Abstract
To explore the changes in actin filaments during the process of myofibrillogenesis, primary cultures of avian skeletal muscle cells were exposed to Latrunculin-A (Lat-A), a drug that binds monomeric actin. Lat-A, at low concentrations (0.2 – 0.5µM) had no effect on actin fibers in the myotubes, but did induce the loss of stress fibers in fibroblasts in the same cultures. Stress fibers reformed when Lat-A was removed. With increasing concentrations of Lat-A, the drug induced the disassembly of premyofibrils, the precursors of mature myofibrils. In addition, 5µM Lat-A induced some disassembly of mature myofibrils that were adjacent to the zone of premyofibrils at the myotube ends. Removal of Lat-A led to the reformation of premyofibrils in the spreading edges of the myotubes, and the subsequent assembly of mature myofibrils. High concentrations of Lat-A (up to 50 µM) had no obvious effect on the mature myofibrils in the central region of the myotubes. The differential sensitivity of premyofibrils and myofibrils to Lat-A suggests that in the stages of myofibril formation, the actin filaments are modified or capped to render them increasingly insensitive to Lat-A. To determine how a change in the actin filaments during myofibrillogenesis might confer greater resistance to depolymerization by Lat-A, we stained the myotubes with an antibody directed against CapZ, a protein that blocks the release of monomer actin from the barbed ends of actin filaments. CapZ was absent from premyofibrils. It was distributed uniformly along nascent myofibrils where F-actin was unstriated, and was localized in a clearly striated Z-band pattern in the mature myofibrils where F-actin patterns were fully striated. These Lat-A and CapZ results are discussed in the context of various models of myofibrillogenesis.
Keywords: Myofibrillogenesis, sarcomere, premyofibril, nascent myofibril, mature myofibril, actin, alpha-actinin, CapZ, latrunculin A
Introduction
Before muscle proteins are expressed in myogenic cells, non-muscle homologues of myofibrillar proteins are present, functioning in motility of the cells and in contractile processes such as cytokinesis. As myogenesis progresses and muscle specific proteins are expressed, the proteins assemble into filament arrays that ultimately become organized into contractile myofibrils. There are several models for how this process of myofibrillogenesis occurs (reviewed in Sanger et al., 2004, 2005). In avian skeletal muscle cultures the process begins when isolated myoblasts begin to fuse with one another to form myotubes. Myofibrils form in the myotubes over several days with the newest myofibrils assembling at the ends and sides of the myotubes and the fully assembled, contractile myofibrils in the central shaft of the multinucleated myotube. This spatially polarized progression of myofibrillogenesis allows properties of the myofibril subunits to be compared at different stages of assembly within a single cell. To test models of myofibrillogenesis, we focused in this study on the actin filaments using the monomer actin-binding agent, Latrunculin-A (Lat-A).
Latrunculin-A is a monomeric actin-binding drug isolated from the Red Sea sponge Latrunculia magnifica. In vivo, it was found to alter cell shape, disrupt microfilament organization, and inhibit microfilament-mediated processes of fertilization and early development (Spector et al., 1983, 1989; Schatten et al., 1986; Ayscough et al., 1997). The structure of Lat-A was determined to be a macrolide containing a 16-membered ring and a 2-thiazolidione moiety with a molecular weight of 422 Daltons (Kashman et al., 1980). Lat-A binds only to actin monomers forming a 1:1 complex with a dissociation constant Kd = 0.2 µM (Coue et al., 1987). The monomeric actin-binding site of Lat-A is located above the actin nucleotide-binding site in the cleft between subdomain II and IV, preventing actin from repolymerizing into filaments (Morton et al., 2000; Yarmola et al., 2000). Lat-A shifts the equilibrium between actin monomers and filamentous actin by binding to monomer and preventing its reincorporation into actin filaments thus leading to the subsequent disassembly of F-actin. Several previous reports showed that Lat-A caused a rapid and reversible disassembly of the filamentous actin structures in nonmuscle cells, but not the microtubular cytoskeleton in those cells (Spector et al., 1983, 1989; Schatten et al., 1986; Ayscough et al., 1997). This specificity and reversibility of Lat-A interaction with actin monomer makes it a powerful agent for analyzing changes in actin organization during myofibrillogenesis.
There are currently several models to explain the steps leading to the assembly of sarcomeres. The model from this lab proposes that there is a stepwise transition from premyofibrils to nascent myofibrils to mature myofibrils (Rhee et al., 1994; Du et al., 2003). The premyofibrils are similar in structure to stress fibers but are composed of muscle specific proteins, excepting the bands of myosin II, which are composed of the non-muscle isoform of myosin II (Rhee et al., 1994; Du et al., 2003). An opposing view is that stress fibers or stress fiber-like structures (SFLS), composed of nonmuscle proteins, serve as transitory templates along which myofibrils form (Dlugosz et al., 1984). The other frequently cited model proposes that actin filaments attached to Z-bands form scattered I-Z-I arrays that are stitched together by titin filaments, and subsequently recruit thick myosin filaments to form a myofibril (Holtzer et al., 1997).
In this study, we followed live quail myotubes in cultures transfected with Green Fluorescent Proteins ligated to either actin or alpha-actinin and analyzed the effect on the actin filaments of the depolymerizing agent Lat-A. The goal was to determine if there was evidence that myofibrils formed along a stress fiber-like template. The expectation was that stress fiber-like structures would be more sensitive to depolymerization than nascent myofibrils, and if they functioned as templates or scaffolds, low concentrations of Lat-A would cause their disassembly and reveal nascent myofibrils forming along them. We found that Lat-A has a differential effect on stress fibers in fibroblasts when compared to its effect on different stages of assembling myofibrils. Stress fibers are very sensitive to Lat-A with submicromolar concentrations inducing their reversible loss in non-muscle cells. In contrast, the premyofibrils and mature myofibrils in skeletal muscle cells were not affected by these low concentrations of Lat-A. With increasing concentrations and incubation times, however, Lat-A induced premyofibrils to disassemble. When premyofibrils disassembled there was no evidence of adjacent fibrils unaffected by Lat-A. Removal of the drug induced the reassembly of premyofibrils in the spreading edges of the myotubes, and resumption of the assembly of mature myofibrils. The mature myofibrils retained their structure after several hours of high doses of Lat-A.
Myotubes were stained with an antibody directed against CapZ, the capping protein that binds to the fast growing end of actin filaments embedded in the Z-bands of mature myofibrils (Casella et al., 1987; Schafer et al., 1993), to determine at which of the different three different stages of myofibrillogenesis this actin stabilizing protein appeared. CapZ is not present in the premyofibrils, but is detected in nascent myofibrils, and becomes localized in the Z-bands of mature myofibrils. These Lat-A and CapZ results are discussed in terms of the various models of myofibrillogenesis.
Materials and Methods
Cell culture and transfection
Skeletal myoblasts were isolated from the breast muscles of 9-day old quail embryos and plated on collagen coated 35 mm MatTek (Ashland, MA) dishes at concentrations of 105 cells per dish according to procedures described in Dabiri et al. (1999). Following two days of growth the cells were transfected with GFP-actin or GFP-α-actinin plasmid DNA using the FuGene6 (Dabiri et al., 1999; Ayoob et al., 2000). DNA-liposome complexes were prepared by combining 3 µl FuGene-6 transfection reagent with 1µg plasmid in 100 µl of serum-free medium for each dish. After incubating for 30 minutes, the complexes were added drop wise to the dishes with 1.5 ml transfection medium. After 24 hours, the cells were washed 3 times with normal skeletal muscle medium so that cells could be followed for further experiments.
Latrunculin-A treatment and recovery
Lat-A (Molecular Probes Inc., Eugene, OR) was dissolved in dimethyl sulfoxide (DMSO) at stock concentrations of 500–2000 µM and stored at −20 C. The stock solution was diluted to the desired concentration in culture medium prior to incubation with cultured cells. Addition of culture medium containing DMSO but lacking Lat-A had no effect on the cytoskeleton of non-muscle and muscle cells over the different periods of observations (data not shown). To reverse the drug effect, the treated cells were carefully washed five times with prewarmed control culture medium and cells monitored for changes.
Immunostaining of fixed cells
Cells intended for immunostaining were grown in dishes on collagen coated cover slips at concentrations of about 105 cells per dish for three days and were incubated in the tissue culture incubator with different concentration of Lat-A. After the desired incubation times, cells were immediately fixed with buffered 3% paraformaldehyde, permeabilized, and stained with antibody against sarcomeric α-actinin (Clone EA-53, Sigma, St. Louis, MO, USA) and a rhodamine-labeled secondary antibody and FITC-phalloidin (Molecular Probes, OR, USA) (Dabiri et al., 1999). CapZ antibodies (MAB 1E5.25.4) were obtained from the Hybridoma Bank (Madison, WI, USA), and detected using fluorescently secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA).
Microscopy of live cells
The cells were observed at various times after Lat-A incubation using a Nikon microscope with a 100X phase-contrast objective. The live transfected cells were maintained at 37 C, 5% CO2 with humidity as previously reported (Dabiri et al, 1997). Images of living and stained cells were acquired with a Hamamatsu Orca CCD camera and processed with Metamorph image processing software and Adobe Photoshop as previously reported (Siebrands et al., 2004; Golson et al., 2004).
Results
Myofibrillogenesis in cells transfected with GFP-alpha-actinin
Myotube cultures were transfected with GFP-actin or GFP-α-actinin on the second day of culture as the myoblasts began to fuse and form multinucleated myotubes. Within 24 hours the fluorescently labeled proteins were expressed in fibroblasts, myoblasts and myotubes and had localized with the endogenous actin or α-actinin in the cells.
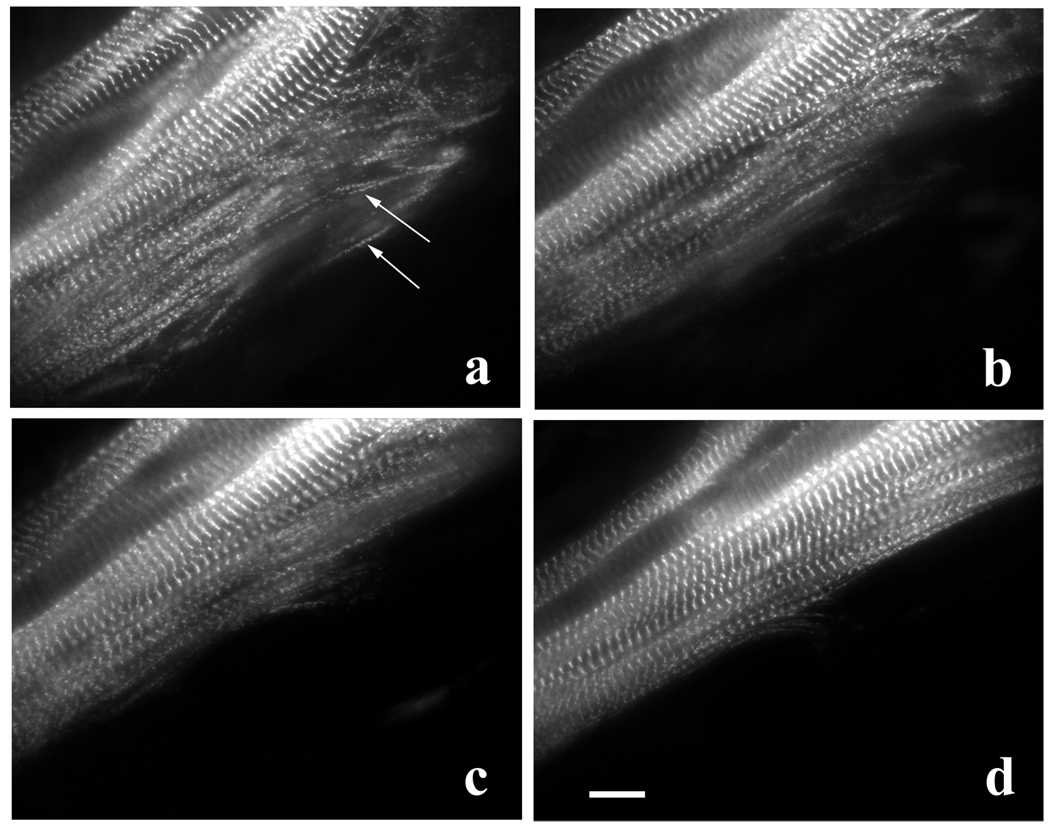
GFP-α-actinin was most prominently localized in banded patterns in myotubes (Z-bands of mature myofibrils) after 1–2 days of transfection (Figure 1). In peripheral areas of transfected myotubes, especially at the sides (arrows, Figure 1 a) and the ends (not shown) linear arrays of closely spaced bodies of α-actinin, i.e. Z-bodies, marked the repeating units of mini-sarcomeres that compose premyofibrils and nascent myofibrils (Sanger et al., 2002). Over the 4 – 6hrs of observation the Z-bodies fused with one another to form Z-bands of mature myofibrils (Figure 1 a–d). During the transformation of the premyofibrils into mature myofibrils, the myotube became cylindrical in shape, and it was difficult to detect premyofibrils at the rounded sides (Figure 1 d).
Figure 1.
Formation of mature myofibrils along the side near the end of a myotube. Myotube expressing GFP-α-actinin is shown at the same microscope stage coordinates at (a) 0, (b) 1 hour, (c) 2 hours, and (d) 4 hours of observation. During this time the Z-bodies of premyofibrils at the edge of the myotube (a, arrows) aligned and fused to form the Z-bands of mature myofibrils (a to d). Bar = 10 microns.
Latrunculin-A Effect on Stress Fibers and Premyofibrils
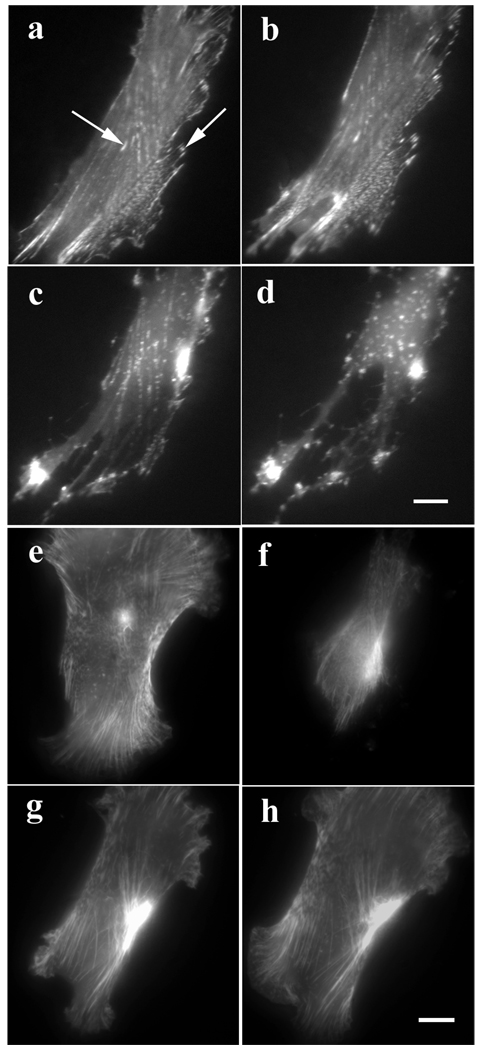
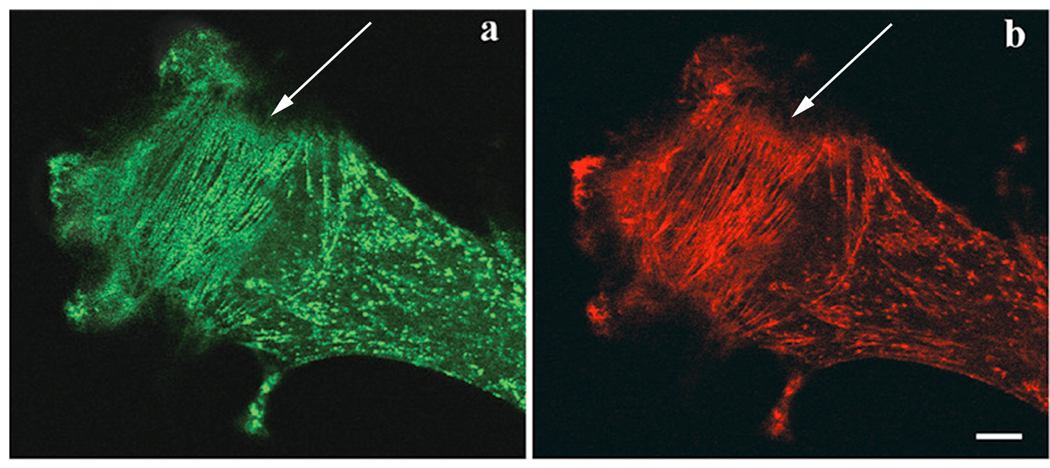
In fibroblasts in the myotube cultures, GFP-actin or GFP-α-actinin localized in stress fibers, adhesion plaques, and membrane ruffles (Figure 2). When exposed to 0.5 µM Lat-A, stress fiber disassembly began at about 10 minutes and by 30 minutes, most of the stress fibers and adhesion plaques had disassembled with the concurrent formation of bright dots and patches of alpha-actinin (Fig. 2 a–d). Application of higher levels of Lat-A (5 µM) led to disassembly of stress fibers and retraction of the cell margins in fewer than 5 minutes (Figure 2 e, f). Reformation of stress fibers and respreading of cells occurred within 1–2 hours when Lat-A was removed from the medium (Figure 2 g, h).
Figure 2.
(a–d) Effect of Lat-A on stress fibers in a fibroblast, which had been transfected with GFP-α-actinin. (a) Untreated control cell. (b–d) The same cell treated with 0.5 µM Lat-A for (b) 10 minutes, (c) 20 minutes, and (d) 30 minutes, lost its stress fibers and focal adhesions. (e–h) Reversal of Lat-A effect on actin in a fibroblast previously transfected with GFP-actin. (e) Untreated control cell. (f) The same cell treated with 5 µM Lat-A for 5 minutes has lost most of its stress fibers. The same cell (g) 30 minutes and (h) 1 hr after Lat-A was removed. The cell spread and regained its polarized shape and stress fibers. Bars = 10 microns.
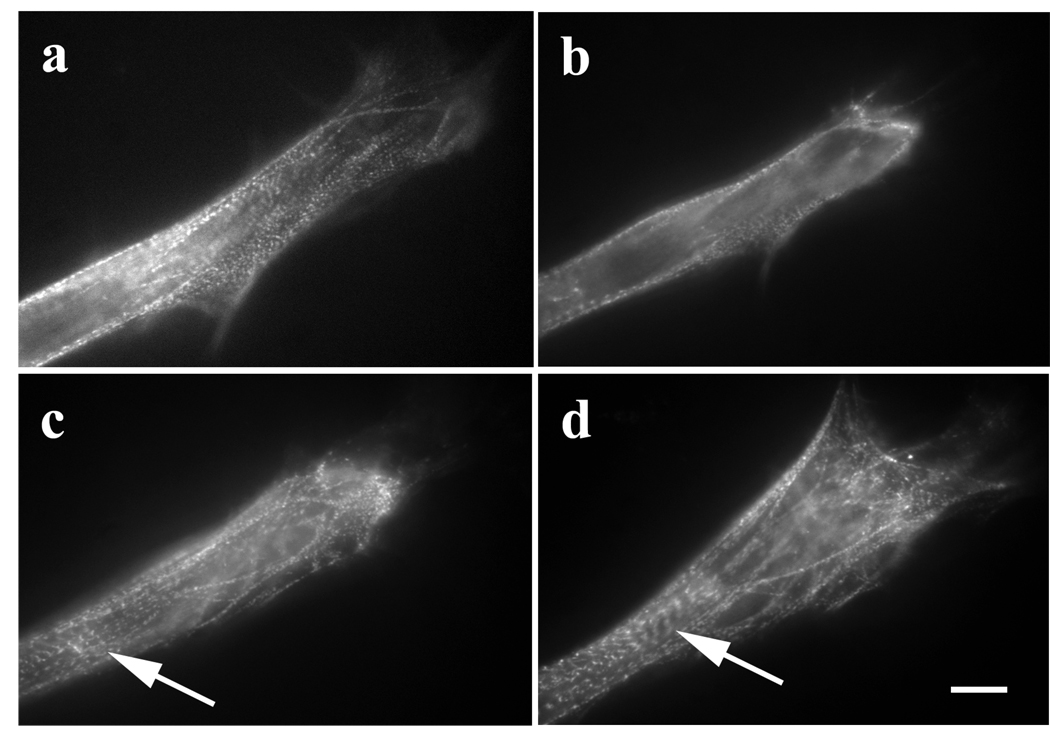
Whereas 5µM Lat-A disrupted stress fibers in fibroblasts within 5 minutes (Figure 2 e, f), it took 25 minutes for the same concentration to cause significant disruption of premyofibrils (Figure 3 a, b). As was the case with stress fibers, premyofibrils reformed after removal of Lat-A (Figure 3 c, d). The release of the myotube from exposure to Lat-A was accompanied by elongation at the end of the myotube (Figure 3 b–d) and myofibril assembly near the ends of the myotubes. In untreated myotubes, the transition from premyofibrils to mature myofibrils took 3–4 hours (Figure 1 b to d). In contrast, in the Lat-A reversed myotubes, new premyofibrils were deposited at the spreading edges of the myotubes, and some of them then fused to form new mature myofibrils during the 60 minute recovery period (Figure 3 c, d).
Figure 3.
Recovery of premyofibrils and formation of myofibrils in a myotube after reversal of Lat-A effect. (a) Control myotube transfected with GFP-alpha-actinin. (b) Same myotube after for 25-minute exposure to 5 µM Lat-A. The premyofibrils close to the end of the myotube were lost. (c) and (d) Recovery after the removal of Lat-A: (c) 30 minutes and then (d) 1 hr recovery. The myotube reformed its premyofibrils, and some of them assembled into mature myofibrils (arrows, c, d). Bar = 10 microns.
Latrunculin-A and Myofibrils
Live cells
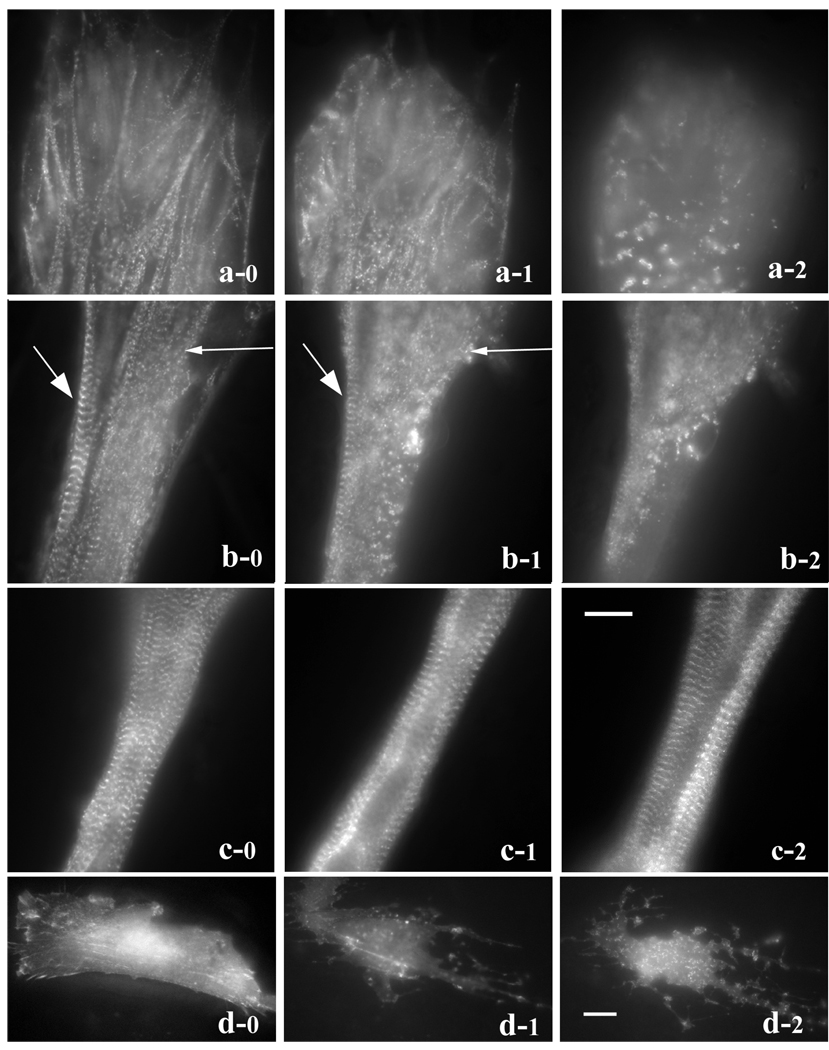
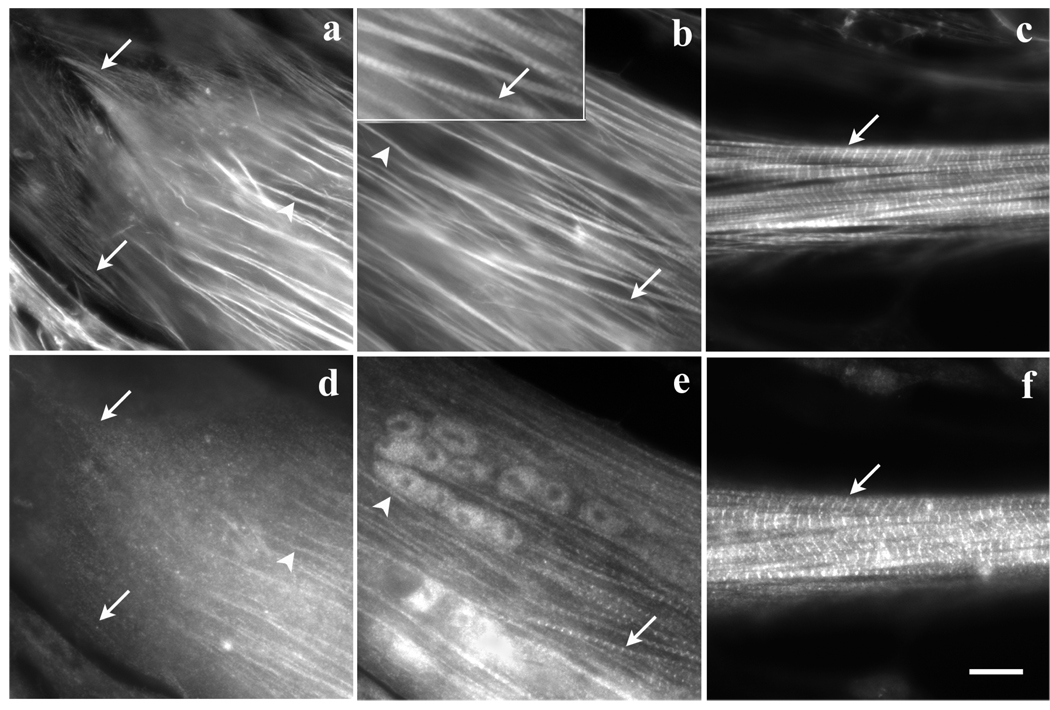
To compare the effects of Lat-A on myofibrils at different stages of assembly we took advantage of the fact that myofibril assembly is spatially polarized in elongated myotubes. The earliest stage of myofibril formation is seen in the population of premyofibrils in the flat ends and sides of the tips of the myotubes. Adjacent to the myotube end is a transition zone with mature myofibrils and premyofibrils, and distal to that is the zone where mature myofibrils fill the rounded shaft of the myotube. Transfected fibroblasts and myotubes in the same cultures were followed at 1-hour time points as the concentration of Lat-A increased from 0.2 µM to 0.5 µM, 1 µM, 2 µM, 5 µM and 10 µM (Figure 4). Three regions of the same myotube were identified: (region a) the flattened end of the myotube containing premyofibrils; (region b) the adjacent region that was partially rounded and contained mature myofibrils as well as premyofibrils along the side of the myotube; and (region c) a more distal region that was cylindrical and filled with mature myotubes (Figure 4). When the concentration of Lat-A was increased to 1 µM, the premyofibrils in the end of the myotube were partially disrupted (Figure 4 a-1). Distal to the myotube end, premyofibrils were disrupted when the Lat-A concentration reached 5µM, but an adjacent mature myofibril remained intact (Figure 4 b-1, large arrow). The premyofibrils in the end of the myotube as well as those in the adjacent region were totally disrupted (Figure 4 a-2, b-2). Unlike the mature myofibril along the side of the myotube in region b that was disrupted when the Lat-A concentration reached 10µM (Figure 4 b-2), mature myofibrils the cylindrical region c of the myotube remained intact after the 10µM exposure step (Figure 5 c-1) continued to retain their Z-band alignment at the end of an additional 4 hrs in 10 µM Lat-A (Figure 4 d-2). By comparison the stress fibers in the fibroblasts in the culture were disrupted after a one-hour exposure to 0.2µM (Figure 4 a-2) followed by a one-hour exposure to 0.5µM Lat-A (Figure 4 a-2), a condition in which the premyofibrils in myotubes were unaffected (not shown).
Figure 4.
Differential effects of Lat-A (a–c) on myofibrils in different stages of assembly in three separate regions of a single myotube and on (d) stress fibers in a fibroblast in the same culture. Cells in the culture were transfected with GFP-alpha-actinin and coordinates of each of the three regions of the myotube were marked so that images at each time point were recorded in the same places. (a) End of myotube where the premyofibrils are located. (b) Transition zone near the end of myotube where mature myofibrils (large arrow) and assembling myofibrils (small arrow) are present. (c) Middle rounded region of the myotube where only mature myofibrils are detected.
| FIGURE | CONCENTRATION STEP | HRS IN LAT-A | |
|---|---|---|---|
| MYOTUBE END | a-1 | 1.0µM | 3 |
| MYOTUBE END | a-2 | 10.0µM | 6 |
| MYOTUBE PROXIMAL TO END | b-1 | 5.0µM | 5 |
| MYOTUBE PROXIMAL TO END | b-2 | 10.0µM | 6 |
| MYOTUBE ROUNDED MIDDLE | c-1 | 10.0µM | 6 |
| MYOTUBE ROUNDED MIDDLE | c-2 | 10.0µM | 10 |
| FIBROBLAST | d-1 | 0.2µM | 1 |
| FIBROBLAST | d-2 | 0.5µM | 2 |
(a–d column 0) before Lat-A treatment.
(a-1) Premyofibrils were partly disrupted after the 1 µM incubation step and completely disassembled (a-2) when the Lat-A concentration reached 10 µM.
(b-1) In the transition zone, premyofibrils (small arrow) disassembled before neighboring mature myofibrils (large arrow) after the 5 µM exposure. (b-2) after the 10 µM exposure step both types of myofibrils had disassembled.
(c-1) In contrast, older mature myofibrils in the rounded region of the myotube remained intact after the exposure step in 10 µM Lat-A and continued to be unaffected at the end of an additional 4 hours in 10 µM Lat-A (c-2).
(d-1) Stress fiber disassembly occurred in a fibroblast after 1hr exposure to 0.2 µM Lat-A, and cell morphology was disrupted after the exposure was increased to 0.5 µM Lat-A for 1hr (d-2). Bar = 10 microns.
Figure 5.
Control (a, b) and Lat-A treated (c, d) myotubes stained with (a, c) anti-sarcomeric alpha-actinin antibody and (b, d) rhodamine phalloidin. (a, b) At the end of one myotube (arrows), premyofibrils have (a) a banded distribution of alpha-actinin with short sarcomeric spacings and (b) unbanded actin staining. Along the length of the other myotube are mature myofibrils with alpha-actinin in Z-bands and actin filaments that are banded with phalloidin staining. (c, d) Parts of two myotubes treated with 5 µM Lat-A for one hour. Multiple patches of alpha-actinin and actin fill the end of one myotube (arrows) with no sign of premyofibrils. The mature myofibrils in the central region of the second myotube were unaffected by Lat-A. Bar = 10 microns.
Immunostained myotubes
Untransfected myotubes stained with sarcomeric alpha-actinin and rhodamine phalloidin either control (Figure 5 a, b) or treated with Lat-A (Figure 5 c, d) showed the same changes in actin filament sensitivity in premyofibrils at the ends of the myotubes and mature myofibrils in the mid-regions of myotubes. Treatment of untransfected muscle cells with 5 to 10 µM Lat-A led to the loss of the premyofibrils at the end of a myotube while mature myofibrils in the central shaft of a second myotube were unaffected (Figure 5 c, d). Removal of Lat-A led to the reformation of rows of premyofibrils that were aligned parallel to the spreading membrane of the myotube end (Figure 6 a, b). This orientation of premyofibrils also occurs in control myotubes.
Figure 6.
End of a myotube previously exposed to 10 µM Lat-A for one hour to disrupt all premyofibrils and then rinsed with control skeletal muscle medium. After a two-hour recovery period, premyofibrils (arrows) formed in the end of the myotube. (a) Alpha-actinin antibody staining. (b) Phalloidin staining. Bar = 10 microns.
CapZ localization in control myotubes
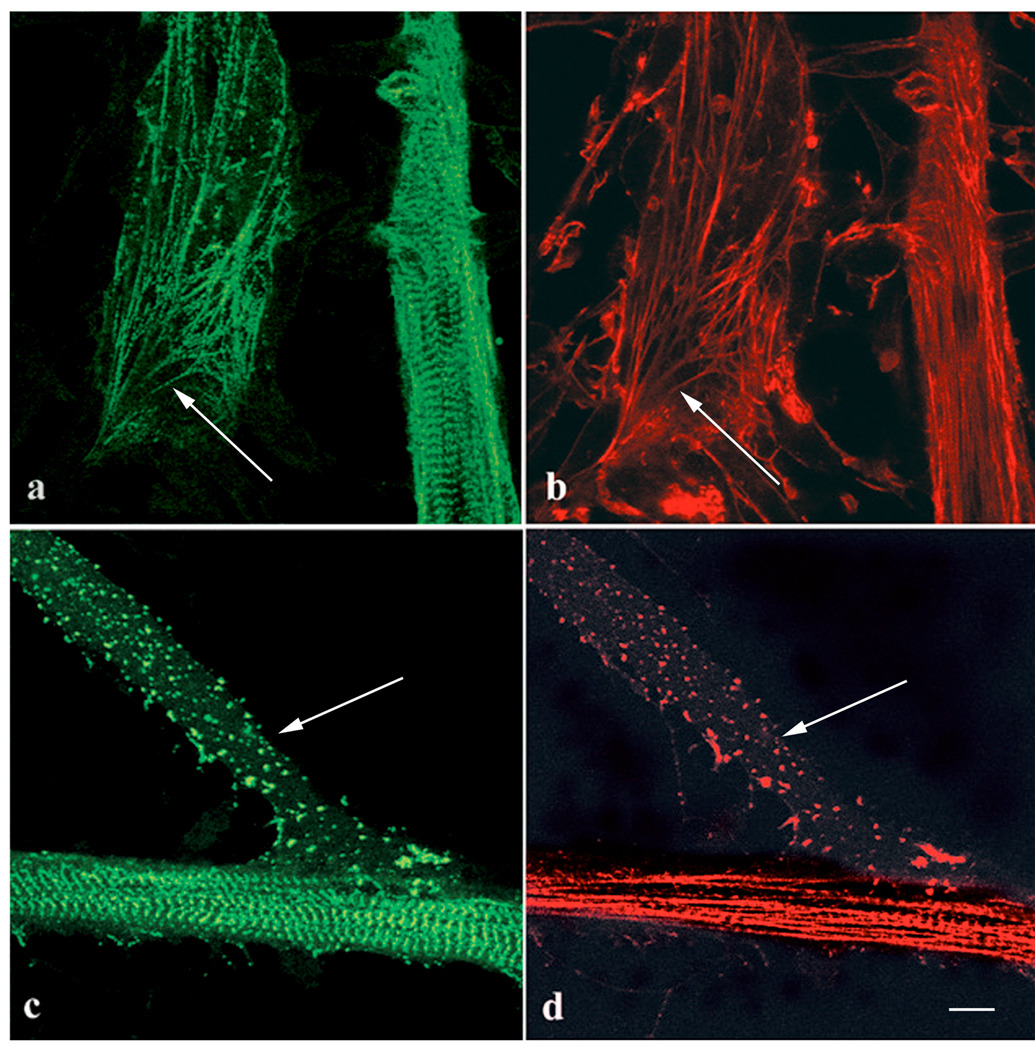
When control myotubes were stained with anti-CapZ antibodies and fluorescently labeled phalloidin (Figure 7 a–d), distinct patterns of staining were seen in three different regions comparable to the three regions described for Figure 4. The immunofluorescent images indicate that Cap-Z staining is absent from the premyofibrils nearest the tips of myotubes and light continuous staining along fibrils immediately proximal to the tip (Figure 7 a, d). In the transition zone phalloidin staining was either continuous or in closely spaced bands (Figure 7 b) and the Cap-Z was localized in narrow bands with 1.5 – 1.7µm spacing (Figure e). Striated patterns of both F-actin and CapZ were always detected in the central rounded parts of the skeletal muscle myotubes (Figure 7 c, f). The bands of Cap-Z colocalize with the bright phalloidin bands that result from overlapping actin filaments in the Z-band. The Z-band spacing in the mature myofibrils measured 1.9 – 2.2µm.
Figure 7.
Three regions of the same myotube stained with (a–c) fluorescently labeled phalloidin and (d–f) anti-CapZ antibodies. (a, d) Actin fibers that extend to the end of the myotube were unstained by the CapZ antibody (arrows in a, d), but some of the more distal fibers (arrowheads) stained with CapZ. (b, e) Fibers in a region of the same myotube near the tip stain with both (b) phalloidin and (e) anti-CapZ antibodies. CapZ was localized in some fibers (arrowheads, b, e) in a continuous pattern and on other fibers (arrows, b, e) it was in a striated pattern with sarcomeric spacings shorter (1.7µm) than the 2 µm sarcomere lengths of mature myofibrils. (c, f) In the rounded central shaft of the same myotube, both (c) phalloidin and (f) anti-CapZ antibodies are in a periodic pattern of 2 µm sarcomeric spacings in the mature myofibrils. The bright band of phalloidin staining is the Z-band where actin filaments overlap. The position of these mature myofibrils corresponds to the region of the myotube where myofibrils were resistant to the effects of Lat-A (see Figure 4 c). Bar = 10 microns.
Discussion
Stress fibers versus premyofibrils
It is clear from these results that the sensitivity of actin filaments to Lat-A in cells in the myotube culture decreased in order from mature myofibrils, to nascent myofibrils/premyofibrils, to stress fibers. We have reported similar differences between stress fibers and premyofibrils in response to the microinjection of vitamin-D binding protein (VDBP), a monomeric actin binding protein in vertebrate blood, and cytochalasin-B, that binds the fast growing end of actin filaments (Sanger et al., 1990). VDBP, injected into cells, or cytochalasin-B each induced stress fiber disassembly in the nonmuscle cells, but did not affect premyofibrils and mature myofibrils in myocytes (Sanger, 1974; Sanger et al., 1990). Structurally stress fibers and premyofibrils are very similar in structure (Rhee et al., 1994). The terms stress fibers and stress fiber-like structures (SFLS) have been used to specify fibrils in myocytes (Dlugosz et al., 1984) that we refer to as premyofibrils. True stress fibers, however, are composed exclusively of non-muscle protein isoforms whereas SFLS and premyofibrils are now known to be composed of muscle protein isoforms with the notable exception of their myosin II filaments that are composed of non-muscle myosin II (Rhee et al., 1994; Sanger et al., 2004, 2005). The effects of drugs that disrupt actin filaments suggest that the muscle proteins associated with actin filaments in myocytes have a greater stabilizing effect on actin than the non-muscle homologues associated with stress fibers.
Premyofibrils versus mature myofibrils
Concentrations of Lat-A that caused premyofibrils to disassemble had no observable effect on mature myofibrils in the rounded central region of myotubes, which were resistant to the effects of Lat-A (Figure 4, Figure 5 c, d), even at 50 µM over several hours. However, mature myofibrils, in regions of myotubes adjacent to the ends where premyofibrils form, could be partially disassembled by very high concentrations of Lat-A (10µM) (Figure 4). These myofibrils presumably represent recently formed mature myofibrils. The regional differences in the effect of Lat-A in myotubes suggest a gradual stepwise increase in the complexity of mature myofibrils.
Two proteins that could confer stability to actin filaments in mature myofibrils are the actin-capping proteins: CapZ at the barbed ends of actin filaments (the usual sites of rapid actin polymerization) embedded in the Z-bands, and tropomodulin at the pointed ends (the usual sites for slow polymerization) of the actin filaments interdigitating with the myosin thick filaments in the A-bands (Casella et al., 1987; Schafer et al., 1993; Almenar-Queralt et al., 1999). However microinjection of rhodamine actin into cardiomyocytes shows that actin monomers are incorporated at both ends of the filament with greater amounts incorporated at the pointed end (Littlefield et al., 2001). This occurred in cardiomyocytes with Cap-Z and tropomodulin at the ends of the thin filaments and involved exchanges of endogenous actin monomers with labeled monomers. The dynamic exchange of actin monomers could be inhibited at the pointed end by overexpression of tropomodulin, and at the barbed end by capping with cytochalasin D (Littlefield et al., 2001). In the presence of Lat-A, as in the present study, actin monomers will be sequestered by Lat-A leading ultimately to the disassembly of the actin filaments. The absence of capping proteins on actin filaments would be expected to increase the dynamic exchange of monomers with the filaments making the filaments more sensitive to disassembly by Lat-A.
Tropomodulin is seen the earliest stages of myofibril assembly (i.e. in premyofibrils) in cultured skeletal muscle cells (Almenar-Queralt et al., 1999), although in cultured cardiomyoctes, it is detected only in mature myofibrils (Gregorio and Fowler, 1995). CapZ is only fully organized in periodic structures later in myofibrillogenesis, when it localizes in the Z-bands of mature myofibrils (Schaefer et al., 1993; Figure 7). In agreement with these observations, our immunofluorescence images show Cap-Z staining absent from the premyofibrils at the tips of myotubes and light staining in a continuous pattern along adjacent fibrils (putative nascent myofibrils (Golson et al., 2004) (Figure 7 d). In the transition zone between tip and central shaft the Cap-Z staining was periodic in a pattern shorter (about 1.7 microns; Figure 7 e) than that in mature myofibrils (sarcomeric spacings of 2.0 to 2.2 microns; Figure 7 f). The absence of CapZ in the premyofibrils and its presence in nascent myofibrils may explain the greater sensitivity of premyofibrils to Lat-A. Other proteins that may stabilize the actin filaments in muscle cells like nebulin, muscle tropomyosin and the troponins, as well as tropomodulin, appear in premyofibrils, but may increase in concentration and/or binding as myofibrils mature.
The presence of tropomodulin in the premyofibrils may also explain their delayed disassembly in Lat-A compared to stress fibers in fibroblasts. The dynamic activity of actin (and other Z-body and Z-band proteins) is necessary for premyofibrils to anneal and remodel to form solid Z-bands typical of mature myofibrils (Dabiri et al., 1997; Wang et al., 2005). The gradual addition of a full complement of capping proteins to mature myofibrils should permit the thin actin filaments to be stabilized and thus become more and more resistant to the actions of Lat-A (Figure 8).
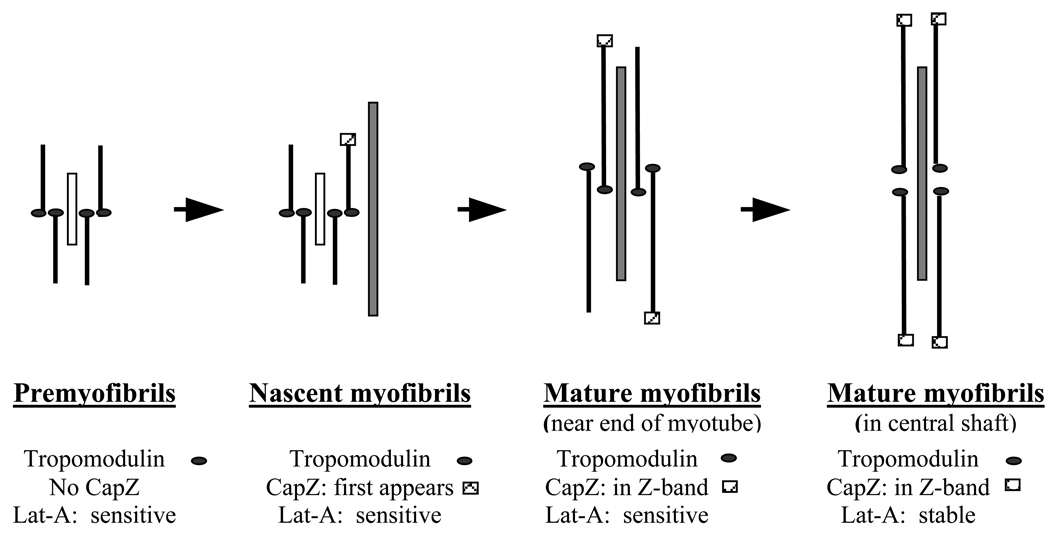
Figure 8.
Diagram summarizing the effects of Lat-A on the sarcomeric repeating units of assembling myofibrils in skeletal muscle cells. Two proteins that may stabilize the actin filaments by capping opposite ends of the filaments: tropomodulin on the slow growing end and Cap-Z on the fast growing end are shown. Tropomodulin is shown to be present on the ends of the premyofibrils based on data that it is on actin filaments at all stages of assembly beginning with premyofibrils (Almenar-Queralt et al., 1999). Premyofibrils and Nascent myofibrils are near the ends of myotubes with premyofibrils closest to the ends. Both types of fibrils stain in a continuous pattern with phalloidin suggesting that their actin filaments overlap along the fibrils, whereas alpha-actinin localization is periodic. Non-muscle myosin II (short white bar) is in both types of fibrils in a periodic distribution, and muscle specific myosin is also associated with the nascent myofibrils (long grey bar) (Sanger et al., 2002; Golson et al., 2004). Based on Lat-A sensitivity, there were two classes of Mature myofibrils. Mature myofibrils in the central shaft of the myotube with well-defined banded concentrations of Cap-Z were stable in 50 µM Lat-A. Intermediate in sensitivity between the nascent myofibrils close to the ends of the myotube were mature myofibrils located between the ends and the rounded central shaft. Myofibrils in these regions disassemble in 10 µM Lat-A after two hours. The decreasing sensitivity of assembling myofibrils to Lat-A during myofibrillogenesis suggests that the thin filaments may not have their full complement of CapZ or other actin associated proteins until late in the formation of mature myofibrils.
Fluorescence Recovery After Photobleaching (FRAP), a quantitative optical technique for measuring the dynamics of molecules leaving and entering macromolecular complexes (White and Stekzer, 1999; Bastiaens and Pepperkok, 2000), demonstrated that actin and alpha-actinin exchanged more rapidly in premyofibrils than in mature myofibrils (McKenna et al., 1985 a, b; Hasebe-Kishi and Shimada, 2000; Shimada et al., 2001; Wang et al., 2005). The greater dynamics of actin in the premyofibrils is correlated with their increased sensitivity to Lat-A. At present there are no data on the dynamic exchange of CapZ from the barbed ends of the thin filaments in the Z-bands, but the binding of tropomodulin to the pointed ends of cardiomyocyte actin filaments is very dynamic with complete exchange in ten minutes measured with FRAP analysis (Littlefield et al., 2001).
Import of Lat-A results for Myofibrillogenesis
Our observations on skeletal myofibrillogenesis indicate that mature myofibrils are gradually assembled from previously deposited premyofibrils (Figure 1, Figure 3; Sanger et al., 2002; Golson et al., 2004). The Lat-A results on mature myofibrils in different regions of the same myotubes suggest that there is a gradual change in the maturation of actin filaments in the assembling myofibrils. It is also notable that reversal from Lat-A treatment led to the reformation of premyofibrils and then their transformation to mature myofibrils. The process of premyofibril fusion detected here in skeletal muscle cells was first detected in transfected avian cardiomyocytes (Dabiri et al., 1997).
It is clear from immunofluorescence staining with isoform specific antibodies that the fibrils at the ends and sides of the myotube are not stress fibers (Holtzer et al., 1997; Sanger et al., 2005) although they are like stress fibers in morphology. These fibrils that we term premyofibrils differ from stress fibers in their response to Lat-A (Figure 4, Figure 5 c, d). Stress fibers or SFLS have been proposed to act as transitory templates along which myofibrils assemble (Dlugosz et al., 1984). At the dose/time range that caused the fibrils at the ends of the myotube to disassemble, mature myofibrils posterior to the myotube ends were unaffected. If SFLS were templates, after their disassembly, assembling myofibrils should have been visible at the ends of the myotubes and we did not see this.
Another model of myofibrillogenesis proposes that scattered I-Z-I aggregates are aligned by titin, with the subsequent recruitment of thick myosin filaments to form myofibrils (Holtzer et al., 1997). We did not see evidence for scattered I-Z-I structures in the live transfected myotubes. Disassembly of premyofibrils by Lat-A followed by removal of the drug led to the formation of linearly aligned Z-bodies and associated actin filaments (Figure 3, Figure 6 a, b) followed by formation of mature myofibrils. It has been shown previously that non-muscle myosin II is localized in premyofibrils between the alpha-actinin rich Z-bodies (Rhee et al., 1994; Sanger et al., 2002). The results in this report are consistent with a transition from premyofibril to myofibril that is accompanied by additions and/or changes in fibril proteins that increase the myofibril stability. It is also consistent with the premyofibril model of myofibrillogenesis in which myofibrils form when premyofibrils, that have a sarcomeric arrangement of non-muscle myosin, actin filaments and Z-bodies, recruit titin, and the non-muscle myosin is replaced by muscle myosin and myosin binding proteins (Rhee et al., 1994; Du et al., 2003; Sanger et al., 2002, 2005).
Acknowledgment
The authors are indebted to Drs Andrea Stout, Nancy Freeman and Ms. Victoria McManus for their comments on this manuscript. This work was supported by grants from the American Heart Association, the Muscular Dystrophy Association and the NIH.
Grant Sponsor: AHA, MDA, and NIH
References
- Almenar-Queralt A, Gregorio CC, Fowler VM. Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J Cell Sci. 1999;112:1111–1123. doi: 10.1242/jcs.112.8.1111. [DOI] [PubMed] [Google Scholar]
- Antin PB, Forry-Schaudies S, Friedman TM, Tapscott SJ, Holtzer H. Taxol induces postmitotic myoblasts to assemble interdigitating microtubule-myosin arrays that exclude actin filaments. J Cell Biol. 1981;90:300–308. doi: 10.1083/jcb.90.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoob JC, Sanger JM, Sanger JW. Visualization of the expression of green fluorescent protein (GFP)- linked proteins [In Process Citation] Methods Mol Biol. 2000;137:153–157. doi: 10.1385/1-59259-066-7:153. [DOI] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens PIH, Pepperkok R. Observing proteins in their natural habitat: the living cell. Trends Biochem. Sci. 2000;25:631–637. doi: 10.1016/s0968-0004(00)01714-x. [DOI] [PubMed] [Google Scholar]
- Casella JF, Craig SW, Maack DJ, Brown AE. Cap Z(36/32), a barbed end actin-capping protein, is a component of the Z-line of skeletal muscle. J Cell Biol. 1987;105:371–379. doi: 10.1083/jcb.105.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ML, Escaleira RC, Rodrigues VB, Manasfi M, Mermelstein CS. Some distinctive features of zebrafish myogenesis based on unexpected distributions of the muscle cytoskeletal proteins actin, myosin, desmin, alpha-actinin, troponin and titin. Mech Dev. 2002;116:95–104. doi: 10.1016/s0925-4773(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc Natl Acad Sci U S A. 1997;94:9493–9498. doi: 10.1073/pnas.94.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri GA, Ayoob JC, Turnacioglu KK, Sanger JM, Sanger JW. Use of green fluorescent proteins linked to cytoskeletal proteins to analyze myofibrillogenesis in living cells. Methods in Enzymology. In: Conn P Michael., editor. Green fluorescent protein. Vol. 302. 1999. pp. 171–186. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Antin PB, Nachmias VT, Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984;99:2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Sanger JM, Linask KK, Sanger JW. Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev Biol. 2003;257:382–394. doi: 10.1016/s0012-1606(03)00104-0. [DOI] [PubMed] [Google Scholar]
- Golson ML, Sanger JM, Sanger JW. Inhibitors arrest myofibrillogenesis in skeletal muscle cells at early stages of assembly. Cell Motil Cytoskeleton. 2004;59:1–16. doi: 10.1002/cm.20017. [DOI] [PubMed] [Google Scholar]
- Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature. 1995;377:83–86. doi: 10.1038/377083a0. [DOI] [PubMed] [Google Scholar]
- Hasebe-Kishi F, Shimada Y. Dynamics of actin and alpha-actinin in nascent myofibrils and stress fibers. J Muscle Res Cell Motil. 2000;21:717–724. doi: 10.1023/a:1010374424143. [DOI] [PubMed] [Google Scholar]
- Holtzer H, Hijikata T, Zhang ZQ, Holtzer S, Protasi SF, Franzini-Armstrong C, Sweeney HL. Independent assembly of 1.6 microns long bipolar MHC filaments and I-Z-I bodies. Cell Structure & Function. 1997;22:83–93. doi: 10.1247/csf.22.83. [DOI] [PubMed] [Google Scholar]
- Kashman Y, Groweiss A, Shmueli U. Latrunculin, a new a-thiazolidinone macrolide from the marine sponge Latrunculia magnifica. Tetrahedron Lett. 1980;21:3629–3632. [Google Scholar]
- Littlefield R, Almenar-Queralt A, Fowler VM. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol. 2001;3:544–551. doi: 10.1038/35078517. [DOI] [PubMed] [Google Scholar]
- Lu MH, DiLullo C, Schultheiss T, Holtzer S, Murray JM, Choi J, Fischman DA, Holtzer H. The vinculin/sarcomeric-alpha-actinin/alpha-actin nexus in cultured cardiac myocytes. J Cell Biol. 1992;117:1007–1022. doi: 10.1083/jcb.117.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NM, Meigs JB, Wang YL. Exchangeability of alpha-actinin in living cardiac fibroblasts and muscle cells. J Cell Biol. 1985;101:2223–2232. doi: 10.1083/jcb.101.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna N, Meigs JB, Wang YL. Identical distribution of fluorescently labeled brain and muscle actins in living cardiac fibroblasts and myocytes. J Cell Biol. 1985;100:292–296. doi: 10.1083/jcb.100.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- Rhee D, Sanger JM, Sanger JW. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motility & the Cytoskeleton. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Sanger JW. The use of cytochalasin B to distinguish myoblasts from fibroblasts in cultures of developing chick striated muscle. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:3621–3625. doi: 10.1073/pnas.71.9.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Mittal B, Sanger JM. Analysis of myofibrillar structure and assembly using fluorescently labeled contractile proteins. J Cell Biol. 1984a;98:825–833. doi: 10.1083/jcb.98.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Mittal B, Sanger JM. Formation of myofibrils in spreading chick cardiac myocytes. Cell Motil. 1984b;4:405–416. doi: 10.1002/cm.970040602. [DOI] [PubMed] [Google Scholar]
- Sanger JM, Dabiri G, Mittal B, Kowalski MA, Haddad JG, Sanger JW. Disruption of microfilament organization in living nonmuscle cells by microinjection of plasma vitamin D-binding protein or DNase I. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5474–5478. doi: 10.1073/pnas.87.14.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM. Fishing out proteins that bind to titin. J Cell Biol. 2001 a;154:21–24. doi: 10.1083/jcb.200106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM. Myofibrillogenesis in Cardiac Muscle. In: Dube DK, editor. Myofibrillogenesis. Boston: Birkhauser; 2001 b. pp. 3–20. [Google Scholar]
- Sanger JW, Chowrashi P, Shaner NC, Spalthoff S, Wang J, Freeman NL, Sanger JM. Myofibrillogenesis in skeletal muscle cells. Clin Orthop. 2002;(403 Suppl):S153–S162. doi: 10.1097/00003086-200210001-00018. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Sanger JM, Franzini-Armstrong C. Assembly of the skeletal muscle cell. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd Edition. New York: McGraw-Hill; 2004. pp. 35–65. [Google Scholar]
- Sanger JW, Kang S, Siebrands CC, Freeman N, Du A, Wang J, Stout AL, Sanger JM. How to build a myofibril. J. Muscle Res. Cell Motility. 2005 doi: 10.1007/s10974-005-9016-7. (In Press) [DOI] [PubMed] [Google Scholar]
- Schafer DA, Waddle JA, Cooper JA. Localization of CapZ during myofibrillogenesis in cultured chicken muscle. Cell Motil Cytoskeleton. 1993;25(4):317–335. doi: 10.1002/cm.970250403. [DOI] [PubMed] [Google Scholar]
- Schatten G, Schatten H, Spector I, Cline C, Paweletz N, Simerly C, Petzelt C. 2001. Latrunculin inhibits the microfilament-mediated processes during fertilization, cleavage and early development in sea urchins and mice. Exp Cell Res. 1986;166:191–208. doi: 10.1016/0014-4827(86)90519-7. [DOI] [PubMed] [Google Scholar]
- Shimada Y, New TM, Hasebe-Kishi F, Suzuki H. Dynamics of contractile proteins constituting myofibrils in living muscle cells. In: Dube D, editor. Myofibrillogenesis. New York: Springer Verlag; pp. 21–39. "Cardiovascular Molecular Morphogenesis. [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins--novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil. Cytoskeleton. 2005;61:34–38. doi: 10.1002/cm.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Stekzer E. Photobleaching GFP reveals protein dynamics inside live cells. Trends Cell Biol. 1999;9:61–65. doi: 10.1016/s0962-8924(98)01433-0. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Forry-Schaudies S, Hoffman B, Holtzer H. Effects of taxol and Colcemid on myofibrillogenesis. Proc Natl Acad Sci U S A. 1982;79:6556–6560. doi: 10.1073/pnas.79.21.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmola EG, Somasundaram T, Boring TA, Spector I, Bubb MR. Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. J Biol Chem. 2000;275:28120–28127. doi: 10.1074/jbc.M004253200. [DOI] [PubMed] [Google Scholar]