.10-10-03369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felleman DJ, Burkhalter A, Van Essen DC. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. J Comp Neurol. 1997;379:21–47. doi: 10.1002/(sici)1096-9861(19970303)379:1<21::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 81.Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- 82.Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- 83.Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- 84.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 85.Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- 87.Distler C, Boussaoud D, Desimone R, Ungerleider LG. Cortical connections of inferior temporal area TEO in macaque monkeys. J Comp Neurol. 1993;334:125–150. doi: 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- 88.Nakamura H, Gattass R, Desimone R, Ungerleider LG. The modular organization of projections from areas V1 and V2 to areas V4 and TEO in macaques. J Neurosci. 1993;13:3681–3691. doi: 10.1523/JNEUROSCI.13-09-03681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18:477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- 90.Bullier J, Schall JD, Morel A. Functional streams in occipito-frontal connections in the monkey. Behav Brain Res. 1996;76:89–97. doi: 10.1016/0166-4328(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 91.Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- 92.Saleem KS, Suzuki W, Tanaka K, Hashikawa T. Connections between anterior inferotemporal cortex and superior temporal sulcus regions in the macaque monkey. J Neurosci. 2000;20:5083–5101. doi: 10.1523/JNEUROSCI.20-13-05083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janssen P, Vogels R, Orban GA. Selectivity for 3D shape that reveals distinct areas within macaque inferior temporal cortex. Science. 2000;288:2054–2056. doi: 10.1126/science.288.5473.2054. [DOI] [PubMed] [Google Scholar]
- 94.Luppino G, Hamed SB, Gamberini M, Matelli M, Galletti C. Occipital (V6) and parietal (V6A) areas in the anterior wall of the parieto-occipital sulcus of the macaque: a cytoarchitectonic study. Eur J Neurosci. 2005;21:3056–3076. doi: 10.1111/j.1460-9568.2005.04149.x. [DOI] [PubMed] [Google Scholar]
- 95.Rozzi S, et al. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex. 2006;16:1389–1417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- 96.Borra E, et al. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex. 2008;18:1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- 97.Galletti C, Gamberini M, Kutz DF, Baldinotti I, Fattori P. The relationship between V6 and PO in macaque extrastriate cortex. Eur J Neurosci. 2005;21:959–970. doi: 10.1111/j.1460-9568.2005.03911.x. [DOI] [PubMed] [Google Scholar]
- 98.Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984;52:1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- 99.DeAngelis GC, Cumming BG, Newsome WT. Cortical area MT and the perception of stereoscopic depth. Nature. 1998;394:677–680. doi: 10.1038/29299. [DOI] [PubMed] [Google Scholar]
- 100.Lappe M, Bremmer F, Pekel M, Thiele A, Hoffmann KP. Optic flow processing in monkey STS: a theoretical and experimental approach. J Neurosci. 1996;16:6265–6285. doi: 10.1523/JNEUROSCI.16-19-06265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siegel RM, Read HL. Analysis of optic flow in the monkey parietal area 7a. Cereb Cortex. 1997;7:327–346. doi: 10.1093/cercor/7.4.327. [DOI] [PubMed] [Google Scholar]
- 102.Desimone R, Schein SJ, Moran J, Ungerleider LG. Contour, color and shape analysis beyond the striate cortex. Vision Res. 1985;25:441–452. doi: 10.1016/0042-6989(85)90069-0. [DOI] [PubMed] [Google Scholar]
- 103.Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Komatsu H, Ideura Y. Relationships between color, shape, and pattern selectivities of neurons in the inferior temporal cortex of the monkey. J Neurophysiol. 1993;70:677–694. doi: 10.1152/jn.1993.70.2.677. [DOI] [PubMed] [Google Scholar]
- 105.Latto R. The role of inferior parietal cortex and the frontal eye-fields in visuospatial discriminations in the macaque monkey. Behav Brain Res. 1986;22:41–52. doi: 10.1016/0166-4328(86)90079-3. [DOI] [PubMed] [Google Scholar]
- 106.Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988.This paper showed that lesions of the MT cortical area result in the impairment of motion perception.
- 107.Orban GA, Saunders RC, Vandenbussche E. Lesions of the superior temporal cortical motion areas impair speed discrimination in the macaque monkey. Eur J Neurosci. 1995;7:2261–2276. doi: 10.1111/j.1460-9568.1995.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 108.Pasternak T, Merigan WH. Motion perception following lesions of the superior temporal sulcus in the monkey. Cereb Cortex. 1994;4:247–259. doi: 10.1093/cercor/4.3.247. [DOI] [PubMed] [Google Scholar]
- 109.Quintana J, Fuster JM. Spatial and temporal factors in the role of prefrontal and parietal cortex in visuomotor integration. Cereb Cortex. 1993;3:122–132. doi: 10.1093/cercor/3.2.122. [DOI] [PubMed] [Google Scholar]
- 110.De Weerd P, Peralta MR, 3rd, Desimone R, Ungerleider LG. Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nat Neurosci. 1999;2:753–758. doi: 10.1038/11234. [DOI] [PubMed] [Google Scholar]
- 111.Merigan WH. Basic visual capacities and shape discrimination after lesions of extrastriate area V4 in macaques. Vis Neurosci. 1996;13:51–60. doi: 10.1017/s0952523800007124. [DOI] [PubMed] [Google Scholar]
- 112.Vogels R, Saunders RC, Orban GA. Effects of inferior temporal lesions on two types of orientation discrimination in the macaque monkey. Eur J Neurosci. 1997;9:229–245. doi: 10.1111/j.1460-9568.1997.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 113.Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46:774–785. doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 114.Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J Neurophysiol. 1983;49:1148–1167. doi: 10.1152/jn.1983.49.5.1148. [DOI] [PubMed] [Google Scholar]
- 115.Roy JP, Komatsu H, Wurtz RH. Disparity sensitivity of neurons in monkey extrastriate area MST. J Neurosci. 1992;12:2478–2492. doi: 10.1523/JNEUROSCI.12-07-02478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Janssen P, Vogels R, Orban GA. Macaque inferior temporal neurons are selective for disparity-defined three-dimensional shapes. Proc Natl Acad Sci U S A. 1999;96:8217–8222. doi: 10.1073/pnas.96.14.8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Uka T, Tanaka H, Yoshiyama K, Kato M, Fujita I. Disparity selectivity of neurons in monkey inferior temporal cortex. J Neurophysiol. 2000;84:120–132. doi: 10.1152/jn.2000.84.1.120. [DOI] [PubMed] [Google Scholar]
- 118.Watanabe M, Tanaka H, Uka T, Fujita I. Disparity-selective neurons in area V4 of macaque monkeys. J Neurophysiol. 2002;87:1960–1973. doi: 10.1152/jn.00780.2000. [DOI] [PubMed] [Google Scholar]
- 119.Krug K, Cumming BG, Parker AJ. Comparing perceptual signals of single V5/MT neurons in two binocular depth tasks. J Neurophysiol. 2004;92:1586–1596. doi: 10.1152/jn.00851.2003. [DOI] [PubMed] [Google Scholar]
- 120.Uka T, DeAngelis GC. Linking neural representation to function in stereoscopic depth perception: roles of the middle temporal area in coarse versus fine disparity discrimination. J Neurosci. 2006;26:6791–6802. doi: 10.1523/JNEUROSCI.5435-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Janssen P, Vogels R, Liu Y, Orban GA. At least at the level of inferior temporal cortex, the stereo correspondence problem is solved. Neuron. 2003;37:693–701. doi: 10.1016/s0896-6273(03)00023-0. [DOI] [PubMed] [Google Scholar]
- 122.Umeda K, Tanabe S, Fujita I. Representation of stereoscopic depth based on relative disparity in macaque area V4. J Neurophysiol. 2007;98:241–252. doi: 10.1152/jn.01336.2006. [DOI] [PubMed] [Google Scholar]
- 123.Durand JB, et al. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron. 2007;55:493–505. doi: 10.1016/j.neuron.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferrera VP, Rudolph KK, Maunsell JH. Responses of neurons in the parietal and temporal visual pathways during a motion task. J Neurosci. 1994;14:6171–6186. doi: 10.1523/JNEUROSCI.14-10-06171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sereno AB, Maunsell JH. Shape selectivity in primate lateral intraparietal cortex. Nature. 1998;395:500–503. doi: 10.1038/26752. [DOI] [PubMed] [Google Scholar]
- 126.Sereno ME, Trinath T, Augath M, Logothetis NK. Three-dimensional shape representation in monkey cortex. Neuron. 2002;33:635–652. doi: 10.1016/s0896-6273(02)00598-6. [DOI] [PubMed] [Google Scholar]
- 127.Tolias AS, Keliris GA, Smirnakis SM, Logothetis NK. Neurons in macaque area V4 acquire directional tuning after adaptation to motion stimuli. Nat Neurosci. 2005;8:591–593. doi: 10.1038/nn1446. [DOI] [PubMed] [Google Scholar]
- 128.Lehky SR, Sereno AB. Comparison of shape encoding in primate dorsal and ventral visual pathways. J Neurophysiol. 2007;97:307–319. doi: 10.1152/jn.00168.2006. [DOI] [PubMed] [Google Scholar]
- 129.Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- 130.Janssen P, Srivastava S, Ombelet S, Orban GA. Coding of shape and position in macaque lateral intraparietal area. J Neurosci. 2008;28:6679–6690. doi: 10.1523/JNEUROSCI.0499-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schlack A, Albright TD. Remembering visual motion: neural correlates of associative plasticity and motion recall in cortical area MT. Neuron. 2007;53:881–890. doi: 10.1016/j.neuron.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 132.Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci. 2004;7:1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- 133.Nassi JJ, Lyon DC, Callaway EM. The parvocellular LGN provides a robust disynaptic input to the visual motion area MT. Neuron. 2006;50:319–327. doi: 10.1016/j.neuron.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nassi JJ, Callaway EM. Multiple circuits relaying primate parallel visual pathways to the middle temporal area. J Neurosci. 2006;26:12789–12798. doi: 10.1523/JNEUROSCI.4044-06.2006.This paper showed that the different pathways by which visual information is carried from V1 to the MT cortical area have different relationships to the magnocellular and parvocellular pathways.
- 135.Movshon JA, Newsome WT. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J Neurosci. 1996;16:7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ponce CR, Lomber SG, Born RT. Integrating motion and depth via parallel pathways. Nat Neurosci. 2008;11:216–223. doi: 10.1038/nn2039.This paper showed that selective inactivation of indirect pathways from V1 (via V2 and/or V3) to the MT cortical area results in a selective reduction in disparity tuning, but not direction tuning of MT neurons. This suggests that the direct pathway from V1 to MT is sufficient for direction, but not disparity tuning.
- 137.Albright TD, Desimone R, Gross CG. Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol. 1984;51:16–31. doi: 10.1152/jn.1984.51.1.16. [DOI] [PubMed] [Google Scholar]
- 138.DeAngelis GC, Newsome WT. Organization of disparity-selective neurons in macaque area MT. J Neurosci. 1999;19:1398–1415. doi: 10.1523/JNEUROSCI.19-04-01398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu J, Newsome WT. Functional organization of speed tuned neurons in visual area MT. J Neurophysiol. 2003;89:246–256. doi: 10.1152/jn.00097.2002. [DOI] [PubMed] [Google Scholar]
- 140.Adams DL, Zeki S. Functional organization of macaque V3 for stereoscopic depth. J Neurophysiol. 2001;86:2195–2203. doi: 10.1152/jn.2001.86.5.2195. [DOI] [PubMed] [Google Scholar]
- 141.Zeki SM. The third visual complex of rhesus monkey prestriate cortex. J Physiol. 1978;277:245–272. doi: 10.1113/jphysiol.1978.sp012271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DeYoe EA, Felleman DJ, Van Essen DC, McClendon E. Multiple processing streams in occipitotemporal visual cortex. Nature. 1994;371:151–154. doi: 10.1038/371151a0. [DOI] [PubMed] [Google Scholar]
- 143.Xiao Y, Zych A, Felleman DJ. Segregation and convergence of functionally defined V2 thin stripe and interstripe compartment projections to area V4 of macaques. Cereb Cortex. 1999;9:792–804. doi: 10.1093/cercor/9.8.792. [DOI] [PubMed] [Google Scholar]
- 144.Zeki S, Shipp S. Modular Connections between Areas V2 and V4 of Macaque Monkey Visual Cortex. Eur J Neurosci. 1989;1:494–506. doi: 10.1111/j.1460-9568.1989.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 145.Gegenfurtner KR, Kiper DC, Levitt JB. Functional properties of neurons in macaque area V3. J Neurophysiol. 1997;77:1906–1923. doi: 10.1152/jn.1997.77.4.1906. [DOI] [PubMed] [Google Scholar]
- 146.Lagae L, Gulyas B, Raiguel S, Orban GA. Laminar analysis of motion information processing in macaque V5. Brain Res. 1989;496:361–367. doi: 10.1016/0006-8993(89)91089-5. [DOI] [PubMed] [Google Scholar]
- 147.Berezovskii VK, Born RT. Specificity of projections from wide-field and local motion-processing regions within the middle temporal visual area of the owl monkey. J Neurosci. 2000;20:1157–1169. doi: 10.1523/JNEUROSCI.20-03-01157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 149.Tan EM, et al. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 150.Wickersham IR, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 152.Zhang F, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 153.Blackshaw SE, Nicholls JG, Parnas I. Expanded receptive fields of cutaneous mechanoreceptor cells after single neurone deletion in leech central nervous system. J Physiol. 1982;326:261–268. doi: 10.1113/jphysiol.1982.sp014190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grueber WB, Graubard K, Truman JW. Tiling of the body wall by multidendritic sensory neurons in Manduca sexta. J Comp Neurol. 2001;440:271–283. doi: 10.1002/cne.1385. [DOI] [PubMed] [Google Scholar]
- 155.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 156.Ma M, Shepherd GM. Functional mosaic organization of mouse olfactory receptor neurons. Proc Natl Acad Sci U S A. 2000;97:12869–12874. doi: 10.1073/pnas.220301797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- 158.Schreiner CE, Read HL, Sutter ML. Modular organization of frequency integration in primary auditory cortex. Annu Rev Neurosci. 2000;23:501–529. doi: 10.1146/annurev.neuro.23.1.501. [DOI] [PubMed] [Google Scholar]
- 159.Sur M, Wall JT, Kaas JH. Modular segregation of functional cell classes within the postcentral somatosensory cortex of monkeys. Science. 1981;212:1059–1061. doi: 10.1126/science.7233199. [DOI] [PubMed] [Google Scholar]
- 160.Yu J, et al. Local-Circuit Phenotypes of Layer 5 Neurons in Motor-Frontal Cortex of YFP-H Mice. Front Neural Circuits. 2008;2:6. doi: 10.3389/neuro.04.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barbour DL, Callaway EM. Excitatory local connections of superficial neurons in rat auditory cortex. J Neurosci. 2008;28:11174–11185. doi: 10.1523/JNEUROSCI.2093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 2006;4:e382. doi: 10.1371/journal.pbio.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu X, Callaway EM. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci. 2009;29:70–85. doi: 10.1523/JNEUROSCI.4104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Belin P, Zatorre RJ. ‘What’, ‘where’ and ‘how’ in auditory cortex. Nat Neurosci. 2000;3:965–966. doi: 10.1038/79890. [DOI] [PubMed] [Google Scholar]
- 165.Dijkerman HC, de Haan EH. Somatosensory processes subserving perception and action. Behav Brain Sci. 2007;30:189–201. doi: 10.1017/S0140525X07001392. discussion 201–39. [DOI] [PubMed] [Google Scholar]
- 166.Kaas JH, Hackett TA. ‘What’ and ‘where’ processing in auditory cortex. Nat Neurosci. 1999;2:1045–1047. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- 167.Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 168.Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]