Abstract
In the present study we addressed the question whether Toxoplasma gondii could promote apoptosis in T lymphocytes in the acute stage of infection. Using in vivo activated T cells and then culturing them for a short time, we observed activation induced cell death in T. gondii infected mice. A higher level of activation induced cell death (AICD) was seen in susceptible C57BL/6 mice than in resistant CBA/J mice following infection with the same P strain of parasite. Apoptosis in T cells of susceptible mice was associated with altered induction of Bcl-2/Bax, loss of Mitochondrial Transmembrane Potential. Both CD4+ and CD8+ T cells were found to be susceptible to apoptosis; CD4+ T cells were sensitive to Fas mediated death whereas CD8+ T cells were insensitive to this signal. Caspase inhibitors had less effect on DNA fragmentation in CD4+ compared to CD8+ T cells. Exposure of CD4+ T cells to anti-IFNγ mAb resulted in an increase in the number of T cells that were positive for anti-apoptotic molecule Bcl-2 and DiOC6, a cationic dye that accumulates in intact mitochondria. These changes were less noticeable in CD8+ T cells following treatment with anti-IFNγ mAb. These findings provide further insight into the mechanisms of T cell apoptosis in T. gondii infection.
Introduction
Detection and quantification of apoptosis induced in activated T cells in response to an infection provides a useful tool for understanding immune system homeostasis. Generalized immunodepression seen in the lymphocytic choriomeningitis virus (LCMV) infected hosts is associated with activation-induced death of T cells [1], and programmed cell death may be one of the factors for the depletion of CD4+ T cells in HIV infection [2,3]. On the other hand, interference in the normal process of apoptosis may cause diseases, such as cancers, autoimmunity diseases, and neurodegenerative disorders [4-6]. Besides the harmful effects, activation induced cell death may serve as a mechanism for the elimination of activated T cells, reducing damage to hosts.
T. gondii is a ubiquitous protozoan intracellular parasite affecting 18-25% of the population, and it is usually benign without substantial morbidity and mortality except during congenital infection and in immunocompromised hosts, in particularly those with HIV infection [7,8]. The impact of Toxoplasma gondii infection on host cell apoptosis has been the subject of intense investigation. Several authors reported inhibition of apoptosis in infected host cells, principally in macrophages by direct interference with apoptosis-signaling cascade, which facilitates the intracellular development of T. gondii [9-11]. However, others have described apoptosis of activated T lymphocytes in Toxoplasmosis [12-14]. The intriguing dual activity of T. gondii to both promote and inhibit apoptosis requires a tight regulation to promote a stable host-parasite interaction and establishment of persistent toxoplasmosis [15].
It is important to understand the mechanisms of apoptosis during the course of T. gondii infection, particularly in early and acute stages as the activation of T cells varies between these two stages [16,17]. Therefore, in the present study we investigated apoptotic response in T cells both in acute and early stages in response to this intracellular pathogen. Since susceptibility to T. gondii varies between different mouse strains, in the current study we examined whether the magnitude of T cell apoptosis could differ between resistant and susceptible mouse strains. Apoptosis of T lymphocytes may be mediated via multiple signaling pathways that include a loss of the inner mitochondrial transmembrane potential, Fas and tumor necrosis factor alpha (TNFα) pathways, caspase dependent/independent pathways. Therefore, we have examined different pathways that could mediate apoptosis in CD4 and CD8 T lymphocytes as a result of T. gondii infection. Our study will provide a more comprehensive understanding of the mechanisms by which the immune system can regulate itself in response to this intracellular microbial infection.
Materials and Methods
Mice, Parasites and Infection
Female C57BL/6 (H-2b) and CBA/J (H-2k) mice, 5–6 wk old, were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were housed in the accredited Animal Research Facility at Dartmouth Medical School (Hanover, NH) and maintained under the guidelines established by the institution for their use. The low virulent PLK used in this study was clonally derived from ME49, which is a type II strain of T. gondii.
Parasites were maintained in our laboratory by in vitro passage in human foreskin fibroblasts at 37°C in MEM medium without calf serum. Parasites were purified from human fibroblast cell culture as previously described [17]. Each mouse received 1 × 105 tachyzoites/i.p injection.
Cell culture and inhibition assays
Mice were killed on days 3 and 6 after infection and spleens were harvested and gently dissociated into single cell suspension. RBCs were removed using lysing buffer (Sigma, St. Louis, MO) and cell suspensions were passed through nylon wool columns to enrich the populations for T cells. These cells were 90% T cells when verified by FACS. Following purification, cells were resuspended in complete medium (RPMI containing 10% FBS, sodium bicarbonate, penicillin/streptomycin, 2-mercaptoethanol, sodium pyruvate and nonessential amino acids). They were cultured at 2×105 cells/well in triplicate in 96-well microtiter plates in complete medium at 37°C in 5% CO2 for different periods of time (0h, 6h or 12h).
For Caspase inhibition assay, two cell permeable peptide fluoromethyl ketone inhibitors of Interleukin-1 B converting enzyme (ICE) family proteases, namely, Cbz-Val-Ala-Asp(OMe)-fluoromethyl ketone (ZVAD-FMK) as well as its truncated analog Boc-Asp(OMe)-fluoromethyl ketone (BD-FMK) were tested as inhibitors of apoptotic cell death of T lymphocytes. The inhibitors ZVAD-fmk, BD-fmk and ZFA-fmk were purchased from Enzyme Systems Products, diluted to a 50 mM working stock in DMSO, and kept at −200C before diluting in complete medium for use in blocking experiments (0μM, 25μM and 50 μM). These dilutions were chosen after conducting a titration curve of these inhibitors for their blocking effect in preliminary experiments. L1210 cells transfected with mouse Fas cDNA was a gift from Dr. Alain Eastman (Dartmouth medical school, Hanover, NH). As controls, the death of L1210 cells was induced by plating cells in wells previously coated with 100 μg/ml anti Fas (Clone Jo2, PharMingen), and HT-2, an IL-2 dependent helper T cell clone was used. Death of HT-2 cells was induced by withdrawal of IL-2 [18].
Flow Cytometry
Antibodies directed against CD4 (mAb RM4.5, FITC-conjugated or PE-conjugated), CD8 (mAb 53-6.7, FITC-conjugated or PE- conjugated), and Bcl-2 (FITCconjugated) were used in our experiments. All Abs were purchased from PharMingen (San Diego, CA). Direct immunofluorescence phycoerythrin (PE)- or FITC-conjugated mAb was added to 1 × 106 cells, followed by a 45-min incubation on ice. Cells were then washed twice with PBS containing 1% BSA and then stained with annexin V to determine cell death. The cells were resuspended in ice-cold binding buffer, and data on the stained cells were acquired on a FACScan flow cytometer.
SubGO DNA analysis
For DNA content assay, 500,000 cells were resuspended in 0.2 ml of a solution containing 0.03 % saponin (Sigma), 10 mg/ml propidium iodide, 50 mg/ml RNAase and 5 mM EDTA, and incubated at room temperature for 25 min in the dark. 20 000 cells were analyzed on a FACScan flow cytometer. In some experiments, cells were first stained with specific surface molecule (as described above) prior to assessment of DNA content. 20 000 cells were analyzed on a FACScan flow cytometer. The FACS data was then analyzed by using cell cycle software Modfit (Verity, Topsham, ME).
Assay with Fas- IgG
Spleen cells were isolated from day 6 post-infected mice, purified through nylon wool column. To evaluate Fas mediated death signal, T cells (2×105) were cultured in 96 well plates with different concentrations (0ug/ml, 25ug/ml and 50ug/ml) of Fas-IgG (provided by Dr. Alain Eastman, Dartmouth medical school, Hanover, NH) for 12 hours at 37°C [19]. Fas-IgG, has been shown to block Fas mediated cell death in several model systems [20,21]. After 12 h in culture, cells were washed twice with PBS and then stained (1×106 cells/samples) with PE-conjugated anti CD4 or anti CD8 mAb. After 40-min incubation on ice, cells were washed twice with PBS containing 1% BSA and assessed for DNA content (subGO DNA) as described earlier.
Measurement of Interferon gamma (IFN ) in the sera from infected mice
Serum samples were collected from mice at days 3 and 6 after infection and uninfected mice and were kept at −200C until use. For detecting cytokine, ELISAs were conducted using paired capture and biotinylated detection antibodies from R&D Systems (Minneapolis, MN) following the manufacturer’s recommendations. The cytokine level was calculated by reference to standard units provided by the manufacturer.
Assay with anti-IFNγ antibody
In this assay, different concentration (0ng/ml, 10ng/ml, 15ng/ml) of anti-IFNγ mAb was used. A titration of anti-IFNγ mAb was carried out in preliminary experiments for inhibitory effect and consequently we have chosen the three dilutions for use in our experiments. The three dilutions of was first added to 96 well plates. Purified splenic T cells (2×105) were then added to the plate and cultured at 37°C in 5% CO2. After 12 h in culture, cells were washed twice with PBS and the stained with immunofluorescence phycoerythrin (PE)-conjugated anti CD4 or anti CD8 mAb. Cells were then incubated with 3,3′-Dihexyloxacarbocyanine iodide (DiOC6) to determine the loss of Mitochondrial Transmembrane Potential (ΔΨm). DiOC6 was purchased from Molecular Probes (Eugene, OR) DNA degradation was analyzed by FACScan as described elsewhere in the methods and materials.
SDS-PAGE and Western blotting
The cellular extracts were prepared from cells collected ex vivo. The whole extract was prepared by boiling pellet cells (1× 108/ml) for 10 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) reducing sample buffer (62.5 mM Tris-CL, pH 6.8, 1% SDS, 1% mercaptoethanol, 10% glycerol, and 0.5% bromophenol blue). Proteins from the extract (1×106 cells equivalents per lane) were resolved by electrophoresis on 10% SDS- PAGE gels, and then transferred (120 V for 1 h) to nitrocellulose membranes. The blot was blocked for 1 h in PBS containing 5% nonfat milk and then incubated in mouse anti Bcl-2 or Bax antibody (1:1.000; Santa Cruz Biotechnology, Inc. CA) overnight at 4°C in PBS/5% nonfat milk. Membranes were rinsed in PBS, and specific reactive proteins were detected using enhanced chemiluminescence (Amersham Pharmacia Biotech). Membranes were stained with Ponceau S and scanned; protein level of each lane was compared to ensure equal protein loading per lane. Bcl-2+ve or Bax+ve cells extract used in the experiment was provided by manufacturer (Santa Cruz Biotechnology, Inc. CA). Relative intensity of Bcl-2/ Bax expression was determined by using NIH software. The level of intensity is relative to T cells under same condition from uninfected mice (given a value of 1).
Assessment of intracellular Bcl-2 expression following mAb treatment
Intracellular detection of Bcl-2 protein within CD4+ and CD8+ T cell subsets was performed as described previously [22]. Splenic T cells were cultured in medium for 12 h in the presence or absence of anti IFNγ mAb, washed in PBS +0.1% BSA and stained with PE conjugated mAb specific for T cell surface antigens. The stained cells were further washed, fixed in 2% paraformaldehyde (Polyscience) for 20 min at room temperature, washed in PBS and then incubated for 15 min at 4°C in PBS-BSA containing 0.05% (w/v) Saponin (Sigma Chemical Co). Intracellular staining with FITC conjugated anti Bcl-2 mAb (PharMingen, San Diego, CA) was done in Saponin buffer for 30 min at room temperature. Finally, the cells were washed in PBS and 20 000 cells analyzed by FACScan.
Statistical analysis
P values were calculated with two-tailed “t” test with 2 sample unequal variance. Mean and Standard deviation (SD) was calculated by a program incorporated in Excel software. Statistical significance was set at P<0.05 for all comparisons.
Results
Apoptosis of activated T lymphocytes is higher in susceptible mice than in resistant mice following infection with T. gondii
It was previously shown that C57BL/6 (H-2b) mice were more susceptible than CBA/J (H-2k) mice when exposed to infection with P strain of T. gondii [23,24]. Splenocytes were isolated from these two strains of mice on day 3 and on day 6 after infection and enriched for T cells. The enriched population was analyzed for evidence of apoptosis after culturing the cells for short periods of time (6 hrs and 12 hrs) or without culture (ex vivo, 0 hr). Apoptosis was assessed by the binding of Annexin V to phosphatidyl serine (PS) and by determining subGO DNA, which detects the level of degraded DNA in the apoptotic cells. As shown in Table 1, the level of apoptosis in T cells was significantly higher in C57BL/6 mice than in CBA/J mice (P<0.001) when they were infected for 6 days. The differences in apoptosis between the two strains of mice are less discernible on day 3 post infection particularly when freshly obtained (ex vivo collected) cells were assayed for apoptosis. However, these differences became slightly significant if the cells from 3 days infected mice were cultured for 6 hr or 12 hr (P <0.05). The susceptible C57BL/6 mice displayed a significantly increased magnitude of apoptosis in T lymphocytes in the acute stage (day 6 postinfection) than in the early stage (day 3 postinfection) of infection (P<0.001). Such difference in apoptosis between two periods of infection was less marked in the resistant CBA/J mice. We observed some death in T cells obtained from both the mouse strains without infection, which were cultured for 12 hrs and then assayed by binding of Annexin V to PS. Another hallmark of apoptosis is rapid DNA fragmentation [18]. The magnitude of apoptosis always remained markedly higher in 6 days infected C57BL/6 mice than in normal C57BL/6 or 6 days infected resistant CBA/J mice. The spontaneous apoptosis in T cells from normal mice was less marked when they were obtained ex vivo or cultured for a shorter period of time (6 hr).
Table 1. Splenic T cells are more susceptible to death in susceptible C57BL/6 mice than in CBA/J mice following T. gondii infection.
Nylon wool purified splenic T cells were cultured in 24 wells at 106 cells/ml at 37°C in 5% CO2.. At indicated times of culture (0h, 6h, 12h), cells were washed twice, and were stained with Annexin V FITC. Significantly increased rate of apoptosis was observed in susceptible C57BL/6 mice than in resistant CBA/J mice in the acute stage of T. gondii infection (6 days postinfection) (p<0.001). This was evident in ex vivo T cells, and the difference was maintained in T cells cultured for extended periods of time (6 hr, 12 hr). The level of apoptosis in susceptible C57BL/6 mice was significantly higher in the acute stage (6 days postinfection) than in the early stage (3 days postinfection) of infection (p<0.001). Similar pattern was seen when the rate of apoptosis was measured by the subG0 DNA assay. Cells with less FL2 fluorescence than the 2N DNA peak were considered having subG0 DNA. Significantly augmented DNA degradation was observed in the susceptible C57BL/6 mice than in resistant CBA/J mice (p<0.001). Data (mean ± SD) are representative of three independent experiments with three mice per group.
| C57Bl/6 | |||||||
|---|---|---|---|---|---|---|---|
| % T cells Annexin FITC+ | % Cell with subGo DNA | ||||||
| C57BL/6 | 0h | 6h | 12h | C57BL/6 | 0h | 6h | 12h |
| Naïve | 8.67±0.16 | 12.4±2.6 | 28.11±3.42 | Naïve | 1.3±0.12 | 2.5±0.21 | 6.0±1.52 |
| Day-3 | 6.96±0.19 | 14.32±1.75 | 32.92±3.26 | Day-3 | 4.25±1.1 | 9.86±2.0 | 16.32±3.2 |
| Day-6 | 14.82±1.75 | 30.42±0.72 | 56.81–0.82 | Day-6 | 10.4±2.1 | 16.82±2.5 | 40.22±2.3 |
| CBA/J | |||||||
|---|---|---|---|---|---|---|---|
| % T cells Annexin FITC+ | % Cell with subGo DNA | ||||||
| CBA/J | 0h | 6h | 12h | CBA/J | 0h | 6h | 12h |
| Naïve | 6.03±0.83 | 12.27±0.75 | 25.02±0.55 | Naïve | 1.25±0.14 | 2.35±0.60 | 4.2±1.1 |
| Day-3 | 8.23±0.31 | 11.09±0.67 | 27.93±2.62 | Day-3 | 4.25±1.1 | 8.6±2.0 | 10.32±3.2 |
| Day-6 | 10.49±0.75 | 23.95±0.41 | 38.13±0.35 | Day-6 | 6.5±0.95 | 9.09±1.5 | 23.22±2.2 |
As DNA degradation assay (subGO DNA) appeared to be more discriminatory in determining apoptosis in T cells between naïve and infected mice, we applied this assay more frequently in the following experiments to examine active process of apoptosis. All experiments to determine the mechanistic aspects of apoptosis were performed using cells from day 6 post infected susceptible C57BL/6 mice unless otherwise specified.
Fas mediated signal is relayed differently in CD4+ and CD8+ T cell subpopulations in T. gondii infected mice
Because Fas/FasL interactions have been shown by many investigators to be involved in T cell death [25,26]. we examined the role of these molecules in the death of CD4+ and CD8+ T cell populations in our experiments. To do this, we assessed the magnitude of apoptosis in both CD4+ and CD8+ T cells in the presence of Fas-IgG, a reagent that blocks Fas-mediated cell death [18-20]. As shown in Figure 1, Fas-IgG inhibited DNA loss in T. gondii activated CD4+ T cells in a dose dependent manner. There was only a slight effect on DNA loss in CD8+ T cells even when they were treated with Fas-IgG at the higher concentration of 50 ug/ml (P > 0.05). The treatment of CD4+ T cells with Fas-Ig at the same concentration resulted into decline in apoptosis from 64% to 17%. These results are shown in Figure 1.
Fig. 1. Fas-IgG inhibits DNA degradation in CD4+ T cells but not in CD8+ T cells.
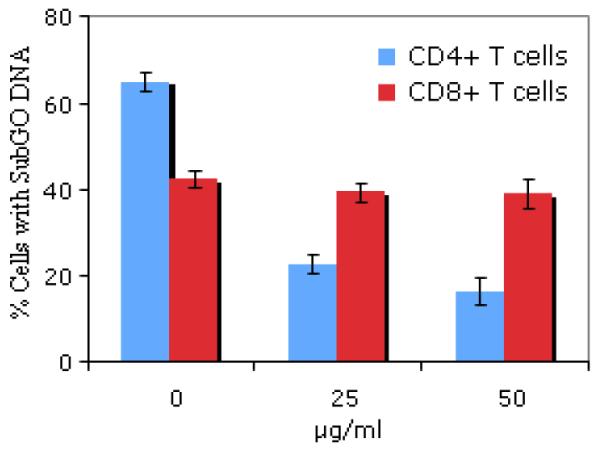
Purified splenic T cells (1.5× 106 cells/ml) were cultured for 12 hrs in the absence (0μg/ml) or in the presence of indicated concentrations (25μg/ml, and 50 μg/ml) of Fas IgG. Another hallmark of apoptosis is rapid DNA fragmentation. The rate of apoptosis was measured by the subG0 DNA assay. There was a significant inhibition in DNA degradation in CD4+ T cells in a dose-dependent manner after the treatment with Fas-IgG (p<0.001). No significant decrease in DNA degradation was observed in CD8+ T cell when Fas-IgG was used at the same concentrations (p> 0.05). The results show the percent of positive cells having subG0 DNA content (mean ± SD). Data are representative of three independent experiments with three mice per group.
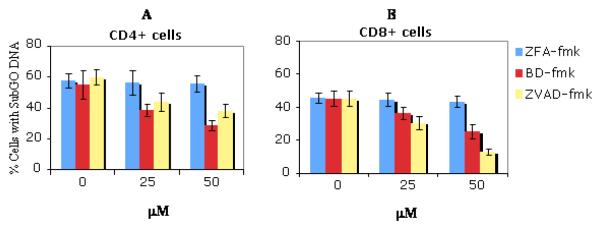
CD8+ T cells are more susceptible to Caspase dependent apoptosis than CD4+ T cells in mice infected with T. gondii
Caspases are required for apoptosis in a number of systems [27]. In this experiment, two broad spectrums caspase inhibitors ZVAD-fmk and BD-fmk were utilized to evaluate whether caspases are involved in T. gondii induced T cell death. Both ZVAD-fmk, and BD-fmk, but not control cathapsin B inhibitor ZFA-fmk prevented DNA loss in activated CD4+ and CD8+ T cells from infected mice in a dose-dependent manner. Although DNA loss was prevented by caspase inhibitors (BD-fmk or ZVADfmk) in both T cell subsets, the effect was less extensive in the CD4+ T cells (Figure 2A) compared to the CD8+ T cells (Figure 2B). The level of apoptosis in CD8+ T cells treated with inhibitor ZVAD-fmk at a concentration of 50 ug/ml decreased from 44% to 13% (Figure 2B) whereas in CD4+ T cells treated with the same inhibitor at the same concentration the level of apoptosis decreased from 56% to 36% (Figure 2A). A similar differential pattern in the sensitivity to BD-fmk, another pan-caspase inhibitor between CD4+ and CD8+ T cells was observed. These results are shown in Figure 2.
Fig 2. Effects of broad spectrum caspase inhibitors ZVAD-fmk and BD-fmk on apoptosis of activated T cells induced by T. gondii infection.
Purified splenic T cells were obtained from C57BL/6 mice infected for 6 days and were put into culture. Caspase inhibitors (BD-fmk, ZVAD-fmk,) and control (ZFA-fmk) inhibitors were added at the indicated concentration at the beginning of culture. After 12hrs of culture, cells were stained with anti CD4 FITC or CD8 FITC mAb, washed twice in PBS-BSA and resuspended in 10 μg/ml propidium iodide in 0.03% saponin, After 25 min incubation at RT, 20 000 cells were analyzed by flow cytometry. Results show the percent of CD4+ (A) or CD8+ (B) T cells that had subG0 DNA±SD. Treatment by both Zvad-fmk and BD-fmk caspase inhibitors significantly diminished DNA degradation in CD4+ and CD8+ T cell subsets but the effect was more marked in CD8+ T cell (p<0.001) than in CD4+ T cell (p<0.05) populations. The experiment is a representative of four independent experiments (each group had three mice) with similar results.
As a control, we also tested whether caspase inhibitors could protect cell death induced by different stimuli. Fas driven death of L1210Fas+ cells and growth factor withdrawal-induced cell death of HT-2 cells was compared to the death of primary T. gondii activated T cells. BD-fmk prevented the death of L1210Fas+ cells treated with anti Fas and HT-2 cells withdrawn from IL-2 as determined by the uptake of 7AAD. ZFA-fmk had no effect on cell death in any of the cell type tested (data not shown).
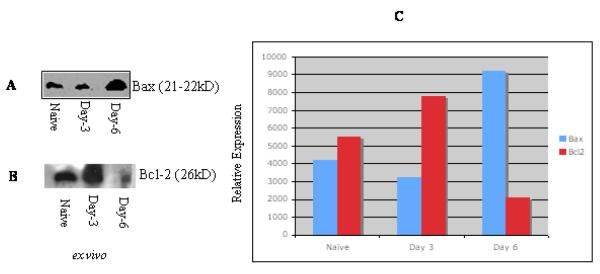
T. gondii activation induced apoptosis is related to uneven levels of Bcl-2 or Bax expression
The members of Bcl-2 regulate apoptosis via their capacity to modulate mitochondrial membrane pore (MMP) [28,29]. To determine whether differential Bcl-2 expression could be involved in T. gondii activation induced apoptosis, purified T cells were analyzed by western blot (see materials and methods) for expression of Bcl-2 or Bax. A higher expression of Bax was observed in T cells from 6 days infected animals than in 3 days infected mice or in naive mice (Figure 3A). On the other hand, the expression level of Bcl-2 was markedly diminished in T cells obtained from 6 days infected mice compared to 3 days postinfected or noninfected mice (Figure 3B). Determination of relative expression of Bax and Bcl-2 pointed to significant changes in the T cells from 6 days infected mice compared to 3 days infected or to uninfected control animals (Figure 3C). The expression of Bax showed 2.2 fold increase in day 6 post infected groups over uninfected groups. In comparison, the relative Bcl-2 expression in day 6 post infected group displayed fall of more than 2.7 fold over the uninfected groups and 3.4 fold over day 3 post infected groups. These results are shown in Figure 3.
Fig 3. The apoptotic process in T cells from T. gondii infected mice is associated with altered induction of Bcl-2 or Bax.
Cell lysates were prepared from purified T lymphocyte populations as described in the materials and methods. Each lane (A-B) contains lysate of 106 cells. The detection of antigen-antibody complexes was conducted by enhanced chemiluminescence. The level of Bcl-2/ Bax expression intensity is relative to the T cells under the same condition from uninfected mice (given a value of 1) (C). Naïve, T cells from uninfected mice; Day-3, T cells from mice infected for 3 days; Day-6, T cells from 6 days infected mice. The relative intensity of Bcl-2/Bax protein expression was determined by using NIH software. Bax induction was higher in T cells from 6 days infected mice compared to naive or 3 days infected mice. Decreased expression of Bcl-2 was observed in cells from mice infected for 6 days compared to animals infected for 3 days or to naïve mice. Similar results were obtained in three repeated experiments (each group had three mice).
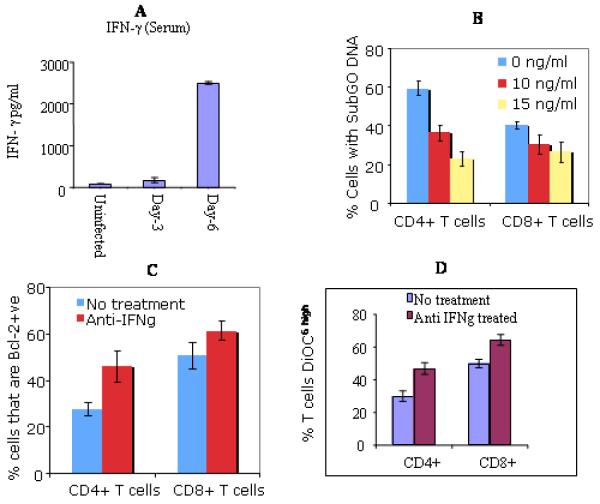
Effect of Anti-IFNγ mAb on T. gondii activated T cell death
We observed significantly higher level of Interferon gamma (IFNγ) cytokine in the serum of C57BL/6 mice at day 6 post infection than in the serum at day 3 post infected or naïve mice (Fig. 4A). Interferon gamma may play a role in the process of death in activated T lymphocytes [30,31]. Thus, we investigated the implication of IFNγ in the apoptosis of T cells in T. gondii infected mice. Addition of anti-IFNγ antibody to 12h culture resulted in significant decrease in DNA loss in CD4+ T cells (P<0.001). The treatment with anti-IFNγ mAb led to significant decrease in DNA loss in CD8+ T cells (P< 0.05), however, the effect was less extensive in this subset of T cells than in CD4+ T cells (Fig. 4B).
Fig 4. Effects of anti-IFNγ mAb on apoptosis of T cells in T. gondii infected mice.
ELISA measured IFNγ cytokine in the sera from indicated groups of mice (A). Sera from: Naïve, uninfected mice; Day-3, mice infected for 3 days; Day-6, 6 days infected mice. Similar results were obtained in three repeated experiments. Treatment with mAb prevented DNA degradation in a dose depended manner (B). Blockade is more marked in CD4+ T cells (p<0.001) compared to CD8+ T cells (p<0.05) at the concentration 10ng/ml of mAb. Results demonstrated the percent of CD4+ or CD8+ T cells that were Bcl-2 positive (C). Expression of Bcl-2 was significantly increased in CD4+ T cells following treatment with mAb (p<0.001). The restoration of Bcl-2 was less extensive in CD8+ T Cells. treatment increased DiOC6high expression (D). Stained cells (CD4+ and CD8+) were resuspended in 25 nM DiOC6 (see methods and materials) and were analyzed by flow cytometry to assess the loss of mitochondrial transmembrane potential (ΔΨm). Higher percent of CD4+ T cells became DiOC6high after the treatment with anti-IFN . mAb (p<0.001). There was also an increase of DiOC6high expression in CD8+ cells but was relatively less marked than in CD4+ T cells. Data shown in A, B, C, and D are representative of three repeated experiments.
Further, we cultured the two subsets of T lymphocytes in the presence of mAb (10ng/ml, 15ng/ml) for a period of 12 hrs and determine the percentage of T cells that were Bcl 2 positive. Anti-apoptotic Bcl-2 expression was significantly less in CD4+ T compared to CD8+ T cells when they were not treated with anti-IFNγ mAb (P < 0.001). After the treatment, the percentage of Bcl-2 positive CD4+ T cells rose from 29 to 47 and in case of CD8+ T cells the same augmented from 52 to 64 (Fig. 4C). In addition, culture of cells in the presence of anti-IFNγ mAb resulted in an increase in the percentage of both CD4+ (from 36 to 47) and CD8+ (from 42 to 57) T cells that became positive for DiOC6 staining (Fig 4D).
Discussion
The mechanisms that drive T lymphocytes death during the contraction phase of the immune response in acute toxoplasmosis are not well understood. The host immune response is crucial for controlling parasite growth during the acute phase of the infection and for preventing the reemergence of acute infection in chronically infected individuals. Mice were infected with the tachyzoites in this study, and they developed an acute phase of infection after 6 days of infection. Mouse models with tachyzoite infections are well documented and have allowed researchers to understand the important mechanisms of immune responses and pathogenesis in toxoplasmosis. Since apoptosis is a very fast process, we have performed analysis with both the directly isolated cells (ex vivo obtained cells) and with the cells cultivated for short periods of time (e.g. 6hr and 12 hr). This study for the first time provides evidence for the correlation of apoptosis with the host’s genetic in toxoplasmosis. The results of our study clearly demonstrate that the level of apoptosis is significantly higher in susceptible C57BL/6 mice than in resistant CBA/J mice. Previous studies have shown that after exposure to PLK, a clonal derivative of ME49 and a type II strain of T. gondii, C57BL/6 mice develop acute infection by day 6 post infection with rapidly replicating parasites, whereas CBA/J mice are much less susceptible with weak parasite replication upon infection with the same dose of PLK strain [23,24]. Further investigations are needed to imply a direct effect of genetic background on splenic T lymphocytes per se.
The results of this study demonstrate marked difference in the death of activated T cells between early (day 3 post infection) and acute (day 6 post infection) stage of T. gondii. The mechanism of hyporesponsiveness in acute phase of infection represents an important unsolved problem in toxoplasmosis. An increased apoptosis of activated T cells may contribute to immunologic hyporesponsiveness in acute T. gondii infection. Decreased production of IL-2 and augmented synthesis of IL-10 during acute stage of T. gondii infection [17] may have a role in the enhanced level of apoptosis. We found significantly fewer parasites in the peripheral organs (spleen, lung and liver) measured by the quantitative competitive polymerase chain reaction (QC-PCR) (data not shown), and detected less amount of in early stage (day 3 postinfection) than in the acute stage (day 6 postinfection) of infection (see Fig. 4A). Poor level of apoptosis in the early stage of infection may be related to the diminished production of IFN (Figure 4, and see discussion below). Of note, following infection with T. gondii, IFNγ response in the resistant CBA/J mice is markedly diminished compared to susceptible C57BL/6 mice (data not shown). It has previously been suggested that apoptosis of T lymphocytes in T. gondii infection is associated with the virulence and density of the parasite in the host [13]. Unlike HIV infection, deletion of T lymphocytes by productive infection has not yet been clearly established in toxoplasmosis.
Different mechanisms may act on different subpopulations of T cells depending upon the level of stimulation and the microenvironment these cells encounter during an infection. In this study, we provide evidence that treatment with Fas-IgG, a fusion protein that binds and blocks FasL with the consequence of inhibiting apoptosis in activated T cells [18,20,33], prevented DNA degradation in CD4+ T cells but not in CD8+ T cells in T. gondii infection. This suggests that there is a component of apoptosis in parasite activated CD8+ T cells that is Fas independent. Fas has the capacity to receive alternate signals in the T cell compartments [34]. It was previously shown that Fas-FasL interactions play a role in the apoptosis of activated CD4+ T cells that are stimulated through the TCR [35]. Likewise, parasite antigen induced stimulation via TCR during acute T. gondii infection is likely to mediate Fas-dependent apoptosis in activated CD4+ T cells. T. gondii activated splenic T cells produce low quantity of IL-2 [17] and high TNFα during acute infection [36]. Whether these two cytokines have a role in accelerated death of T. gondii primed CD8+ T cells is currently under investigation in our laboratory.
The mechanism for activated CD8+ T cell death seen in acute T. gondii infection is probably triggered through other signaling pathways rather than through Fas. Caspases are the proteases when activated cleave several molecules involved in cell structure and integrity. Caspase inhibitors have been developed and shown to inhibit most cell death [27,37,38]. In the present study, we examined the effect of blocking T cell apoptosis in the presence of two broad-spectrum caspase inhibitors ZVAD-fmk, and BD-fmk. The ZVAD-fmk peptide has a broader spectrum of inhibition, effective against all caspases tested to date, including caspases-3, -6, -8, -1, -2 and -4. The truncated ZVAD-fmk analogue BD-fmk is a general cysteine protease inhibitor and has a still wider spectrum of inhibition against the caspases. The results of our experiment demonstrate that caspase inhibitors ZVAD-fmk and BD-fmk could inhibit DNA degradation (Fig 2A) in both CD4+ and CD8+ T cell subsets but the effect was more extensive in CD8+ T cells than in CD4+ T cells. This difference may be related to the expression of cell surface death receptors in the two subpopulations of activated T cells. We observed lesser extent of inhibition by ZVAD-fmk than by BD-fmk in cell cultures. There are several examples of T cell death, which could be blocked by the one of the inhibitors but is less sensitive to the other [32,39,40]. It is also possible that ZVAD-fmk is less stable than BD-fmk in cell culture. We tested if caspase inhibitors could protect cell death mediated by different stimuli as a control. We found that BD-fmk prevented the death of L1210 Fas+ cells treated with anti-Fas and HT-2 cells withdrawn from IL-2 when assessed by uptake of 7AAD (data not shown).
Little is known in parasitic infections concerning the role of Bcl2/Bax in regulating cell survival or death. The Bcl-2 family includes both pro-apoptotic (Bax) as well as anti-apoptotic (Bcl-2) molecules that determine, in part, the susceptibility of cells to a death signal [28]. Western blot results of this study demonstrate increased Bax expression in T cells in mice infected for 6 days than in mice infected for 3 days or in naïve mice (Fig-3A). This increase in Bax expression is associated with decrease in Bcl-2 expression (Fig 3B). Some increase in Bcl-2 in 3 day postinfected mice over naïve mice may reflect host’s enhanced response to maintain survivability in rapidly expanding parasite sensitized T cells in an early stage of immune activation. Over expression of Bax induces or enhances spontaneous cell death without additional factors [41]. It is conceivable that the signal for Bax activation emanates from the mitochondria, although other sources are not excluded.
Previous studies in murine toxoplasmosis have shown the implication of Th1 cytokines, particularly of in the death of activated T cells [13,14]. In this study we have confirmed the important role of IFN in the apoptosis of activated T cells in T. gondii infection and provided new information concerning the effect of IFNγ on the DNA loss in CD4+ T cells and CD8+ T cells, on the expression of anti-apoptotic Bcl-2 molecule and on the loss of mitochondrial transmembrane potential. Sera from mice infected for 6 days contained significantly higher level of IFNγ compared to mice infected for 3 days or to naïve mice (Fig 4A). Denkers et al. demonstrated that SAg, the surface antigen of T. gondii could drive over production of IFNγ during T. gondii infection [42]. We showed that DNA degradation in activated T cells was significantly diminished by treatment with anti-IFNγ mAb, this diminution is more pronounced in CD4+ T cells than in CD8+ T cells (Fig 4B). has been demonstrated to play a critical role in cell death induced by anti TCR mAbs in the absence of co-stimulatory molecules [30]. The percentage of Bcl-2 (an anti-apoptotic protein) positive cells and the cells staining DiOC6 (that accumulates in intact mitochondria) were significantly less in the CD4+ population than in CD8+ population (see non-treated groups in Figs 4C and 4D). These findings clearly show increased apoptosis in CD4+ T cells than in CD8+ T cells in the acute stage of T. gondii infection although both subsets of activated T lymphocytes are susceptible to apoptosis. Enhanced apoptosis of CD4+ T cells might be responsible at least partially for the development of hyporesponsiveness during the acute phase of toxoplasmosis [43].
The treatment with anti-IFN mAb increased the percentage of CD4+ and CD8+ T cells that are Bcl-2 positive (Fig. 4C) and CD4+ and CD8+ T cells that have accumulated DiOC6 (Fig. 4D). However, these changes after anti-IFNγ mAb treatment is less marked in CD8+ T cells than in CD4+ T cells. It has recently been reported that IFNγ producing cells were related to decreased expression of Bcl-2 in HIV infection [22]. In T gondii infection, IFNγ locally produced in payer’s patches contributes to the induction of apoptosis in Peyer’s patch T cells [31]. In this study, anti-IFNγ mAb treatment at least partially restored loss of mitochondrial transmembrane potential as DiOC6 accumulated in cells that have intact mitochondria. Taken together, our observations on the survival/death of T lymphocytes (CD4+ and CD8+) provide further insight into the homeostasis of T cells in the immune response to this intracellular parasite.
Acknowledgments
We thank Jacqueline Y. Smith for discussion and Chaitali Dutta for help in Western blot technique. The authors have no financial conflict of interest. A. Haque is supported by CNRS (France). This work was supported by National Institutes of Health Grant AI19613.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Razvi E, Welsh R. Programmed cell death of T lymphocytes during acute viral infection: a mechanism for virus-induced immune deficiency. J Virol. 1993;67:5754–5765. doi: 10.1128/jvi.67.10.5754-5765.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen J. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4 Tcell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- [4].Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- [5].Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–415. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- [6].Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29:303–309. doi: 10.1016/j.jaut.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Remington JS, McLeod R, Desmonts G. In: Toxoplasmosis. Infectious diseases of the fetus and newborn infant. Remington JS, Klein JO, editors. Saunders Company; Philadelphia: 1995. pp. 140–267. [Google Scholar]
- [8].Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shapira S, Harb OS, Caamano J, Hunter CA. The NF-kappaB signaling pathway: immune evasion and immunoregulation during toxoplasmosis. Int J Parasitol. 2004;34:393–400. doi: 10.1016/j.ijpara.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [10].Luder CG, Gross U. Apoptosis and its modulation during infection with Toxoplasma gondii: molecular mechanisms and role in pathogenesis. Curr Top Microbiol Immunol. 2005;289:219–237. doi: 10.1007/3-540-27320-4_10. [DOI] [PubMed] [Google Scholar]
- [11].Carmen JC, Sinai AP. Suicide prevention: disruption of apoptotic pathways by protozoan parasites. Mol Microbiol. 2007;64:904–916. doi: 10.1111/j.1365-2958.2007.05714.x. [DOI] [PubMed] [Google Scholar]
- [12].Caamano J, Tato C, Cai G, Villegas EN, Speirs K, Craig L, et al. Identification of a role for NF-kappa B2 in the regulation of apoptosis and in maintenance of T cell mediated immunity to Toxoplasma gondii. J Immunol. 2000;165:5720–5728. doi: 10.4049/jimmunol.165.10.5720. [DOI] [PubMed] [Google Scholar]
- [13].Gavrilescu LC, Denkers EY. IFN-γ overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J. Immunol. 2001;167:902–909. doi: 10.4049/jimmunol.167.2.902. [DOI] [PubMed] [Google Scholar]
- [14].Mordue DG, Monroy F, La Regina MM, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol. 2001;167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- [15].Schaumburg F, Hippe D, Vutova P, Luder CG. Pro- and anti-apoptotic activities of protozoan parasites. Parasitology. 2006;132:S69–85. doi: 10.1017/S0031182006000874. [DOI] [PubMed] [Google Scholar]
- [16].Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, et al. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- [17].Haque S, Khan I, Haque A, Kasper LH. Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin 2 production and macrophage mediated inhibitory effects. Infect Immun. 1994;62:2908–2916. doi: 10.1128/iai.62.7.2908-2916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hildeman D, Mitchell T, Teague T, Henson P, Day B, Kappler J, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- [19].Wolf CM, Eastman A. The Temporal Relationship between Protein Phosphatase, Mitochondrial Cytochrome c Release, and Caspase Activation in Apoptosis. Exp Cell Res. 1999;247:505–513. doi: 10.1006/excr.1998.4380. [DOI] [PubMed] [Google Scholar]
- [20].Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, et al. Cellautonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- [21].Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT. Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem. 1999;274:7987–7992. doi: 10.1074/jbc.274.12.7987. [DOI] [PubMed] [Google Scholar]
- [22].Ledru E, Lecoeur H, Garcia S, Debord T, Gougeon M. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J Immunol. 1998;160:3194–3206. [PubMed] [Google Scholar]
- [23].Lee YH, Channon JY, Matsuura T, Schwartzman JD, Shin DW, Kasper LH. Functional and quantitative analysis of splenic T cell immune responses following oral Toxoplasma gondii infection in mice. Exp Parasitol. 1999;91:212–221. doi: 10.1006/expr.1998.4359. [DOI] [PubMed] [Google Scholar]
- [24].Lee YH, Kasper LH. Immune responses of different mouse strains after challenge with equivalent lethal doses of Toxoplasma gondii. Parasite. 2004;11:89–97. doi: 10.1051/parasite/200411189. [DOI] [PubMed] [Google Scholar]
- [25].Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Armstrong R, Aja T, Xiang J, Gaur S, Krebs JF, Hoang K, et al. Fas-induced activation of the cell death-related protease CPP32 Is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- [27].Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- [28].Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- [29].Hildeman D, Jorgensen T, Kappler J, Marrack P. Apoptosis and the homeostatic control of immune responses. Curr Opin Immunol. 2007;19:516–521. doi: 10.1016/j.coi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu Y, Janeway CA. Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liesenfeld O, Kosek JC, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer’s patch T cells in mice following per oral infection with Toxoplasma gondii. Infect Immun. 1997;65:4682–4689. doi: 10.1128/iai.65.11.4682-4689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McCarthy NJ, Whyte MK, Gilbert CS, Evan GI. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- [34].Suzuki I, Fink PJ. The dual functions of Fas ligand in the regulation of peripheral CD8+ and CD4+ T cells. Proc Natl Acad Sci U S A. 2000;97:1707–1712. doi: 10.1073/pnas.97.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- [36].Haque S, Franck J, Dumon H, Kasper LH, Haque A. Protection against lethal toxoplasmosis in mice by an avirulent strain of Toxoplasma gondii : stimulation of IFNγ and TNFα response. Expt Parasitol. 1999;93:231–240. doi: 10.1006/expr.1999.4457. [DOI] [PubMed] [Google Scholar]
- [37].Cohen G. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nicholson DW. Apoptosis. Baiting death inhibitors. Nature. 2001;410:33–34. doi: 10.1038/35065201. [DOI] [PubMed] [Google Scholar]
- [39].Sarin A, Wu M-L, Henkart PA. Different Interleukin-1β Converting Enzyme (ICE) Family Protease Requirements for the Apoptotic Death of T Lymphocytes Triggered by Diverse Stimuli. J Exp Med. 1996;184:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang Y, Zhao S, Song J. Caspase-dependent apoptosis and -independent poly(ADPribose) polymerase cleavage induced by transforming growth factor β1. Int J Bioch Cell Biol. 2004;36:223–234. doi: 10.1016/s1357-2725(03)00215-2. [DOI] [PubMed] [Google Scholar]
- [41].Cerezo A, Martinez AC, Gonzalez A, Gomez J, Rebollo A. IL-2 deprivation triggers apoptosis which is mediated by c-Jun N-terminal kinase 1 activation and prevented by Bcl-2. Cell Death Differ. 1999;6:87–94. doi: 10.1038/sj.cdd.4400458. [DOI] [PubMed] [Google Scholar]
- [42].Denkers EY, Caspar P, Hieny S, Sher A. Toxoplasma gondii infection induces specific nonresponsiveness in lymphocytes bearing the V beta 5 chain of the mouse T cell receptor. J Immunol. 1996;156:1089–1094. [PubMed] [Google Scholar]
- [43].Khan IA, Matsuura T, Kasper LH. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol. 1996;8:887–896. doi: 10.1093/intimm/8.6.887. [DOI] [PubMed] [Google Scholar]