Abstract
A retrospective review of pediatric lung transplant recipients at fourteen sites in North America and Europe was conducted to evaluate the impact of adding cytomegalovirus immunoglobulin (CMVIG) prophylaxis to at least three weeks of intravenous ganciclovir in pediatric lung transplant recipients. Data were recorded for the first year after transplantation. Associations between time to CMV and risk factors, including CMVIG use, were assessed by multivariable Cox proportional hazards models. Of 599 subjects whose charts were reviewed, 329 received at least three weeks of ganciclovir with 62 (19%) receiving CMVIG. CMVIG was administered more frequently with CMV donor-positive/recipient-negative (D+/R−) serostatus (p<0.05). In multivariable models, subjects who did not receive CMVIG as part of their prophylaxis were three times more likely to develop CMV infection (HR 3.4; 95% CI 1.2, 9.5) independent of CMV serostatus. However, CMVIG administration was not associated with decreased risk of episodes of CMV disease. Receipt of CMVIG was not associated with decreased risks of post-transplant morbidities (acute rejection, respiratory viral infection or early bronchiolitis obliterans) or morbidity within the first year after pediatric lung transplantation. The use of CMVIG in addition to antiviral prophylaxis in pediatric lung transplantation requires further evaluation in pediatric lung transplant recipients.
Keywords: Lung transplantation, Cytomegalovirus, Cytomegalovirus hyperimmune globulin, Pediatrics
Introduction
Lung transplant recipients experience significant morbidity and mortality, often due to adverse events of infectious origin, with up to 90% experiencing an infectious complication. [1, 2] Cytomegalovirus (CMV) is a common cause of infection and has been associated with morbidity including chronic allograft rejection in transplant recipients. [3–6] Despite the availability of newer antimicrobial agents, the incidence of CMV infection has not declined substantially over the past decade.[5] In addition, only recently has the long-term survival in pediatric lung transplant recipients improved overall, although it still remains considerably worse compared to other organ transplantation. [7, 8]
Strategies to prevent CMV infection and disease have evolved over time; however, the optimal preventative tactic is uncertain. Data from adult centers indicate that CMV hyperimmune globulin (CMVIG) and ganciclovir, in combination, are superior in preventing CMV viremia compared to ganciclovir alone. [9] Prophylactic CMVIG has decreased the rates of both CMV disease and CMV pneumonitis rates by 50%, leading to a concomitant decrease in the average length of hospital stay for liver transplant recipients.[10] In addition, a recent meta-analysis of CMVIG use in solid organ transplant recipients demonstrated a lower incidence of CMV-associated death and clinically significant disease in subjects who received CMVIG-based CMV prophylaxis [11]. However, other studies have found no significant impact of CMVIG on the incidence of CMV disease after liver transplant [12]. To date, a comprehensive evaluation of CMVIG use in pediatric lung transplant recipients has not been performed. The goal of this multi-center cohort study was to evaluate the impact of CMVIG, when added to at least three weeks of ganciclovir prophylaxis, on CMV infection and disease in pediatric lung transplant recipients.
Methods
Fourteen pediatric lung transplant centers in North America and Europe, who are members of the International Pediatric Lung Transplant Collaborative, performed a multi-center retrospective cohort study. The population included all subjects younger than 21 years of age who received a primary lung or heart-lung transplant at any of the centers between January 1988 and the time of data collection (August 2004–January 2007), survived at least two weeks after surgery, and had data available from the date of transplantation to one year post-transplant or until death or retransplantation, whichever occurred first. Chart reviews, performed by a single investigator, examined demographic data, antiviral agents, and CMV episodes. Identification of CMV episodes depended upon the diagnostic methodology available at each individual institution, and included demonstration of antigenemia, positive viral culture or positive polymerase chain reaction (PCR).
Definitions
Post transplant CMV infection definitions are adapted from those proposed by the American Society of Transplantation Infectious Disease Working Group on Infectious Diseases Monitoring and Ljungman et al. [13, 14]
CMV infection
Presence of active replicating virus without associated symptoms or signs of illness. This could represent new infection or reactivation of previously latent virus. Viremia was determined by the standard method used at each institution: conventional viral culture, shell vial viral culture, pp65 antigenemia testing or CMV polymerase chain reaction (PCR) in whole blood or peripheral blood mononuclear cells
“CMV disease” is a combination of CMV syndrome and proven, probable and possible CMV disease as defined by [13]:
CMV syndrome: Constellation of symptoms consisting of fever, fatigue, thrombocytopenia and/or leukopenia in association with evidence of CMV infection and without alternate explanations.
Proven CMV invasive disease: Histological demonstration of CMV inclusions in biopsy tissue from lung, liver, or other body site, with associated clinical signs and symptoms.
Probable CMV disease: Clinically compatible symptoms, evidence of CMV infection without concurrent infections or rejection, but without definitive histopathology on biopsy tissue.
Possible CMV Disease: Clinically compatible symptoms, evidence of CMV infection, but occurring in the presence of concurrent infections or rejection.
Other post transplant outcomes
Post transplant outcome measures including acute rejection (AR), bronchiolitis obliterans syndrome (BOS), and bronchiolitis obliterans (BO), based on definitions proposed by working groups from the International Society for Heart and Lung Transplantation (ISHLT). [15–18] Diagnosis and grading of AR was performed at each individual center without a central pathology review. Post-transplant lymphoproliferative disease (PTLD) was diagnosed by histologic examination of tissue biopsy [19].
Statistical analyses
Data were entered into an ORACLE database, analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC), and graphics were produced using R version 2.0.1 software (The R Foundation for Statistical Computing, Vienna, Austria). Associations between CMVIG and continuous and categorical risk factors were assessed using Wilcoxon rank-sum tests and Chi-square and Fisher’s exact tests, respectively. Associations between CMVIG and time to first CMV episode, and between CMVIG and time to post-transplant morbidities and mortality, were assessed by univariable and multivariable Cox proportional hazards models, censored at death, re-transplant, or one year post-transplant. CMV infection was considered separately from CMV syndrome/disease (designated CMV disease), and subjects with CMV disease without prior infection were excluded from the analysis of CMV infection. For each proportional hazards model, the proportional hazards assumption was assessed by entering risk-factor-by-time interactions into the model; this assumption was also assessed graphically using log-log-survival plots. Events that occurred during post-transplant follow-up, such as pulmonary fungal infection (PFI), rejection and cessation of post-transplant prophylaxis were modeled as time-dependent covariates. The functional form for age and era of transplant was chosen by modeling the quintiles of these variables as categorical variables and assessing the resulting parameter estimates. Multivariable models were chosen by performing backwards selection, with a significance criterion of 0.05, on initial models containing all risk factors while forcing CMVIG into the model. Interactions suggested by the data or by clinical importance were included in the model selection process. All tests were two-tailed and performed at a significance level of 0.05.
Results
Patient demographics
Of the 599 pediatric lung transplant recipients enrolled in the study, 329 received at least three weeks of ganciclovir prophylaxis and were included in this analysis. Recipients ranged in age from 49 days to 20.9 years (median 13.1 years) and 189 (57%) were female. Indications for lung transplantation included cystic fibrosis (55%), idiopathic pulmonary hypertension (10%), complex congenital heart disease with pulmonary hypertension (14%), bronchiolitis obliterans (3%) and other diagnoses (18%).
CMVIG administration
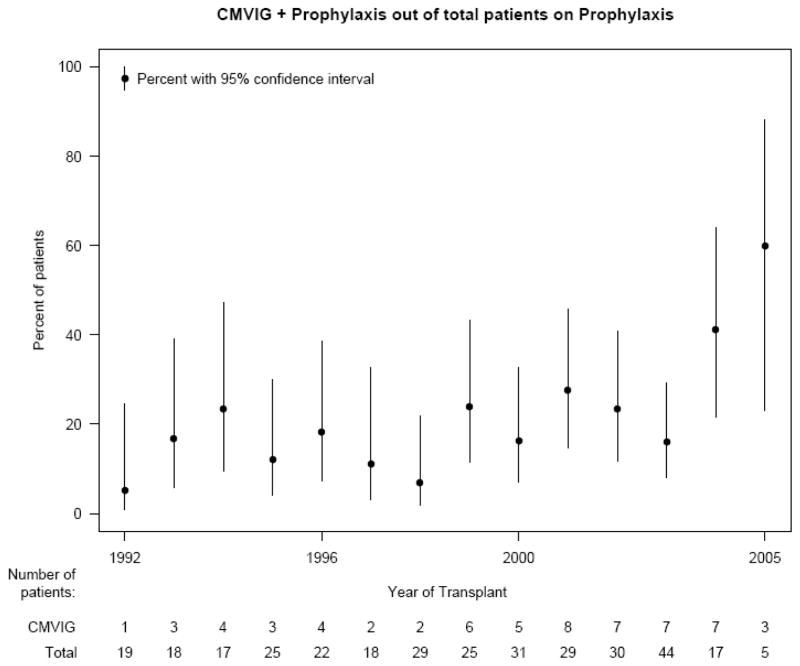
CMVIG was administered to 62 (19%) subjects, who did not differ significantly from those who did not receive CMVIG, except for CMV donor-recipient serostatus and race (Table 1). Dosing regimens for CMVIG varied widely. Subjects received a mean of 5 doses of CMVIG for prophylaxis (range 1–12). Dosing intervals ranged from 1 day to 1 month, most commonly every two weeks. Median duration of therapy after transplant was 84 days (range 1–192) and median dose administered each time was 150 mg/kg (mean 133 mg/kg). No significant complications of CMVIG administration were reported in the medical records. Furthermore, duration of antiviral prophylaxis other than CMVIG was longer for patients administered CMVIG compared to those patients taking prophylactic ganciclovir alone (p = 0.025, Table 1). The administration of CMVIG was not significantly associated with gender, underlying etiology for transplant, or age (p>.05). Subjects with CMV D+/R− were more likely to receive CMVIG. The proportion of subjects receiving prophylactic CMVIG increased between the early 1990s and after 2000 (Figure 1).
Table 1.
Demographics for subjects with and without CMVIG administration
| CMVIG | No CMVIG | ||||
|---|---|---|---|---|---|
| N | Median (P25, P75) | N | Median (P25, P75) | P value | |
| Age at transplant (years) | 61 | 14.0 (10.8, 16.4) | 267 | 12.8 (8.5, 16.2) | 0.098* |
| Months of antiviral prophylaxis | 62 | 2.9 (1.9, 4.3) | 267 | 1.6 (1.4, 3.6) | 0.025* |
| N | (%) | N | (%) | P value | |
| Female | 36 | 58 | 153 | 57 | 0.91 |
| Caucasian/White | 59 | 95 | 228 | 86 | 0.043 |
| Cystic fibrosis | 38 | 61 | 142 | 53 | 0.26 |
| Donor and recipient CMV status | 0.039 | ||||
| Donor +/Recipient + | 10 | 16 | 67 | 26 | |
| Donor +/Recipient − | 33 | 54 | 100 | 39 | |
| Donor −/Recipient + | 5 | 8 | 48 | 19 | |
| Donor −/Recipient − | 13 | 21 | 44 | 17 | |
| Initial immunosuppression regimen: mycophenolate | 19 | 31 | 53 | 20 | 0.064 |
Wilcoxon Rank Sum test
Figure 1.
Proportion of subjects receiving CMVIG by year of transplantation
Incidence of CMV episodes
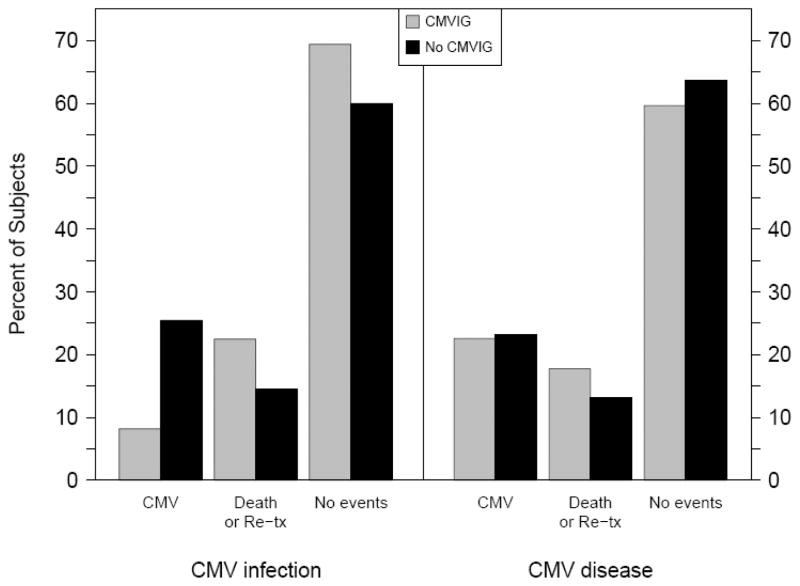
CMV episodes, including infection and disease as defined above, were common after pediatric lung transplant with an incidence in the first year of 36% (120 of 329 subjects). The 120 subjects developed 155 episodes (range 1–4 episodes) within the first year after transplantation. Of 62 patients who received CMVIG, 14 (23%) developed CMV disease compared to 62 (23.2%) of the 267 who did not receive CMVIG. Excluding subjects who developed CMV disease without prior infection, four (8%) subjects who received CMVIG developed CMV infection, compared to 56 (27%) who did not receive CMVIG, Table 2 and Figure 2. The mean and median times to first CMV infection were 125 and 86 days respectively. The difference in time to first infection in subjects with and without CMVIG was not statistically significant (85 days and 87 days respectively). In addition, time to first episode of CMV disease with and without CMVIG occurred at a median of 122 days (mean 137 days) and 96 days (mean 118 days) (p > 0.05).
Table 2.
Outcome by one year post-transplant for subjects with and without CMVIG administration by category of CMV episode
| Status at 1 year post-transplant | CMV infection1, N (%) | CMV disease, N (%) | ||
|---|---|---|---|---|
| No CMVIG | CMVIG | No CMVIG | CMVIG | |
| CMV episode | 56 (25) | 4 (8) | 62 (23) | 14 (23) |
| Retransplant or Death without prior CMV | 32 (15) | 11 (22) | 35 (13) | 11 (18) |
| No Events (CMV, Death, or Retransplant) | 132 (60) | 34 (69) | 170 (64) | 37 (60) |
| Total | 220 | 49 | 267 | 62 |
CMV infection, excluding those with CMV disease without prior infection
Figure 2.
Outcome including any CMV episodes, death or retransplantation, or no events before one year post-transplant categorized by the receipt of CMVIG and type of CMV episode. Reported as percentages of the total in each group A: CMV infection (excluding those with CMV disease without prior infection) (CMVIG n=49; no CMVIG n=220) B: CMV disease (CMVIG n=62, no CMVIG n=267)
Risk of CMV by type of CMV episode
Risk factors for CMV episode were assessed for both CMV infection and CMV disease, Table 3. Univariable risk factors for CMV infection included not receiving CMVIG (HR 3.3; 95% CI 1.2–9.0), donor or recipient CMV seropositivity at transplant (HR 2.8; 95% CI 1.5–5.2 and 2.2; 95% CI 1.3–3.6, respectively), and initial treatment at hospital discharge with cyclosporine (HR 2.1; 1.09–3.9). For CMV disease, donor CMV seropositivity (HR 3.8; 95% CI 2.0–7.5), earlier era of transplant (p= 0.01) and transplant type (p=0.039) were risks. CMVIG administration was not protective against CMV disease. Age at transplant, induction therapy, underlying etiology for transplant, gender, prior acute rejection and prior fungal or viral infection were not associated with increased risk of CMV infection or CMV disease.
Table 3.
Univariable hazard ratios for time to first CMV episode by type of episode (CMV infection or CMV disease)
| Risk factor | CMV infection1 | CMV disease | |||||
|---|---|---|---|---|---|---|---|
| N | Hazard ratio (95% CI) | P-value | N | Hazard ratio (95% CI) | P-value | ||
| No CMVIG | 269 | 3.3 (1.2, 9.0) | 0.022 | 329 | 1.01 (0.57, 1.8) | 0.97 | |
| Donor and recipient | 264 | 0.002 | 320 | 0.002 | |||
| CMV status | |||||||
| Donor −Recipient− | Reference | Reference | |||||
| Donor+Recipient+ | 12.5 (3.0, 52.7) | 5.4 (1.6, 18.2) | |||||
| Donor+Recipient− | 7.0 (1.6, 29.7) | 6.9 (2.1, 22.2) | |||||
| Donor−Recipient+ | 6.3 (1.4, 28.6) | 2.4 (0.63, 9.4) | |||||
| Transplant type | 269 | 0.42 | 329 | 0.039 | |||
| Heart and lung | Reference | Reference | |||||
| Tx type Single | 3.8 (0.48, 29.9) | 4.5 (1.4, 14.3) | |||||
| Tx type Double/Other | 1.3 (0.64, 2.7) | 1.4 (0.72, 2.7) | |||||
| Era of transplant | 269 | 0.14 | 329 | 0.010 | |||
| Year 2003–2005 | Reference | Reference | |||||
| Year 1992–1994 | 1.5 (0.73, 3.2) | 4.3 (1.8, 10.3) | |||||
| Year 1995–1997 | 0.71 (0.33, 1.5) | 2.2 (0.90, 5.5) | |||||
| Year 1998–1999 | 0.54 (0.21, 1.4) | 3.4 (1.4, 8.4) | |||||
| Year 2000–2003 | 0.68 (0.33, 1.4) | 2.3 (0.97, 5.4) | |||||
| Female gender | 269 | 1.3 (0.76, 2.2) | 0.34 | 329 | 1.2 (0.77, 1.9) | 0.40 | |
| Age at transplant | 268 | 0.88 | 328 | 0.11 | |||
| Age 0– 7.5 yrs | Reference | Reference | |||||
| Age 7.6–12.1 yrs | 1.5 (0.66, 3.4) | 1.9 (0.79, 4.4) | |||||
| Age 12.1–14.3 yrs | 1.3 (0.58, 3.0) | 2.0 (0.87, 4.7) | |||||
| Age 14.3–17.0 yrs | 1.4 (0.62, 3.2) | 1.6 (0.67, 3.8) | |||||
| Age 17.0–21.0 yrs | 1.2 (0.47, 2.8) | 2.9 (1.3, 6.5) | |||||
| Cystic Fibrosis etiology | 268 | 1.07 (0.64, 1.8) | 0.79 | 328 | 1.01 (0.64, 1.6) | 0.96 | |
| Donor CMV positive | 267 | 2.8 (1.5, 5.2) | 0.002 | 325 | 3.8 (2.0, 7.5) | <0.001 | |
| Recipient CMV positive | 266 | 2.2 (1.3, 3.6) | 0.004 | 324 | 0.85 (0.53, 1.4) | 0.49 | |
| Any induction treatment | 251 | 1.2 (0.73, 2.1) | 0.43 | 304 | 1.2 (0.72, 1.9) | 0.53 | |
| Immunosuppressive regimen: cyclosporine | 269 | 2.1 (1.09, 3.9) | 0.026 | 329 | 1.10 (0.68, 1.8) | 0.71 | |
| A2 rejection prior to CMV | 269 | 1.1 (0.68, 1.9) | 0.62 | 329 | 1.00 (0.64, 1.6) | 0.99 | |
| BOS prior to CMV | 269 | 0.20 (0.05, 0.84) | 0.027 | 329 | 0.19 (0.05, 0.76) | 0.019 | |
CMV infection, excluding those with CMV disease without prior infection
Multivariable models were explored to include the risks reported in the univariable models as well as common demographic variables. For CMV infection, independent risks in the multivariable model include not receiving CMVIG (HR 3.4; 95% CI 1.2–9.5), CMV donor/recipient status other than CMV D−/R−, and transplant in the earliest era (1992–1994; HR 2.1; 1.1–3.8), Table 4. Increased risk for CMV disease was associated with receipt of a single lung transplant (HR 5.3; 95% CI1.8–15.3), receipt of any induction therapy (HR 1.8; 95% CI 1.1–2.9), donor CMV seropositivity and transplant before 2002 (HR 5.5; 95% CI 1.9–15.5). CMVIG administration was associated with decreased risk of CMV infection but not CMV disease in this cohort.
Table 4.
Multivariable risks for CMV infection or CMV disease
| Multivariable risks for CMV INFECTION (59/264 with CMV INFECTION) | ||
|---|---|---|
| Risk factor | HR (95% CI) | P-value |
| No CMVIG | 3.4 (1.2, 9.5) | 0.022 |
| CMV status, vs. D−R− | ||
| D+R+ | 12.3 (2.9, 52.0) | <0.001 |
| D+R− | 9.2 (2.1, 39.2) | 0.003 |
| D−R+ | 8.6 (1.8, 39.8) | 0.006 |
| Transplant 1992–1994 versus 2003–2005 | 2.1 (1.1, 3.8) | 0.022 |
| Prophylaxis completed before CMV infection | 0.32 (0.18, 0.56) | <0.001 |
| Multivariable risks for CMV DISEASE (74/325 with CMV DISEASE) | ||
| Risk factor | HR (95% CI) | P-value |
| No CMVIG | 1.4 (0.70, 2.6) | 0.37 |
| CMV status, verses D−R− | ||
| D+R+ | 4.9 (1.4, 16.9) | 0.012 |
| D+R− | 6.0 (1.8, 19.8) | 0.003 |
| D−R+ | 2.0 (0.51, 8.0) | 0.32 |
| Single Lung Transplant versus all others | 5.3 (1.8, 15.3) | 0.002 |
| Any induction therapy | 1.8 (1.07, 2.9) | 0.025 |
| Transplant 1992–2002 versus 2003–2005 | 5.5 (1.9, 15.5) | 0.001 |
| Prophylaxis completed before CMV disease | 0.52 (0.30, 0.90) | 0.021 |
Risk of transplant morbidity and mortality with CMVIG
The administration of CMVIG did not significantly affect morbidity. In a multivariable model, receipt of CMVIG was not associated with morbidities including acute rejection, respiratory viral infections, post-transplant lymphoproliferative disease, or bronchiolitis obliterans. CMVIG administration was not associated with a decrease in the risk for death or retransplantation within the first post-transplant year.
Discussion
The impact of CMV after transplantation is well recognized; however, the optimal regimens to prevent CMV infection and disease, as well as their direct and indirect effects, remain difficult to identify. In pediatric lung transplant, the impact of adding CMVIG to standard antiviral prophylaxis has not been previously explored. The risks for CMV infection and disease in this cohort are similar to those reported previously in both the adult and pediatric literature, with increased risk during the early transplant era and with donor/recipient CMV seropositivity [5, 20–23].
Similar to previously reported assessments of risks, the data collected in this study indicate that ganciclovir prophylaxis along with CMVIG as adjunct therapy was associated with a decreased risk of CMV infection but not CMV disease within the first year after pediatric lung transplantation. In addition, CMVIG did not appear to impact occurrence of acute rejection, BOS, or survival. Results reported from adult lung transplant recipients have been variable with decreased CMV infection and/or disease without a control group or with historical controls [24, 25]. Alternatively, some reports in adult liver transplant recipients have demonstrated that administration of CMVIG resulted in decreased episodes of tissue-invasive CMV disease [10]. Additional studies in liver transplant recipients from the same group reported that the addition of ganciclovir to CMVIG is superior to CMVIG alone and is cost effective [26, 27]. Similarly, in adult heart transplant patients, CMVIG alone can decrease the risk of CMV disease [28]; further, the prophylactic administration of CMVIG with ganciclovir significantly reduced rates of CMV disease in comparison to rates in those who received ganciclovir alone[9, 20]. The difference between the results in the pediatric lung transplant population, which does not have a decrease in CMV disease but does have a decrease in CMV infection, may be related to the definitions applied in this study compared to those previously reported studies as many were performed prior to the development of the currently accepted definitions that were used in this study.
More recent analyses indicate that CMVIG administration decreases CMV-related morbidity and mortality [3, 11]. The benefit conferred may be due either to a correction of hypogammaglobulinemia or directly from CMV prevention or amelioration, but this has not been investigated [29]. In this cohort, CMVIG administration was not associated with decreased CMV-related morbidity or mortality. The observational nature of this cohort including a lack of uniformity for the schedule and dosage of CMVIG prevent drawing definitive conclusions.
As with any retrospective cohort, limitations regarding the available data exist. In this study, information regarding immunoglobulin levels was not recorded; however at the time of data collection, no center routinely collected serial measurement of immunoglobulin levels in their patients for the duration of the study period. As with previously reported data from this cohort, the definitions for risks and outcomes applied were determined prior to the initiation of data collection and were applied uniformly across all participating sites.
As reported above, the participating centers did not use a standard dosing schedule for CMVIG. Possible explanations for the wide range of dosing schedules include duration of the study period, variability in clinician preference and the lack of centrally available standardized dosing regimens. However, details of CMVIG administration, including date of infusion and dosing regimens, were captured through substantive review of pharmacy records and clinical charts. Descriptive information regarding the administration of CMVIG in the 62 pediatric lung transplant recipients who received the therapy is presented for inference. In addition, with the follow-up ending at one year post-transplant, associations with remote outcomes including BOS may be underestimated by this analysis.
The impact of concurrent ganciclovir administration also requires comment. To limit the complexity of the analysis, subjects who received less than three weeks or no ganciclovir prophylaxis were excluded from the analysis. In addition, subjects who received CMVIG also received a slightly longer average duration of ganciclovir which was statistically significant (2.9 months verses 1.6 months). However, the addition of prophylaxis duration to the multivariable models did not significantly alter the models.
Conclusions
The optimal preventative regimen for CMV after pediatric lung transplantation remains elusive. However, CMV donor and recipient serostatus is a non-modifiable risk factor that clinicians can use to guide their concern for CMV infection and disease. The adjunct administration of CMVIG in this population is associated with decreased risk of CMV infections but not disease in patients who receive at least three weeks of ganciclovir. While the schedule and dosing of CMVIG administration was not uniform in this cohort, further prospective investigation into the use of CMVIG as an adjunct to ganciclovir prophylaxis in pediatric lung transplant recipients is warranted.
Acknowledgments
Supported by Thrasher Research Fund, National Institutes of Health/K23 RR022956 (LDI), CSL Behring (previously MedImmune, Inc. with unrestricted research grant funding)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kramer MR, Marshall SE, Starnes VA, et al. Infectious complications in heart-lung transplant. Archives of Internal Medicine. 1993;153:2010–2016. [PubMed] [Google Scholar]
- 2.Kanj SS, Tapson V, Davis DR, et al. Infections in Patients with Cystic Fibrosis following Lung Transplantation. Chest. 1997;112:924–930. doi: 10.1378/chest.112.4.924. [DOI] [PubMed] [Google Scholar]
- 3.Ruttmann E, Geltner C, Bucher B, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81:1415–1420. doi: 10.1097/01.tp.0000209439.27719.ed. [DOI] [PubMed] [Google Scholar]
- 4.Chmiel C, Speich R, Hofer M, et al. Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clin Infect Dis. 2008;46:831–839. doi: 10.1086/528689. [DOI] [PubMed] [Google Scholar]
- 5.Danziger-Isakov LA, Delamorena M, Hayashi RJ, et al. Cytomegalovirus viremia associated with death or retransplantation in pediatric lung transplant recipients. Transplantation. 2003;75:1538–1543. doi: 10.1097/01.TP.0000061607.07985.BD. [DOI] [PubMed] [Google Scholar]
- 6.Valentine VG, Weill D, Gupta MR, et al. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. J Heart Lung Transplant. 2008;27:875–881. doi: 10.1016/j.healun.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Aurora P, Boucek MM, Christie J, et al. Registry of the International Society for Heart and Lung Transplantation: tenth official pediatric lung and heart/lung transplantation report--2007. J Heart Lung Transplant. 2007;26:1223–1228. doi: 10.1016/j.healun.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Aurora P, Edwards LB, Christie J, et al. Registry of the International Society for Heart and Lung Transplantation: eleventh official pediatric lung and heart/lung transplantation report--2008. J Heart Lung Transplant. 2008;27:978–983. doi: 10.1016/j.healun.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Valantine HA, Luikart H, Doyle R, et al. Impact of Cytomegalovirus Hyperimmune Globulin on Outcome after Cardiothoracic Transplantation. Transplantation. 2001;72:1647–1652. doi: 10.1097/00007890-200111270-00012. [DOI] [PubMed] [Google Scholar]
- 10.Snydman DR, Werner BG, Dougherty NN, et al. Cytomegalovirus immune globulin prophylaxis in liver transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1993;119:984–991. doi: 10.7326/0003-4819-119-10-199311150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bonaros N, Mayer B, Schachner T, et al. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: a meta-analysis. Clin Transplant. 2008;22:89–97. doi: 10.1111/j.1399-0012.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- 12.Hodson EM, Craig JC, Strippoli GF, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2008:CD003774. doi: 10.1002/14651858.CD003774.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Humar A, Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 14.Ljungman P, Griffiths P, Paya C. Definition of Cytomegalovirus Infection and Disease in Transplant Recipients. Clinical Infectious Diseases. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of the nomenclature and for clinical staging of chronic dysfunction in lung allografts. Journal of Heart and Lung Transplantation. 1993;12:713–716. [PubMed] [Google Scholar]
- 16.Yousem SA, Berry G, Brunt E, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection. Journal of Heart Transplantation. 1990;9:593–601. [PubMed] [Google Scholar]
- 17.Yousem SA, et al. Revision of the 1990 working formulation of the classification of pulmonary allograft rejection: Lung rejection study group. Journal of Heart and Lung Transplantation. 1996;15:1–15. [PubMed] [Google Scholar]
- 18.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi RJ, Kraus MD, Patel AL, et al. Posttransplant lymphoproliferative disease in children: Correlation of histology to clinical behavior. Journal of Pediatric Hematology/Oncology. 2001;23:7–9. doi: 10.1097/00043426-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Opelz G, Dohler B, Ruhenstroth A. Cytomegalovirus prophylaxis and graft outcome in solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:928–936. doi: 10.1111/j.1600-6143.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 21.Kranz B, Vester U, Wingen AM, et al. Acute rejection episodes in pediatric renal transplant recipients with cytomegalovirus infection. Pediatr Transplant. 2008;12:474–478. doi: 10.1111/j.1399-3046.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuypers DR, Evenepoel P, Maes BD, et al. Role of immunosuppressive drugs in the development of tissue-invasive cytomegalovirus infection in renal transplant recipients. Transplant Proc. 2002;34:1164–1170. doi: 10.1016/s0041-1345(02)02812-9. [DOI] [PubMed] [Google Scholar]
- 23.Kamar N, Mengelle C, Esposito L, et al. Predictive factors for cytomegalovirus reactivation in cytomegalovirus-seropositive kidney-transplant patients. J Med Virol. 2008;80:1012–1017. doi: 10.1002/jmv.21176. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez CA, Chaparro C, Krajden M, et al. Cytomegalovirus viremia in lung transplant recipients receiving ganciclovir and immune globulin. Chest. 1998;113:924–932. doi: 10.1378/chest.113.4.924. [DOI] [PubMed] [Google Scholar]
- 25.Weill D, Lock BJ, Wewers DL, et al. Combination prophylaxis with ganciclovir and cytomegalovirus (CMV) immune globulin after lung transplantation: effective CMV prevention following daclizumab induction. Am J Transplant. 2003;3:492–496. doi: 10.1034/j.1600-6143.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 26.Snydman DR, Falagas ME, Avery R, et al. Use of combination cytomegalovirus immune globulin plus ganciclovir for prophylaxis in CMV-seronegative liver transplant recipients of a CMV-seropositive donor organ: a multicenter, open-label study. Transplant Proc. 2001;33:2571–2575. doi: 10.1016/s0041-1345(01)02101-7. [DOI] [PubMed] [Google Scholar]
- 27.Arbo MD, Snydman DR, Wong JB, et al. Cytomegalovirus immune globulin after liver transplantation: a cost-effectiveness analysis. Clin Transplant. 2000;14:19–27. doi: 10.1034/j.1399-0012.2000.140105.x. [DOI] [PubMed] [Google Scholar]
- 28.Kocher AA, Bonaros N, Dunkler D, et al. Long-term results of CMV hyperimmune globulin prophylaxis in 377 heart transplant recipients. J Heart Lung Transplant. 2003;22:250–257. doi: 10.1016/s1053-2498(02)00474-6. [DOI] [PubMed] [Google Scholar]
- 29.Goldfarb NS, Avery RK, Goormastic M, et al. Hypogammaglobulinemia in lung transplant recipients. Transplantation. 2001;71:242–246. doi: 10.1097/00007890-200101270-00013. [DOI] [PubMed] [Google Scholar]