Abstract
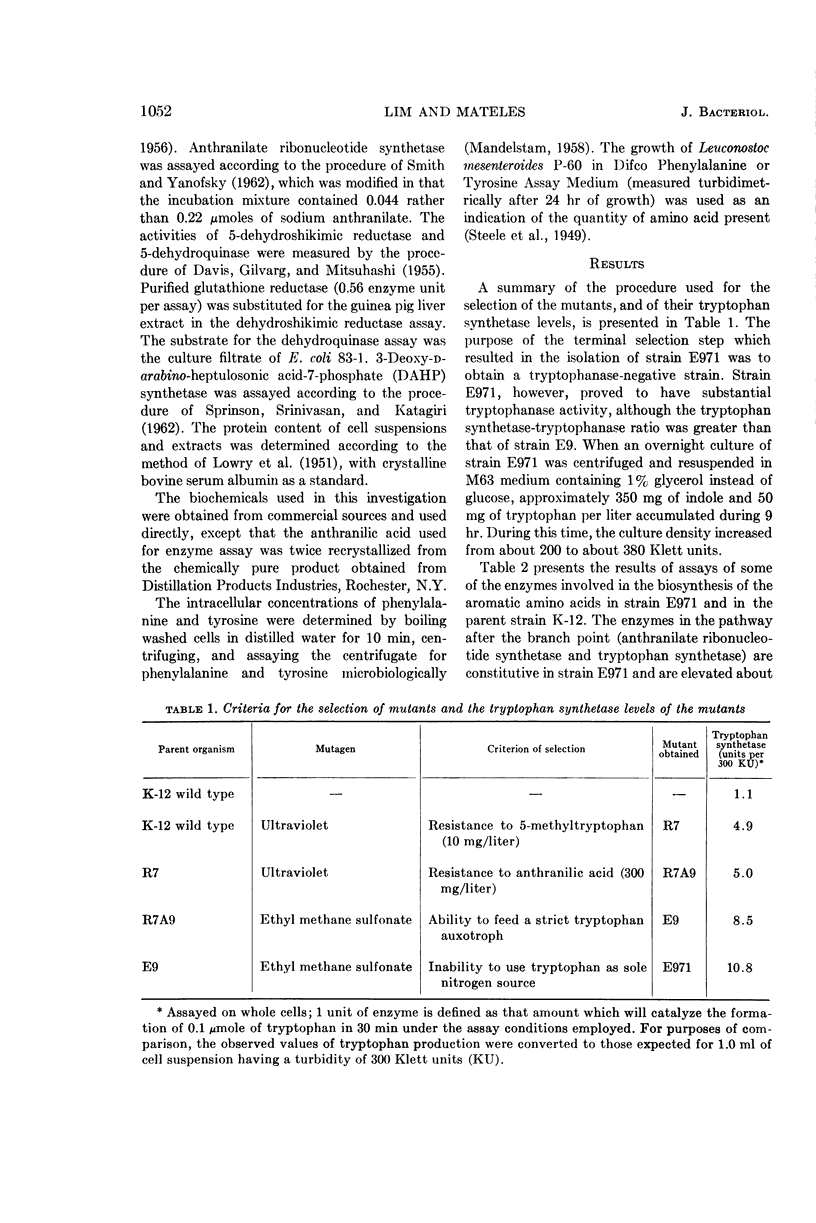
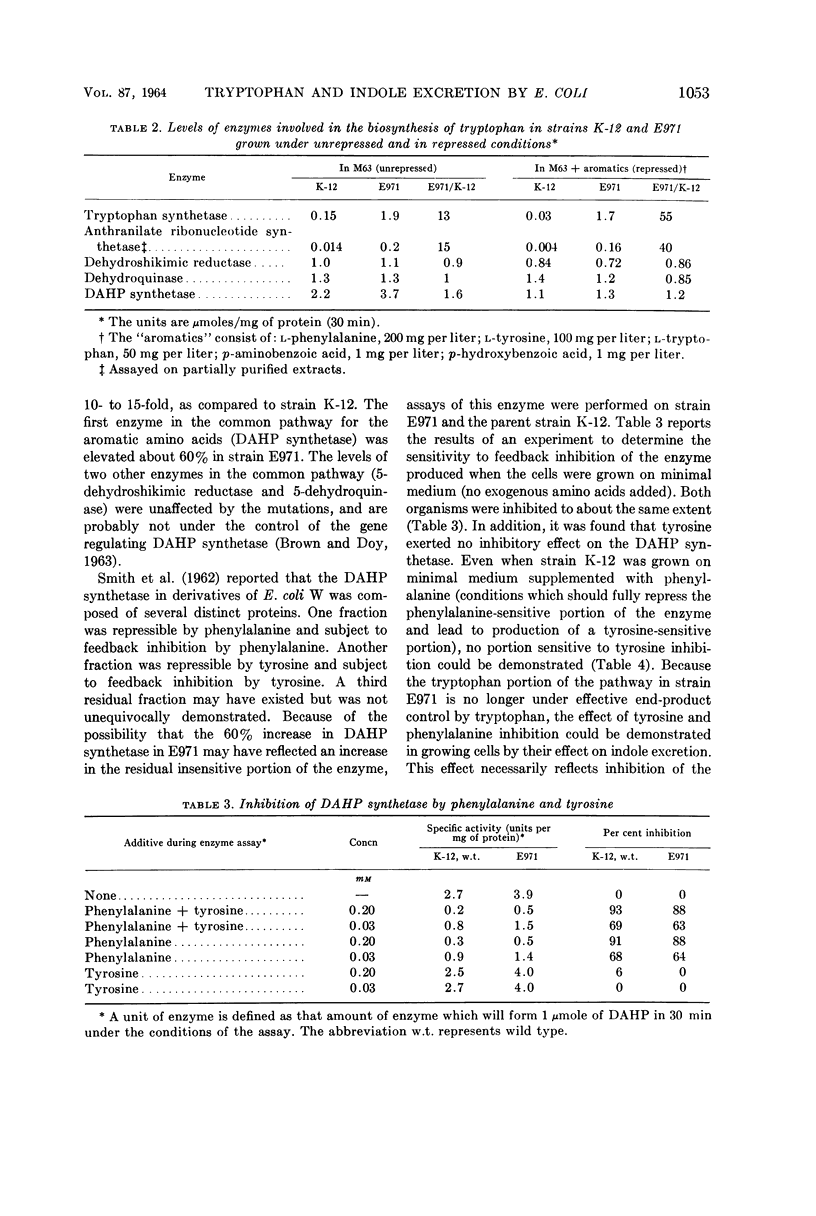
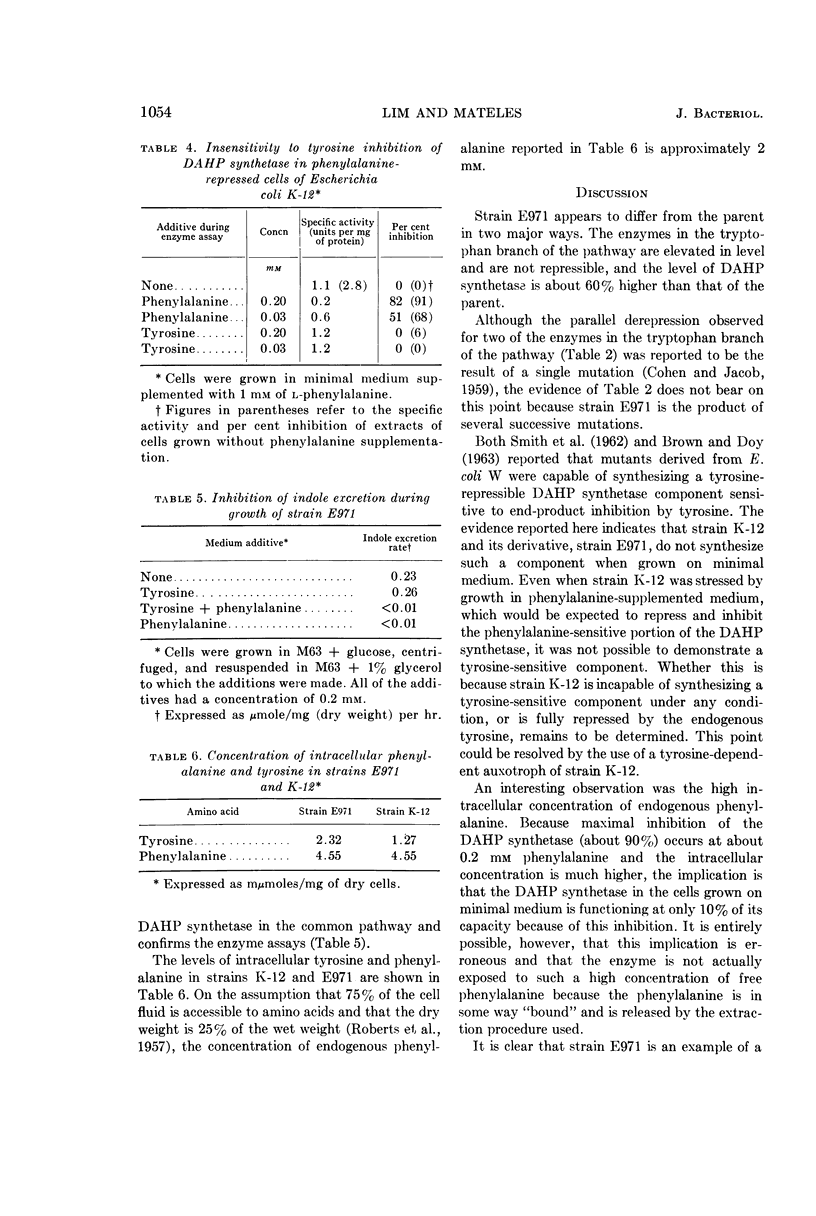
Lim, P. G. (Massachusetts Institute of Technology, Cambridge), and R. I. Mateles. Tryptophan- and indole-excreting prototrophic mutant of Escherichia coli. J. Bacteriol. 87:1051–1055. 1964.—A mutant of Escherichia coli K-12, capable of excreting 350 mg of indole and 50 mg of tryptophan per liter when grown on minimal medium, was found to have a level of 3-deoxy-d-arabino-heptulosonic acid-7-phosphate (DAHP) synthetase 60% higher than the parent, and to have a 10- to 15-fold elevation of the levels of enzymes in the tryptophan branch of the pathway for aromatic amino acid biosynthesis. Contrary to what previous investigators found in E. coli W, the presence of a tyrosine-repressible component of DAHP synthetase sensitive to end-product inhibition by tyrosine could not be demonstrated in either strain K-12 or the mutant. The mutant strain is an example of a microorganism which excretes biosynthetic end products solely because of genetic derepression, as opposed to most previously reported amino acid accumulators which require a combination of genetic and physiological manipulation to achieve derepression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN K. D., DOY C. H. END-PRODUCT REGULATION OF THE GENERAL AROMATIC-PATHWAY IN ESCHERICHIA COLI W. Biochim Biophys Acta. 1963 Sep 3;77:170–172. doi: 10.1016/0006-3002(63)90489-x. [DOI] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- DICKMAN S. R., CROCKETT A. L. Reactions of xanthydrol. IV. Determination of tryptophan in blood plasma and in proteins. J Biol Chem. 1956 Jun;220(2):957–965. [PubMed] [Google Scholar]
- HUANG H. T. Accumulation of 1-homolanthionine by an Escherichia coli mutant. Biochemistry. 1963 Mar-Apr;2:296–298. doi: 10.1021/bi00902a018. [DOI] [PubMed] [Google Scholar]
- HUANG H. T. Production of L-threonine by auxotrophic mutants of Escherichia coli. Appl Microbiol. 1961 Sep;9:419–424. doi: 10.1128/am.9.5.419-424.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G., YANOFSKY C. Influence of 3-methylanthranilic and anthranilic acids on the formation of tryptophan synthetase in Escherichia coli. J Bacteriol. 1961 Jan;81:81–90. doi: 10.1128/jb.81.1.81-90.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIM P. G., MATELES R. I. Tryptophan- and indole-excreting bacterial mutants. Science. 1963 Apr 26;140(3565):388–389. doi: 10.1126/science.140.3565.388. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELSTAM J. The free amino acids in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):103–110. doi: 10.1042/bj0690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIIO I., OTSUKA S. I., KATSUYA N. Cellular permeability and extracellular formation of glutamic acid in Brevibacterium flavum. J Biochem. 1963 May;53:333–340. doi: 10.1093/oxfordjournals.jbchem.a127706. [DOI] [PubMed] [Google Scholar]
- SMITH L. C., RAVEL J. M., LAX S. R., SHIVE W. The control of 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthesis by phenylalanine and tyrosine. J Biol Chem. 1962 Nov;237:3566–3570. [PubMed] [Google Scholar]
- VELDKAMP H., van den BERG, ZEVENHUIZEN L. P. Glutamic acid production by Arthrobacter globiformis. Antonie Van Leeuwenhoek. 1963;29:35–51. doi: 10.1007/BF02046037. [DOI] [PubMed] [Google Scholar]



