Abstract
In August 2007, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health Office of Rare Diseases, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics cosponsored a 2-day workshop to reassess the body of evidence supporting antepartum assessment of fetal well-being, identify key gaps in the evidence, and formulate recommendations for further research. Participants included experts in obstetrics and fetal physiology, and representatives from relevant stakeholder groups and organizations. This article is a summary of the discussions at the workshop, including synopses of oral presentations on the epidemiology of stillbirth and fetal neurological injury, fetal physiology, techniques for antenatal monitoring, and maternal and fetal indications for monitoring. Finally, a synthesis of recommendations for further research compiled from three breakout workgroups is presented.
Since the development of technologies for electronic fetal heart rate monitoring in the 1970s, and with increasing sophistication of ultrasound and Doppler imaging, an array of techniques for antenatal assessment of fetal well being have been introduced into clinical practice. The primary goal of antenatal testing is to identify fetuses at risk for intrauterine injury or death, so that these adverse outcomes can be prevented. Despite widespread use of these technologies, however, there is limited evidence to guide their appropriate application, or to demonstrate their effectiveness at improving perinatal outcomes.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), along with cosponsors, the NIH Office of Rare Diseases, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics, held a workshop on antepartum fetal monitoring on August 27-28, 2007 to critically assess the existing evidence and identify key gaps in knowledge. Experts were invited to summarize the current state of the art in antenatal testing methodology and indications, and to identify pressing research needs. Evidence for a number of important issues was reviewed, including the extent to which antenatal testing decreases fetal death and long term neurological disability and how antenatal testing impacts gestational age at delivery and mode of delivery. This manuscript is an executive summary of the proceedings of the workshop. Detailed manuscripts by the individual attendees, based on their presentations, were collectively published in a recent issue of Seminars in Perinatology (1).
The ultimate goal of antepartum fetal monitoring is to improve perinatal outcome, specifically by decreasing stillbirth and longer term neurologic impairments such as injury to the fetal central nervous system. The rate of stillbirth is 6.2/1000 live births and fetal deaths in the U.S., accounting for more than 55% of perinatal mortality (2). Injury to the fetal central nervous system (CNS) is expressed after delivery in a number of clinical entities and syndromes with cerebral palsy the most common. In contrast to stillbirth, where rates have declined, rates of cerebral palsy have been increasing, primarily due to increased survival of low birth weight and premature infants (3). It is widely held that 90% or more of neonatal encephalopathy cases arise prior to the onset of labor (4), but most antenatal causes of CNS injury are not detected during routine prenatal care (5).
Both stillbirth and cerebral palsy have been associated with extremes of maternal age and parity, maternal obesity, African American race, prenatal smoking, maternal medical disease, use of assisted reproductive technologies, previously affected pregnancy, fetal anomalies, multiple pregnancy, fetal growth restriction, and male fetal sex (6). These similarities in risk factors suggest that fetal CNS injury and stillbirth may share a common pathway. Some authors have postulated that observed trends in decreasing stillbirth rates may be contributing to increasing cerebral palsy rates, i.e. neurologically injured fetuses that would have previously succumbed to in utero death now survive with permanent neurological impairment.
Fetal hypoxia and acidosis represent the final common pathway to fetal injury and death in many high risk pregnancies (7). The basis for antepartum testing relies on the premise that the fetus whose oxygenation in utero is challenged will respond with a series of detectable physiologic adaptive or decompensatory signs as hypoxemia or frank metabolic acidemia develop. In one adaptive response to hypoxemia, blood flow is redirected to the brain, heart, and adrenals with subsequent decreased renal perfusion and fetal urine production, which may result in decreased amniotic fluid volume. Fetal movement is an indirect indicator of central nervous system integrity and function (8). During acute hypoxemia, fetal movements decrease, as the fetus attempts to conserve energy. Loss of fetal movement raises concern for ongoing central nervous system hypoxia and injury. A chemoreceptor response to hypoxemia leads to vagally-mediated reflex slowing of the fetal heart rate (FHR), which may appear clinically as late decelerations associated with uterine contractions.
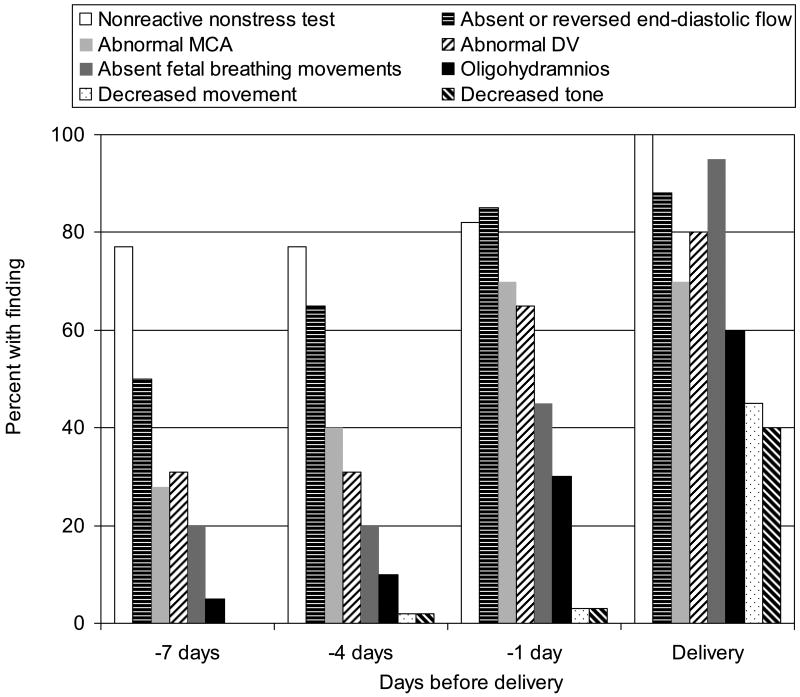
A number of investigators have described sequences of measurable changes in fetal blood flow and biophysical parameters that occur as placental insufficiency worsens and fetal hypoxemia and acidemia develop (9;10). Although the precise sequences of observed characteristics differ slightly in these reports, a general pattern of fetal response to intrauterine challenge emerges (Figure 1). Loss of FHR reactivity and abnormal blood flow in the umbilical artery are often the earliest signs of fetal compromise. Sequential changes in other fetal vessels are detectable next, followed by abnormalities in biophysical parameters such as fetal breathing movements, amniotic fluid levels, fetal body movements, and fetal tone. Not all fetuses exhibiting the full range of these findings, however, will exhibit significant metabolic acidosis at birth. In a group of 34 liveborn infants with intrauterine growth restriction delivered because of progressive deterioration in Doppler and biophysical parameter assessments, while all had abnormal arterial cord blood pH (median 7.23, range 6.95 – 7.29), the median base excess was -4.6 (range −14.5 to 0.9), and 3/34 (8.8%) had an Apgar < 7 at 5 minutes (11).
Figure 1.
Progression of Doppler and biophysical findings in severe fetal growth restriction. NST, nonstress test; absent or reversed end-diastolic flow; MCA, middle cerebral artery; DV, ductus venosus; fetal breathing movements. Data from Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol 2001;18:571-7.
Antenatal Testing Methodologies
Fetal movement counting
In the normal fetus, fetal movements are first perceptible at 17 – 20 weeks and reach peak frequency at or before 38 weeks. Fetal movement decreases in response to hypoxemia, making formalized maternal assessment of fetal movements a potentially simple method of monitoring fetal oxygenation and well being. Results of trials of routine fetal movement assessment for reduction of stillbirth have been mixed. In a randomized trial conducted in Denmark (12), fetal movement counting (FMC) was associated with a 73% reduction in avoidable stillbirths (RR 0.27, 95% CI 0.08-0.93). However, a subsequent large (N=68,654) international trial showed no difference in potentially avoidable late fetal deaths between women who were instructed to count routinely and controls (difference in mean rate -0.06/1000, 95% CI −0.76 to 0.64) (13). The results of these trials are difficult to compare because of methodologic differences, particularly in how women were instructed to count movements and how decreased fetal movement was defined. Though the “count to 10” method (14) is frequently employed, it is not clear from the existing evidence whether there is a specific fetal movement threshold or “alarm limit” below which fetal risk is increased. Some authors suggest that a more important predictor may be an overall maternal sense that fetal activity is reduced, and that any such report warrants further evaluation (15). A recent systematic review (16) concluded that there is insufficient evidence to recommend routine fetal movement counting to prevent stillbirth.
Cardiotocographic techniques: contraction stress test, nonstress test (Table 1)
Table 1. Antenatal Testing Methodologies.
| Name | Components | Results/Scoring | False Negative | False Positive | References |
|---|---|---|---|---|---|
| Contraction Stress Test (Oxytocin Challenge Test) |
|
|
0.04% | 35 - 65% | (17;101) |
| Nonstress Test |
|
|
0.2 – 0.65% | 55 - 90% | (20-22;102-104) |
| Biophysical Profile | Presence or absence of 5 components within 30 min:
|
Each component present is assigned score of 2 points; maximum score is 10/10
|
0.07 - 0.08% | 40 - 50% | (28;105;106) |
| Modified Biophysical Profile |
|
|
0.08% | 60% | (30;75;107;108) |
The contraction stress test (CST) is based on the premise that uterine contractions transiently restrict oxygen delivery to the fetus and that a hypoxic fetus will demonstrate recurrent late decelerations. The rate of antepartum stillbirth within one week of a negative CST (i.e., the false negative rate) is 0.04% (17); however, of positive tests, up to 30% have been reported to be false positive (that is, patients tolerate labor without FHR changes indicating intervention.) (18). Drawbacks to the CST include the need to stimulate contractions and the fact that inducing contractions is contraindicated in a number of conditions (e.g., placenta previa). A less intensive method, the nonstress test (NST), grew from the observations that the presence of two or more fetal heart rate accelerations during a CST most often predicted a negative CST and that absence of accelerations on a baseline FHR tracing was associated with adverse perinatal outcomes (19). The NST false negative rate is about 0.3% (20). Non reactive NSTs have about a 55% false positive rate (i.e., a backup test is normal) (21). NSTs should be performed at least twice weekly (22).
Ultrasonographic assessments: Amniotic fluid volume, biophysical profile and modified biophysical profile (Table 1)
Amniotic fluid volume (AFV) is commonly estimated by either the maximum vertical pocket (MVP) or the 4-quadrant amniotic fluid index (AFI) (23;24). By dye dilution studies, both AFI < 5 cm and MVP <2 cm had poor sensitivity for detecting true oligohydramnios (sensitivity 10% and 5%, respectively). Similarly, AFI > 20 cm and MVP > 8 cm were poor predictors of true polyhydramnios (sensitivity 29% for both) (25). The biophysical profile (BPP) combines the ultrasonographic estimation of AFV and assessments of fetal breathing, body, and reflex/tone/flexion-extension movements with the NST. (26). This test is felt to assess indicators of both acute (NST, breathing, body movement) and chronic (AFV) hypoxia, and the BPP score is linearly correlated with fetal pH (27). The risk of fetal death within one week of a normal biophysical assessment is 1 in 1300 (28). The modified biophysical profile (mBPP) relies on the NST as a measure of acute oxygenation, and the AFI as a measure of longer term oxygenation (29). In a large observational study, the false negative rate was 0.8/1000, but 60% of abnormal modified BPP's are false positive (30).
Doppler velocimetry
Measurement of blood flow velocities in the maternal and fetal vessels gives information about uteroplacental blood flow and fetal responses to physiologic challenges. Of all the antenatal assessment methods, Doppler-based tests have been most rigorously evaluated in randomized trials. The information derived from velocity waveforms in different vessels varies according to the specific vessel assessed (see Table 2).
Table 2. Doppler assessment of maternal/fetal circulation and clinical information.
| Vessel examined | Clinical Information |
|---|---|
| Uterine artery | Maternal (flow resistance to the uterus) |
| Umbilical artery | Placental (flow resistance to placenta) |
| Arterial circulation (MCA) | Fetal (fetal adaptation to flow resistance change) |
| Venous circulation (umbilical vein, inferior vena cava, ductus venosus) | Fetal (fetal cardiac function) |
Reprinted from Am J Obstet Gynecol, Vol. 191, Kontopoulos EV, Vintzileos AM, Condition-specific antepartum fetal testing, Pages 1546–51, Copyright 2004, with permission from Elsevier.
Uterine artery (UtA)
Failure of adequate trophoblast invasion and remodeling of maternal spiral arteries is characterized by persistent high-pressure uterine circulation and increased impedance to uterine artery blood flow. Elevated resistance indices and/or persistent UtA waveform notching at 22-24w indicate reduced blood flow in the maternal compartment of the placenta and have been associated with future preeclampsia, fetal growth restriction (FGR), and perinatal death (31). A number of investigators have explored the use of UtA Doppler for third trimester fetal assessment among women with complicated pregnancies (32-34), but its role in this setting has not been clearly defined.
Umbilical artery (UA)
Umbilical artery flow velocity waveforms of normally growing fetuses are characterized by high-velocity diastolic flow, while in growth-restricted fetuses, UA diastolic flow is diminished, absent, or even reversed in severe cases (35). This progressive reduction of UA diastolic flow is associated with worsening destruction of placental villous vasculature (36). In the growth restricted fetus, absent or reversed end diastolic flow is associated with fetal hypoxia (37) and increased perinatal morbidity and mortality (38). In a systematic review of 11 randomized trials enrolling approximately 7000 high-risk patients, the use of Doppler ultrasound was associated with a trend toward decreased perinatal mortality (OR 0.71, 95% CI 0.50 – 1.01) (39). UA Doppler assessments are considered most useful for monitoring of early-onset growth restriction due to uteroplacental insufficiency (40). Several randomized trials have demonstrated that routine UA Doppler screening of all pregnancies does not improve perinatal outcomes (41). Current American College of Obstetricians and Gynecologists (ACOG) practice guidelines support the use of UA Doppler assessments only in the management of suspected intrauterine growth restriction, stipulating that decisions regarding the timing of delivery should be based on UA Doppler results in combination with other tests of fetal well-being (18).
Middle cerebral artery (MCA)
In the compromised fetus, systemic blood flow is redistributed from the periphery to the brain. Doppler measurement of flow velocity in the fetal middle cerebral artery can detect this “brain-sparing effect” and has gained recent attention as an assessment tool. The limited data available currently are mixed.
Fetal veins (umbilical vein, inferior vena cava, ductus venosus)
Blood flow in the umbilical vein is continuous in normal pregnancies after 15 weeks gestation. Pathological states, such as FGR, may be associated with pulsatile flow in the umbilical vein, which is a reflection of cardiac dysfunction against increased afterload. The ductus venosus regulates oxygenated blood in the fetus (42) and is resistant to alterations in flow except in the most severely growth restricted fetuses. Recent evidence suggests that Doppler evaluation of fetal veins combined with arterial assessments is useful for predicting outcomes in growth restricted fetuses (43;44).
Emerging Methods of Fetal Assessment
Fetal physiology assessment
As the fetal central nervous system matures, there are distinctive alterations in fetal physiological and behavioral parameters, such as heart rate patterns, motor activity, and sleep-wake cycles. One important developmental feature is the increased coupling between fetal movement (FM) and FHR that normally occurs with advancing gestational age and reflects maturation of the parasympathetic and sympathetic components of the fetal autonomic nervous system. The use of a fetal actocardiograph, which electronically records FHR and FM, and novel analytic techniques allow computation of time-dependent cross-correlation coefficients between FHR and FM (45). Studies have suggested that high levels of maternal stress (46), preterm birth, and other pregnancy complications (45) are associated with alterations in FM/FHR coupling as well as FHR reactivity (47). Potential impairment or maturational delay of the fetal autonomic nervous system from a variety of insults or exposures may be detected by monitoring movement-related patterns of FHR in combination with FM/FHR coupling measures.
Fetal magnetoencephalography
Aims for direct assessment of fetal cortical and brainstem function. A specialized apparatus incorporating an array of ultrasensitive magnetic field detectors allows noninvasive, direct continuous recording of fetal electro-cortical signals, and can record fetal brain activity in response to auditory and visual stimuli applied to the maternal abdomen (48;49). This technology may contribute to future clinically important assessments of the CNS status of the fetus.
Indications for Antenatal Testing (Table 3)
Table 3. Maternal risk factors and estimated risk of stillbirth, and reported strategies for antepartum fetal surveillance.
| Condition | Prevalence | Estimated rate of stillbirth | Odds Ratio | GA to initiate testing | Testing mode and schedule | References |
|---|---|---|---|---|---|---|
| All pregnancies | -- | 6.4/1000 | 1.0 | -- | -- | (109) |
| Low-risk pregnancies | 80% | 4.0 – 5.5/1000 | 0.86 | -- | -- | (109) |
| Diabetes | ||||||
| Treated with diet (A1) | 2.5 – 5% | 6 – 10/1000 | 1.2 – 2.2 | Not indicated | -- | (54;109) |
| Treated with insulin | 2.4% | 6 – 35/1000 | 1.7 – 7.0 | A2, B, C, D without HTN, renal disease, or FGR: 32 w | CST/w, midweek NST | (52;109) |
| 32 w | NST or BPP 2x/w | (110) | ||||
| 34 w | NST 2x/w + AFI/w | (51) | ||||
| R, F: 26w | CST/w, midweek NST | (52) | ||||
| Any class with HTN, renal disease, FGR: 26 w | CST/w, midweek NST | (52) | ||||
| 28 w | NST or BPP 2x/w | (110) | ||||
| Hypertensive disorder | ||||||
| Chronic hypertension | 6 – 10% | 6 – 25/1000 | 1.5 – 2.7 | 26 w | NST, AFI 2x/w | (109;111) |
| 33 w | MBPP 2x/w | (29;112) | ||||
| With SLE or FGR or DM or PIH: 26 w | NST, AFI 2x/w | (112;113) | ||||
| Pregnancy-induced hypertension | ||||||
| Mild | 5.8 – 7.7% | 9 – 51/1000 | 1.2 – 4.0 | At diagnosis | MBPP 2x/w | (29;30;109) |
| Severe | 1.3 – 3.3% | 12 – 29/1000 | 1.8 – 4.4 | At diagnosis | NST/day with BPP if nonreactive; AFI 2x/w | (57;109) |
| Growth restricted fetus | 2.5 - 10% | 10 – 47/1000 | 7 – 11.8 | Suspected: at diagnosis | NST, AFI/w | (113-115) |
| UAD 1-2x/w | (18;116) | |||||
| Confirmed | MBPP 2x/w | (29;30) | ||||
| UAD 1-2x/w | (18;116) | |||||
| Multiple gestation | 2 – 3.5% | |||||
| Twins | 2.7% | 12/1000 | 1.0 – 2.8 | Concordant growth: 32 w | NST, AFI/w | (109;113) |
| Discordant growth: at diagnosis | MBPP 2x/w | (30) | ||||
| Triplets | 0.14% | 34/1000 | 2.8 – 3.7 | 28 w | BPP, 2x/w | (109;117) |
| Oligohydramnios | 2% | 14/1000 | 4.5 | At diagnosis | NST, AFI 2x/w | (113;118) |
| PPROM | At diagnosis | NST/day | (69;119) | |||
| BPP/day | (108;120) | |||||
| Postterm pregnancy (compared to 40w) | ||||||
| 41w | 9% | 1.6/1000 | 1.5 | 41 w | BPP 2x/w | (72;121;122) |
| 41 w | MBPP/w | (30) | ||||
| ≥ 42w | 5% | 2 – 3.5/1000 | 1.8 – 2.9 | 42 w | MBPP 2x/w | (30;72;121) |
| Previous stillbirth | 0.5 – 1.0% | 9 – 20/1000 | 1.4 – 3.2 | 32 w | MBPP 2x/w or BPP/w or CST/w | (29;82;109;120) |
| 34 w or 1 w prior to previous stillbirth | MBPP/w | (30) | ||||
| Decreased fetal movement | 4 – 15% | 13/1000 | 2.5 – 5.6 | At diagnosis | MBPP | (15;29;30;84;109) |
| SLE | < 1% | 40 – 150/1000 | 6 – 20 | 26 w | CST, BPP, or NST/w | (109;123) |
| Renal disease | < 1% | 15 – 200/1000 | 2.2 – 30 | 30 – 32 | BPP 2x/w | (109;124) |
| Cholestasis of pregnancy | < 0.1% | 12 – 30/1000 | 1.8 – 4.4 | 34 w | MBPP/w | (30;109) |
| Advanced maternal age (reference < 35 y) | ||||||
| 35 – 39 y | 15 – 18% | 11 – 14/1000 | 1.8 – 2.2 | ID | ID | (109) |
| 40 y + | 2% | 11 – 21/1000 | 1.8 – 3.3 | ID | ID | (109) |
| Black women compared with white women | 15% | 12 – 14/1000 | 2.0 – 2.2 | ID | ID | (109) |
| Maternal age < 20 | 4% | 7 – 13/1000 | 1.1 – 1.6 | ID | ID | (121;125) |
| Nulliparity | 40% | 3.8 (Sweden) | 1.2 (Sweden) | ID | ID | (93;126) |
| Very high (10-14) or extremely high (≥ 15) parity | 0.1% | 14 – 22/1000 | 2.0 – 2.2 | ID | ID | (94) |
| Assisted reproductive technology | 1% | 12/1000 | 2.6 | ID | ID | (127) |
| Abnormal serum markers | ||||||
| 1st trimester PAPP-A <5th Percentile | 5% | 9/1000 | 2.2 – 4.0 | ID | ID | (98;128) |
| 2 or more abnormal 2nd trimester quad screen markers | 0.1 – 2% | 8 – 18/1000 | 4.3 – 9.2 | ID | ID | (99) |
| Obesity (prepregnancy) | ||||||
| BMI 25 – 29.9 kg/m2 | 21% | 12 – 15/1000 | 1.9 – 2.7 | ID | ID | (109) |
| BMI ≥ 30 kg/m2 | 20% | 13 – 18/1000 | 2.1 – 2.8 | ID | ID | (109) |
| Low educational attainment (< 12 y vs. 12 y +) | 30% | 10 – 13/1000 | 1.6 – 2.0 | ID | ID | (109) |
| Smoking > 10 cigarettes/day | 10 – 20% | 10 – 15/1000 | 1.7 – 3.0 | ID | ID | (109) |
| Thrombophilia | 1 – 5% | 18 – 40/1000 | 2.8 – 5.0 | ID | ID | (109) |
| Thyroid disorders | 0.2 – 2% | 12 – 20/1000 | 2.2 – 3.0 | ID | ID | (109) |
GA, gestational age; CST, contraction stress test; w, week; HTN, hypertension; FGR, fetal growth restriction; NST, nonstress test; BPP, biophysical profile; AFI, amniotic fluid index; mBPP, modified biophysical profile; SLE, systemic lupus erythematosus; DM, diabetes mellitus; PIH, pregnancy-induced hypertension; y, years; ID, insufficient data; BMI, body-mass index.
Diabetes
Historically, insulin-dependent diabetes has been a major contributor to perinatal mortality; however, due to both improved treatment and antepartum monitoring, the stillbirth rate in pregnancies complicated by diabetes now is equivalent to or lower than uncomplicated pregnancies (50). Poorly controlled maternal diabetes is associated with increased perinatal mortality largely related to congenital anomalies and indicated preterm deliveries, but also to sudden unexplained fetal death. Though observational studies have described the use of the NST (51), CST (52), and BPP (53) in management of diabetic pregnancy, no method(s) have been assessed in well-designed clinical trials and it is not clear which method, if any, is superior. There is no evidence supporting routine antepartum fetal assessment in diet-controlled gestational diabetes (54).
Hypertensive Disorders
Maternal hypertension in pregnancy, whether chronic, pregnancy-induced, or a combination, is a risk factor for perinatal death, and is a common indication for antenatal testing (20). There are insufficient data to recommend one testing modality over another, or to make conclusions about when testing should begin and how frequently it should be repeated. Some authorities hold that mild to moderate chronic hypertension in the absence of growth restriction or superimposed preeclampsia is not an indication for routine fetal surveillance (55), and a recent systematic review concluded that benefits and harms of routine antenatal assessment in women with chronic hypertension cannot be determined with current evidence (56). No randomized trials have assessed the best method for antenatal testing in the preeclamptic patient for whom delayed delivery is desired. The National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy has recommended daily fetal movement assessment, and weekly nonstress tests and/or biophysical profiles for patients with mild preeclampsia before term (55). If fetal growth restriction or decreased AFV are present, testing is recommended twice weekly. Daily fetal cardiotocographic or ultrasound surveillance may be useful in conservative management of severe preterm preeclampsia (57).
Fetal Growth Restriction
FGR is a well-recognized risk factor for fetal death. Abnormalities in Doppler velocimetry indices may help distinguish between fetal growth restriction due to placental insufficiency, in which impedance indices tend to be increased, and growth restriction from other causes (e.g. congenital infection) or constitutional smallness, which are less frequently associated with increased impedance to blood flow (58;59). There are no data from randomized trials indicating the optimal mode and frequency of antenatal testing of the fetus with growth restriction. Given the limitations in predictive value, timing delivery based on results of antenatal testing in preterm FGR presents a particular problem, as the risks of fetal loss must be balanced against the risks of iatrogenic prematurity.
Multiple pregnancy
The greater prevalence of maternal risk factors (e.g. advanced maternal age, preterm labor, preeclampsia) and fetal risk factors (e.g. abnormal growth, abnormal placentation, congenital anomalies) contribute to higher perinatal mortality rates in multiple gestations than in singletons. Population based evidence indicates that the lowest risk for intrauterine death in multiple gestations occurs at 37-38w (60). Chorionicity is an important consideration in the assessment of risk, with higher rates of adverse outcomes among monochorionic twins. Limited data specific to twin pregnancy suggest that weekly simultaneous NSTs (60), BPPs (61), mBPPs, and UAD, alone or in combination, (62) may be of benefit in predicting outcomes in twin pregnancies. There are scant data to indicate the gestational age at which testing should start; some suggest that fetal surveillance among diamniotic-dichorionic twins with concordant growth may not be needed before 38 weeks. There is insufficient evidence to support specific recommendations for any antenatal testing strategy in triplets and higher-order multiples.
Amniotic fluid abnormalities
Abnormalities of AFV have long been viewed as risk factors for poor perinatal outcomes (63;64), although this concept has recently been called into question (65). Polyhydramnios (AFI > 24 OR MVP > 8) and oligohydramnios (AFI < 5 or MVP < 2 cm) each frequently coexist with other maternal or fetal problems, such as congenital anomalies, diabetes, hypertension, postterm pregnancy, and fetal growth restriction. There is some controversy over whether isolated oligohydramnios (66) or polyhydramnios (67) near term is associated with adverse pregnancy outcomes. In a large retrospective study, approximately 40% of repeat assessments of oligohydramnios (AFI ≤5 cm) revealed AFI > 5 cm within 3 – 4 days (68). There are few data on which to base recommendations for antenatal testing in pregnancies with abnormalities of AFV.
Preterm premature rupture of membranes (PPROM) is associated with oligohydramnios and subclinical intrauterine infection. The goal of antenatal testing in this setting is early recognition of chorioamnionitis necessitating delivery. Most experts recommend daily antenatal testing in patients with PPROM, either with the NST (69) or the BPP (70).
Roughly 40% of third-trimester polyhydramnios cases diagnosed by ultrasound have normal or near-normal AFV on subsequent assessments; outcomes in these cases are generally good (71). Persistent polyhydramnios is associated with poorer pregnancy outcomes, including fetal anomalies, maternal diabetes, and perinatal death (71); these pregnancies may benefit from antenatal surveillance, but there are little data to support specific methodologies (67).
Postterm pregnancy
Postterm pregnancy is associated with increased fetal mortality and neonatal seizures, especially if growth restriction is present (72). The optimal gestational age at which to initiate testing has not been established. Some investigators have recommended ≥ 41w, (73;74). Because adverse pregnancy outcomes increase after 40w, ACOG guidelines support initiating antenatal assessment after 40 w, though there are no randomized trial data to show that testing improves perinatal outcomes (74). Several investigators have evaluated the mBPP for monitoring postterm pregnancy (30;75).
Oligohydramnios in postterm pregnancy is associated with poorer outcomes. However, recent studies have questioned the utility of AFV estimation as an independent predictor of adverse outcomes in prolonged pregnancies (76;77). Use of the AFI versus the MVP may increase the diagnosis of oligohydramnios without impacting perinatal outcome (78). Twice-weekly assessment of AFV is commonly recommended for patients at ≥ 41 weeks gestation (74).
Elevated risk in postdates pregnancy is related to impaired placental gas exchange, therefore Doppler assessments of placental circulation would not be expected to be helpful (40). Correlation of UA Doppler results with outcome is poor (79) and sensitivity is low. There is no clear role for Doppler velocimetry in monitoring postterm pregnancies given the existing evidence.
History of prior stillbirth
A history of previous stillbirth is associated with a two- to tenfold increased risk of fetal death in subsequent pregnancy, depending in part on the etiology of the previous loss (80). Previous stillbirth has long been considered an indication for antepartum testing (81); however, there are no randomized trial data and scant other data on when to initiate testing, or whether antepartum surveillance by any method is effective at reducing the risk of recurrent stillbirth. Some authors recommend initiating testing at 34 weeks, or 1 week prior to the previous loss (30). Weeks, et al. (82) followed 300 otherwise healthy patients for whom history of prior stillbirth was the only indication for antenatal testing with weekly CSTs or semiweekly mBPPs. In this cohort, there was one perinatal death (recurrent stillbirth 3 days after a negative CST and <24 hours after a reactive NST), and 13.6% of patients were delivered for positive or equivocal fetal testing results. All 3 of the patients whose first abnormal test result occurred at <32w were delivered at term without complications. Additionally, there was no association between gestational age at previous stillbirth and the incidence of an abnormal test or cesarean delivery for fetal distress. The authors concluded that it is reasonable to initiate antenatal testing for history of stillbirth at 32 weeks. Comparing the recurrent fetal death rate in this study (1/300 or 3.3/1000) to that in another relatively low-risk population (19.0/1000, (83)), suggests that serial CSTs or mBPPs may reduce the risk of recurrent stillbirth, but this has not been rigorously tested.
Decreased fetal movement
By a number of definitions, decreased fetal movement (DFM) has been associated with adverse pregnancy outcomes such as congenital malformations (84), FGR (85), preterm delivery (86), and perinatal death (87). However, not all (88) studies link DFM to adverse outcome. DFM requires evaluation (18), but there are no randomized trials and little other data to support a specific protocol for such evaluation. Most authors (8) recommend an NST at a minimum. ACOG/AAP Guidelines for Perinatal Care recommend an NST and AFI for evaluation of DFM (89). Patients in whom an NST and ultrasound/AFV assessment are normal do not appear to require further testing (90). There is no clear evidence that adding UA or UtA Doppler assessments in the evaluation of DFM in otherwise low-risk women improves perinatal outcomes (91).
Newer indications for antenatal testing
The ACOG practice bulletin on antepartum fetal surveillance suggests that antepartum testing may be appropriate for any “pregnancies in which the risk of antepartum fetal demise is increased,” including the conditions described (18) (Table 3). Recent research has highlighted increased stillbirth risk for a number of additional conditions, including: advanced maternal age (92), nulliparity (93), grand multiparity (94), obesity (95), conception with assisted reproductive technologies (96), hereditary and acquired thrombophilias such as factor V Leiden mutation (97), and abnormalities in first and second trimester serum screening results (98;99). Whether a program of antenatal testing in women with these risk factors can reduce the incidence of stillbirth is unknown.
Benefits and costs of antenatal testing
The gaps in the evidence regarding the efficacy of antepartum testing in preventing fetal death or injury make it difficult to assess the large-scale benefits of antepartum testing in general. Limitations of the existing evidence also prevent a comprehensive understanding of the costs of antenatal fetal surveillance. Potential costs include the actual dollars spent on tests and their interpretation; opportunity costs of patients' and practitioners' time spent in testing; and maternal and infant morbidity (or even mortality), e.g. from labor inductions, cesarean deliveries, or iatrogenic prematurity, especially given the chances for false positive tests. Very little is known about the effects of antenatal testing on maternal mental states—does testing provoke anxiety or rather offer reassurance? How these potential costs balance against potential benefits is uncertain.
Challenges and Opportunities
The existing literature on the ideal use of antenatal testing and its benefit in reducing fetal death or injury is characterized by a number of overarching limitations. Importantly, much of the existing evidence is observational, and recommendations are often based on expert opinion. There is a clear need for additional randomized trial data; however, conducting well-designed randomized trials could be challenging. For one thing, despite weaknesses in evidence, antepartum testing is an accepted and expected component of prenatal care in many cases, making it difficult or impossible to design definitive trials comparing outcomes among pregnancies assigned to testing versus no testing. Furthermore, even among pregnancies at increased risk, stillbirth and CNS injury are rare outcomes, and multiple potential confounding factors must be taken into account; it is thus difficult to conduct adequately powered trials. In attempts to overcome this barrier, many investigators have assessed more common surrogate endpoints (e.g., cesarean delivery for fetal distress or meconium staining), but it is not clear which, if any, are most appropriate. Thus, for many antenatal testing strategies, there are few data directly indicating that their use reduces rates of fetal death or long-term neurological impairment. It is worth considering whether the development of alternate definitions of false-negative and false-positive tests would serve to advance research in the field.
To date, most studies on the predictive value of antenatal testing methods have been conducted in heterogeneous groups of “high-risk” pregnancies (100) (Table 3). It may not be appropriate to generalize one testing methodology to all conditions. Rather, testing protocols should be specific to the underlying risk condition prompting the assessment. Effectiveness of antenatal fetal testing in preventing stillbirth may be improved by targeting specific testing modalities to specific pathophysiologic process. Kontopoulos and Vintzileos (40) reported that condition-specific fetal testing in 12,766 high risk pregnancies at their institution resulted in a fetal death rate of 1/3191, a threefold decrease from rates where the same assessments are used without condition-specificity. Persistent gaps in our understanding of fetal disease processes and their progression limit further condition-specific application and interpretation of tests.
Condition-specific testing, however, cannot, however, fully address the scope of potentially preventable fetal death and injury. As many as 50% of late fetal deaths occur in women without identifiable risk conditions (14). It is especially difficult to design studies and strategies for using antenatal testing to prevent these unexpected losses. Some method of maternal assessment of fetal movement appears to be a promising candidate for a universal screening test, but it is not clear that this or any of the other existing methodologies can have an impact in these pregnancies at subclinical risk, at least not in the ways that they are currently applied.
For the most part, studies of antenatal testing have focused on stillbirth prevention— the body of research examining long term outcomes among surviving infants is substantially underdeveloped. Future work should adopt a wider view to investigate the role of antepartum testing in prevention of disability in addition to prevention of perinatal death. Such research must employ long term, high quality follow up, evaluate other composite short and long term outcomes (neurologic injury, neurodevelopmental outcomes, etc), and must also account for environmental and external influences after delivery.
Conclusion: Defining a Research Agenda
Priority areas for future research are highlighted in Box 1. For all areas of research, workshop participants stressed the need for well-designed randomized controlled trials whenever appropriate. For example, it would be both feasible and important to conduct trials comparing the effectiveness of different combinations of primary and secondary assessment techniques on improving perinatal outcomes.
Box 1: Recommendations for future research.
Epidemiology of stillbirth and cerebral palsy
Development of national active surveillance programs
Routine thorough etiologic investigations after stillbirth
Emphasize long term neurodevelopmental followup
Fetal/placental pathophysiology
Enhance knowledge of placental dysfunction
Observational studies of changes in fetal physiology and test results by specific disease processes
Understand possible subtypes of fetal growth restriction
Definitions and significance of amniotic fluid abnormalities
Fetal movement assessment
Improve discrimination between normal and abnormal fetal movement
Develop effective algorithm for fetal movement assessment
Identify role in universal screening or as adjunct assessment
Fetal testing technologies
Identify most appropriate method for primary surveillance and backup testing
Establish best testing intervals
Further research on ages at which to initiate testing
Best methodologies in the fetus <32w
Benefits of matching testing methods to indication and specific pathophysiology
Development of risk profiles incorporating testing results and additional pregnancy exposures and characteristics
Evaluate combinations of assessments
Research and development of technologies for early identification of the fetus at risk for neurologic injury
Indications for antenatal testing
Role of antenatal surveillance in well-controlled diabetes
Utility of Doppler ultrasound in management of preeclampsia
Use of customized growth percentiles for evaluation of fetal growth and implications for antenatal testing
Further study of testing in twins and higher-order multiples
Investigate new indications for testing: advanced maternal age, obesity, nulliparity, thrombophilia, assisted reproduction, tobacco use, previous poor pregnancy outcome
Researchers should evaluate newer systems of test interpretation on a number of levels. For example, perhaps the binary classification of NST results is an oversimplification. The implications of antepartum testing results for individual patients may be improved if they are considered in combination with pretest odds and likelihood ratios. Determination of pretest odds may be based on multiple factors, such as severity of underlying disease, socioeconomic status, previous obstetric history, obesity, and tobacco use.
Further attention to developing evidence-based testing intervals and appropriate ages to initiate testing is needed. There is little evidence to allay concerns that early onset of antepartum fetal surveillance may lead to situations in which false positive test results lead to inductions of labor, potentially higher cesarean delivery rates, and iatrogenic prematurity. Clearer data on application and interpretation of antenatal tests in fetuses < 32 weeks gestation is an important research need. A common theme was that future studies should investigate application of testing technologies and strategies specifically targeted to the underlying disease process warranting surveillance. To this end, additional observational studies to better understand placental pathophysiology and fetal reactions to specific maternal disease states are warranted. Randomized trials could compare performance of different testing strategies between groups of women with similar underlying pathology. Attention should be given to development of preconceptional and interconceptional practices that may mitigate the risk of fetal injury and death.
In summary, participants at the NICHD workshop, Antenatal Testing: A Reevaluation, identified numerous gaps in the evidence guiding the clinical application of most antepartum assessments commonly in use today. Existing data are primarily observational, and neglect some potentially important questions, such as the appropriate gestational age to initiate testing, the adaptations needed for assessment of infants at lower gestational ages, the optimal frequency of testing, and the targeting of technologies to underlying pathophysiology. Though there are challenges to designing and conducting adequately powered studies of antenatal testing strategies, further research is clearly needed.
Acknowledgments
The workshop was jointly sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the NIH Office of Rare Diseases, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics.
Appendix. Participant Listing
Stephanie Archer, MA, National Institute of Child Health and Human Development, National Institutes of Health
Ahmet A. Baschat, MB, BCH, Associate Professor, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Maryland, Baltimore
David Burchfield, MD, Professor and Chief, Department of Pediatrics—Neonatology, University of Florida
Lawrence D. Devoe, MD, Professor, Department of Obstetrics and Gynecology, Medical College of Georgia
Michael Y. Divon, MD, Chair, Department of Obstetrics and Gynecology, Lenox Hill Hospital
Eric Eichenwald, MD, Associate Professor, Department of Pediatrics—Neonatology, Baylor College of Medicine
William P. Fifer, PhD, Professor and Assistant Director, Columbia University, Sackler Institute for Developmental Psychobiology, New York State Psychiatric Institute
Christopher Harman, MD, Professor, Department of Obstetrics and Gynecology, University of Maryland, Baltimore
Rosemary Higgins, MD, National Institute of Child Health and Human Development, National Institutes of Health
John Ilekis, PhD, National Institute of Child Health and Human Development, National Institutes of Health
Hal C. Lawrence, MD, Vice President Designee, Practice Activities, American College of Obstetricians and Gynecologists
Kenneth Leveno, MD, Jack A. Pritchard Chair in Obstetrics and Gynecology, UT Southwestern Medical Center
Curtis L. Lowery, MD, Professor, Director Maternal Fetal Medicine, University of Arkansas for Medical Sciences, Department of Obstetrics and Gynecology
George A. Macones, MD, MSCE, Chairman, Department of Obstetrics and Gynecology, Washington University School of Medicine
Giancarlo Mari, MD, Professor, Department of Obstetrics and Gynecology, Wayne State University, Hutzel Hospital
Chester B. Martin, Jr., MD, Profesor Emeritus, Department of Obstetrics and Gynecology, University of Wisconsin
Pamela Murphy, BSN, RNP, Department of Obstetrics and Gynecology, University of Arkansas for Medical Sciences
Michael P. Nageotte, MD, Magella Medical Group, Long Beach, CA 90806
Nancy O'Reilly, MHS, Director, Practice Bulletins, American College of Obstetricians and Gynecologists
Susan Pagliaro, BS, National Institute of Child Health and Human Development, National Institutes of Health
Tonse Raju, MD, National Institute of Child Health and Human Development, National Institutes of Health
Uma M. Reddy, MD, MPH, National Institute of Child Health and Human Development, National Institutes of Health
Dwight J. Rouse, MD, Professor, Department of Obstetrics and Gynecology, University of Alabama at Birmingham
Hamisu Salihu, MD, PhD, Associate Professor, Department of Epidemiology and Biostatistics, University of South Florida School of Public Health
Christy Scifres, MD, Fellow, Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Washington University School of Medicine
Michael Terrin, MD, Professor of Epidemiology and Preventive Medicine, Division of Pulmonary and Critical Care, University of Maryland, Baltimore
Jonathan Weeks, MD, Norton Maternal Fetal Medicine Specialists, Louisville, KY 40207
Marian Willinger, PhD, National Institute of Child Health and Human Development, National Institutes of Health
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
For a list of workshop participants, see the Appendix online at http://links.lww.com/xxx.
Reference List
- 1.Signore C, Spong CY, editors. Antenatal Testing: A Reevaluation. Semin Perinatol. 2008;32:323–376. doi: 10.1053/j.semperi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 2.MacDorman MF, Munson ML, Kirmeyer S. Fetal and perinatal mortality, United States, 2004. Natl Vital Stat Rep. 2007;56:1–19. [PubMed] [Google Scholar]
- 3.Vincer MJ, Allen AC, Joseph KS, Stinson DA, Scott H, Wood E. Increasing prevalence of cerebral palsy among very preterm infants: a population-based study. Pediatrics. 2006;118:e1621–e1626. doi: 10.1542/peds.2006-1522. [DOI] [PubMed] [Google Scholar]
- 4.Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1549–53. doi: 10.1136/bmj.317.7172.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists' Task Force on Neonatal Encephalopathy and Cerebral Palsy ACoOaG, American Academy of Pediatrics. Neonatal encephalopathy and cerebral palsy: Defining the pathogenesis and pathophysiology. Washington, DC: American College of Obstetricians and Gynecologists; 2003. [Google Scholar]
- 6.MacDorman MF, Hoyert DL, Martin JA, Munson ML, Hamilton BE. Fetal and perinatal mortality, United States, 2003. Natl Vital Stat Rep. 2007;55:1–17. [PubMed] [Google Scholar]
- 7.Vintzileos AM, Campbell WA, Rodis JF, McLean DA, Fleming AD, Scorza WE. The relationship between fetal biophysical assessment, umbilical artery velocimetry, and fetal acidosis. Obstet Gynecol. 1991;77:622–6. [PubMed] [Google Scholar]
- 8.Olesen AG, Svare JA. Decreased fetal movements: background, assessment, and clinical management. Acta Obstet Gynecol Scand. 2004;83:818–26. doi: 10.1111/j.0001-6349.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- 9.Hecher K, Bilardo CM, Stigter RH, Ville Y, Hackeloer BJ, Kok HJ, et al. Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet Gynecol. 2001;18:564–70. doi: 10.1046/j.0960-7692.2001.00590.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrazzi E, Bozzo M, Rigano S, Bellotti M, Morabito A, Pardi G, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet Gynecol. 2002;19:140–6. doi: 10.1046/j.0960-7692.2002.00627.x. [DOI] [PubMed] [Google Scholar]
- 11.Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571–7. doi: 10.1046/j.0960-7692.2001.00591.x. [DOI] [PubMed] [Google Scholar]
- 12.Neldam S. Fetal movements as an indicator of fetal well-being. Dan Med Bull. 1983;30:274–8. [PubMed] [Google Scholar]
- 13.Grant A, Elbourne D, Valentin L, Alexander S. Routine formal fetal movement counting and risk of antepartum late death in normally formed singletons. Lancet. 1989;2:345–9. doi: 10.1016/s0140-6736(89)90535-7. [DOI] [PubMed] [Google Scholar]
- 14.Moore TR, Piacquadio K. A prospective evaluation of fetal movement screening to reduce the incidence of antepartum fetal death. Am J Obstet Gynecol. 1989;160:1075–80. doi: 10.1016/0002-9378(89)90164-6. [DOI] [PubMed] [Google Scholar]
- 15.Froen JF. A kick from within--fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13–24. doi: 10.1515/JPM.2004.003. [DOI] [PubMed] [Google Scholar]
- 16.Mangesi L, Hofmeyr GJ. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database Syst Rev. 2007:CD004909. doi: 10.1002/14651858.CD004909.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Freeman RK, Anderson G, Dorchester W. A prospective multi-institutional study of antepartum fetal heart rate monitoring. II. Contraction stress test versus nonstress test for primary surveillance. Am J Obstet Gynecol. 1982;143:778–81. doi: 10.1016/0002-9378(82)90009-6. [DOI] [PubMed] [Google Scholar]
- 18.ACOG practice bulletin. Antepartum fetal surveillance. Number 9, October 1999 (replaces Technical Bulletin Number 188, January 1994). Clinical management guidelines for obstetrician-gynecologists. Int J Gynaecol Obstet. 2000;68:175–85. [PubMed] [Google Scholar]
- 19.Evertson LR, Gauthier RJ, Schifrin BS, Paul RH. Antepartum fetal heart rate testing. I. Evolution of the nonstress test. Am J Obstet Gynecol. 1979;133:29–33. doi: 10.1016/0002-9378(79)90406-x. [DOI] [PubMed] [Google Scholar]
- 20.Freeman RK, Anderson G, Dorchester W. A prospective multi-institutional study of antepartum fetal heart rate monitoring. I. Risk of perinatal mortality and morbidity according to antepartum fetal heart rate test results. Am J Obstet Gynecol. 1982;143:771–7. doi: 10.1016/0002-9378(82)90008-4. [DOI] [PubMed] [Google Scholar]
- 21.Rochard F, Schifrin BS, Goupil F, Legrand H, Blottiere J, Sureau C. Nonstressed fetal heart rate monitoring in the antepartum period. Am J Obstet Gynecol. 1976;126:699–706. doi: 10.1016/0002-9378(76)90523-8. [DOI] [PubMed] [Google Scholar]
- 22.Boehm FH, Salyer S, Shah DM, Vaughn WK. Improved outcome of twice weekly nonstress testing. Obstet Gynecol. 1986;67:566–8. [PubMed] [Google Scholar]
- 23.Phelan JP, Ahn MO, Smith CV, Rutherford SE, Anderson E. Amniotic fluid index measurements during pregnancy. J Reprod Med. 1987;32:601–4. [PubMed] [Google Scholar]
- 24.Rutherford SE, Phelan JP, Smith CV, Jacobs N. The four-quadrant assessment of amniotic fluid volume: an adjunct to antepartum fetal heart rate testing. Obstet Gynecol. 1987;70:353–6. [PubMed] [Google Scholar]
- 25.Magann EF, Chauhan SP, Barrilleaux PS, Whitworth NS, Martin JN. Amniotic fluid index and single deepest pocket: weak indicators of abnormal amniotic volumes. Obstet Gynecol. 2000;96:737–40. doi: 10.1016/s0029-7844(00)01020-6. [DOI] [PubMed] [Google Scholar]
- 26.Manning FA, Platt LD, Sipos L. Antepartum fetal evaluation: development of a fetal biophysical profile. Am J Obstet Gynecol. 1980;136:787–95. doi: 10.1016/0002-9378(80)90457-3. [DOI] [PubMed] [Google Scholar]
- 27.Manning FA, Snijders R, Harman CR, Nicolaides K, Menticoglou S, Morrison I. Fetal biophysical profile score. VI. Correlation with antepartum umbilical venous fetal pH. Am J Obstet Gynecol. 1993;169:755–63. doi: 10.1016/0002-9378(93)90002-z. [DOI] [PubMed] [Google Scholar]
- 28.Manning FA, Morrison I, Harman CR, Lange IR, Menticoglou S. Fetal assessment based on fetal biophysical profile scoring: experience in 19,221 referred high-risk pregnancies. II. An analysis of false-negative fetal deaths. Am J Obstet Gynecol. 1987;157:880–4. doi: 10.1016/s0002-9378(87)80077-7. [DOI] [PubMed] [Google Scholar]
- 29.Nageotte MP, Towers CV, Asrat T, Freeman RK. Perinatal outcome with the modified biophysical profile. Am J Obstet Gynecol. 1994;170:1672–6. [PubMed] [Google Scholar]
- 30.Miller DA, Rabello YA, Paul RH. The modified biophysical profile: antepartum testing in the 1990s. Am J Obstet Gynecol. 1996;174:812–7. doi: 10.1016/s0002-9378(96)70305-8. [DOI] [PubMed] [Google Scholar]
- 31.Papageorghiou AT, Yu CK, Cicero S, Bower S, Nicolaides KH. Second-trimester uterine artery Doppler screening in unselected populations: a review. J Matern Fetal Neonatal Med. 2002;12:78–88. doi: 10.1080/jmf.12.2.78.88. [DOI] [PubMed] [Google Scholar]
- 32.Gudmundsson S, Korszun P, Olofsson P, Dubiel M. New score indicating placental vascular resistance. Acta Obstet Gynecol Scand. 2003;82:807–12. doi: 10.1034/j.1600-0412.2003.00203.x. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez-Andrade E, Brodszki J, Lingman G, Gudmundsson S, Molin J, Marsal K. Uterine artery score and perinatal outcome. Ultrasound Obstet Gynecol. 2002;19:438–42. doi: 10.1046/j.1469-0705.2002.00665.x. [DOI] [PubMed] [Google Scholar]
- 34.Soregaroli M, Valcamonico A, Scalvi L, Danti L, Frusca T. Late normalisation of uterine artery velocimetry in high risk pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001;95:42–5. doi: 10.1016/s0301-2115(00)00358-4. [DOI] [PubMed] [Google Scholar]
- 35.Gudmundsson S, Marsal K. Umbilical and uteroplacental blood flow velocity waveforms in pregnancies with fetal growth retardation. Eur J Obstet Gynecol Reprod Biol. 1988;27:187–96. doi: 10.1016/0028-2243(88)90122-0. [DOI] [PubMed] [Google Scholar]
- 36.Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985;92:31–8. doi: 10.1111/j.1471-0528.1985.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 37.Nicolaides KH, Bilardo CM, Soothill PW, Campbell S. Absence of end diastolic frequencies in umbilical artery: a sign of fetal hypoxia and acidosis. BMJ. 1988;297:1026–7. doi: 10.1136/bmj.297.6655.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karsdorp VH, van Vugt JM, van Geijn HP, Kostense PJ, Arduini D, Montenegro N, et al. Clinical significance of absent or reversed end diastolic velocity waveforms in umbilical artery. Lancet. 1994;344:1664–8. doi: 10.1016/s0140-6736(94)90457-x. [DOI] [PubMed] [Google Scholar]
- 39.Neilson JP, Alfirevic Z. Doppler ultrasound for fetal assessment in high risk pregnancies. Cochrane Database Syst Rev. 2000:CD000073. doi: 10.1002/14651858.CD000073. [DOI] [PubMed] [Google Scholar]
- 40.Kontopoulos EV, Vintzileos AM. Condition-specific antepartum fetal testing. Am J Obstet Gynecol. 2004;191:1546–51. doi: 10.1016/j.ajog.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Bricker L, Neilson JP. Routine doppler ultrasound in pregnancy. Cochrane Database Syst Rev. 2000:CD001450. doi: 10.1002/14651858.CD001450. [DOI] [PubMed] [Google Scholar]
- 42.Kiserud T, Eik-Nes SH, Blaas HG, Hellevik LR, Simensen B. Ductus venosus blood velocity and the umbilical circulation in the seriously growth-retarded fetus. Ultrasound Obstet Gynecol. 1994;4:109–14. doi: 10.1046/j.1469-0705.1994.04020109.x. [DOI] [PubMed] [Google Scholar]
- 43.Baschat AA, Gembruch U, Reiss I, Gortner L, Weiner CP, Harman CR. Relationship between arterial and venous Doppler and perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol. 2000;16:407–13. doi: 10.1046/j.1469-0705.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 44.Kiserud T, Kessler J, Ebbing C, Rasmussen S. Ductus venosus shunting in growth-restricted fetuses and the effect of umbilical circulatory compromise. Ultrasound Obstet Gynecol. 2006;28:143–9. doi: 10.1002/uog.2784. [DOI] [PubMed] [Google Scholar]
- 45.DiPietro JA, Irizarry RA, Hawkins M, Costigan KA, Pressman EK. Cross-correlation of fetal cardiac and somatic activity as an indicator of antenatal neural development. Am J Obstet Gynecol. 2001;185:1421–8. doi: 10.1067/mob.2001.119108. [DOI] [PubMed] [Google Scholar]
- 46.DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TR. Development of fetal movement--fetal heart rate coupling from 20 weeks through term. Early Hum Dev. 1996;44:139–51. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- 47.Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, et al. Fetal heart rate reactivity differs by women's psychiatric status: an early marker for developmental risk? J Am Acad Child Adolesc Psychiatry. 2004;43:283–90. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Eswaran H, Preissl H, Wilson JD, Murphy P, Robinson SE, Rose D, et al. Short-term serial magnetoencephalography recordings offetal auditory evoked responses. Neurosci Lett. 2002;331:128–32. doi: 10.1016/s0304-3940(02)00859-5. [DOI] [PubMed] [Google Scholar]
- 49.Holst M, Eswaran H, Lowery C, Murphy P, Norton J, Preissl H. Development of auditory evoked fields in human fetuses and newborns: a longitudinal MEG study. Clin Neurophysiol. 2005;116:1949–55. doi: 10.1016/j.clinph.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Pregnancy outcomes in the Diabetes Control and Complications Trial. Am J Obstet Gynecol. 1996;174:1343–53. doi: 10.1016/s0002-9378(96)70683-x. [DOI] [PubMed] [Google Scholar]
- 51.Kjos SL, Leung A, Henry OA, Victor MR, Paul RH, Medearis AL. Antepartum surveillance in diabetic pregnancies: predictors of fetal distress in labor. Am J Obstet Gynecol. 1995;173:1532–9. doi: 10.1016/0002-9378(95)90645-2. [DOI] [PubMed] [Google Scholar]
- 52.Lagrew DC, Pircon RA, Towers CV, Dorchester W, Freeman RK. Antepartum fetal surveillance in patients with diabetes: when to start? Am J Obstet Gynecol. 1993;168:1820–5. doi: 10.1016/0002-9378(93)90696-g. [DOI] [PubMed] [Google Scholar]
- 53.Dicker D, Feldberg D, Yeshaya A, Peleg D, Karp M, Goldman JA. Fetal surveillance in insulin-dependent diabetic pregnancy: predictive value of the biophysical profile. Am J Obstet Gynecol. 1988;159:800–4. doi: 10.1016/s0002-9378(88)80139-x. [DOI] [PubMed] [Google Scholar]
- 54.Landon MB, Vickers S. Fetal surveillance in pregnancy complicated by diabetes mellitus: is it necessary? J Matern Fetal Neonatal Med. 2002;12:413–6. doi: 10.1080/jmf.12.6.413.416. [DOI] [PubMed] [Google Scholar]
- 55.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 56.Mulrow CD, Chiquette E, Ferrer RL, Sibai BM, Stevens KR, Harris M, et al. Management of chronic hypertension during pregnancy. Evid Rep Technol Assess (Summ) 2000:1–4. [PMC free article] [PubMed] [Google Scholar]
- 57.Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514–9. doi: 10.1016/j.ajog.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Baschat AA, Weiner CP. Umbilical artery doppler screening for detection of the small fetus in need of antepartum surveillance. Am J Obstet Gynecol. 2000;182:154–8. doi: 10.1016/s0002-9378(00)70505-9. [DOI] [PubMed] [Google Scholar]
- 59.Soothill PW, Ajayi RA, Campbell S, Nicolaides KH. Prediction of morbidity in small and normally grown fetuses by fetal heart rate variability, biophysical profile score and umbilical artery Doppler studies. Br J Obstet Gynaecol. 1993;100:742–5. doi: 10.1111/j.1471-0528.1993.tb14265.x. [DOI] [PubMed] [Google Scholar]
- 60.Devoe LD, Azor H. Simultaneous nonstress fetal heart rate testing in twin pregnancy. Obstet Gynecol. 1981;58:450–5. [PubMed] [Google Scholar]
- 61.Lodeiro JG, Vintzileos AM, Feinstein SJ, Campbell WA, Nochimson DJ. Fetal biophysical profile in twin gestations. Obstet Gynecol. 1986;67:824–7. doi: 10.1097/00006250-198606000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Devoe LD, Ware DJ. Antenatal assessment of twin gestation. Semin Perinatol. 1995;19:413–23. doi: 10.1016/s0146-0005(05)80018-7. [DOI] [PubMed] [Google Scholar]
- 63.Chamberlain PF, Manning FA, Morrison I, Harman CR, Lange IR. Ultrasound evaluation of amniotic fluid volume. I. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcome. Am J Obstet Gynecol. 1984;150:245–9. doi: 10.1016/s0002-9378(84)90359-4. [DOI] [PubMed] [Google Scholar]
- 64.Chamberlain PF, Manning FA, Morrison I, Harman CR, Lange IR. Ultrasound evaluation of amniotic fluid volume. II. The relationship of increased amniotic fluid volume to perinatal outcome. Am J Obstet Gynecol. 1984;150:250–4. doi: 10.1016/s0002-9378(84)90360-0. [DOI] [PubMed] [Google Scholar]
- 65.Ott WJ. Reevaluation of the relationship between amniotic fluid volume and perinatal outcome. Am J Obstet Gynecol. 2005;192:1803–9. doi: 10.1016/j.ajog.2004.12.062. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Troendle J, Meikle S, Klebanoff MA, Rayburn WF. Isolated oligohydramnios is not associated with adverse perinatal outcomes. BJOG. 2004;111:220–5. doi: 10.1111/j.1471-0528.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- 67.Magann EF, Chauhan SP, Doherty DA, Lutgendorf MA, Magann MI, Morrison JC. A review of idiopathic hydramnios and pregnancy outcomes. Obstet Gynecol Surv. 2007;62:795–802. doi: 10.1097/01.ogx.0000290349.58707.e0. [DOI] [PubMed] [Google Scholar]
- 68.Lagrew DC, Pircon RA, Nageotte M, Freeman RK, Dorchester W. How frequently should the amniotic fluid index be repeated? Am J Obstet Gynecol. 1992;167:1129–33. doi: 10.1016/s0002-9378(12)80054-8. [DOI] [PubMed] [Google Scholar]
- 69.Harding JA, Jackson DM, Lewis DF, Major CA, Nageotte MP, Asrat T. Correlation of amniotic fluid index and nonstress test in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1991;165:1088–94. doi: 10.1016/0002-9378(91)90477-9. [DOI] [PubMed] [Google Scholar]
- 70.Vintzileos AM, Bors-Koefoed R, Pelegano JF, Campbell WA, Rodis JF, Nochimson DJ, et al. The use of fetal biophysical profile improves pregnancy outcome in premature rupture of the membranes. Am J Obstet Gynecol. 1987;157:236–40. doi: 10.1016/s0002-9378(87)80141-2. [DOI] [PubMed] [Google Scholar]
- 71.Golan A, Wolman I, Sagi J, Yovel I, David MP. Persistence of polyhydramnios during pregnancy--its significance and correlation with maternal and fetal complications. Gynecol Obstet Invest. 1994;37:18–20. doi: 10.1159/000292513. [DOI] [PubMed] [Google Scholar]
- 72.Divon MY, Haglund B, Nisell H, Otterblad PO, Westgren M. Fetal and neonatal mortality in the postterm pregnancy: the impact of gestational age and fetal growth restriction. Am J Obstet Gynecol. 1998;178:726–31. doi: 10.1016/s0002-9378(98)70482-x. [DOI] [PubMed] [Google Scholar]
- 73.Guidetti DA, Divon MY, Langer O. Postdate fetal surveillance: is 41 weeks too early? Am J Obstet Gynecol. 1989;161:91–3. doi: 10.1016/0002-9378(89)90240-8. [DOI] [PubMed] [Google Scholar]
- 74.ACOG Practice Bulletin. Clinical management guidelines for obstetricians-gynecologists. Number 55, September 2004 (replaces practice pattern number 6, October 1997). Management of Postterm Pregnancy. Obstet Gynecol. 2004;104:639–46. doi: 10.1097/00006250-200409000-00052. [DOI] [PubMed] [Google Scholar]
- 75.Clark SL, Sabey P, Jolley K. Nonstress testing with acoustic stimulation and amniotic fluid volume assessment: 5973 tests without unexpected fetal death. Am J Obstet Gynecol. 1989;160:694–7. doi: 10.1016/s0002-9378(89)80062-6. [DOI] [PubMed] [Google Scholar]
- 76.Lam H, Leung WC, Lee CP, Lao TT. Amniotic fluid volume at 41 weeks and infant outcome. J Reprod Med. 2006;51:484–8. [PubMed] [Google Scholar]
- 77.Morris JM, Thompson K, Smithey J, Gaffney G, Cooke I, Chamberlain P, et al. The usefulness of ultrasound assessment of amniotic fluid in predicting adverse outcome in prolonged pregnancy: a prospective blinded observational study. BJOG. 2003;110:989–94. [PubMed] [Google Scholar]
- 78.Alfirevic Z, Walkinshaw SA. A randomised controlled trial of simple compared with complex antenatal fetal monitoring after 42 weeks of gestation. Br J Obstet Gynaecol. 1995;102:638–43. doi: 10.1111/j.1471-0528.1995.tb11402.x. [DOI] [PubMed] [Google Scholar]
- 79.Guidetti DA, Divon MY, Cavalieri RL, Langer O, Merkatz IR. Fetal umbilical artery flow velocimetry in postdate pregnancies. Am J Obstet Gynecol. 1987;157:1521–3. doi: 10.1016/s0002-9378(87)80255-7. [DOI] [PubMed] [Google Scholar]
- 80.Reddy UM. Prediction and prevention of recurrent stillbirth. Obstet Gynecol. 2007;110:1151–64. doi: 10.1097/01.AOG.0000287616.71602.d0. [DOI] [PubMed] [Google Scholar]
- 81.Freeman RK, Dorchester W, Anderson G, Garite TJ. The significance of a previous stillbirth. Am J Obstet Gynecol. 1985;151:7–13. doi: 10.1016/0002-9378(85)90414-4. [DOI] [PubMed] [Google Scholar]
- 82.Weeks JW, Asrat T, Morgan MA, Nageotte M, Thomas SJ, Freeman RK. Antepartum surveillance for a history of stillbirth: when to begin? Am J Obstet Gynecol. 1995;172:486–92. doi: 10.1016/0002-9378(95)90561-8. [DOI] [PubMed] [Google Scholar]
- 83.Sharma PP, Salihu HM, Kirby RS. Stillbirth recurrence in a population of relatively low-risk mothers. Paediatr Perinat Epidemiol. 2007;21 1:24–30. doi: 10.1111/j.1365-3016.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 84.Valentin L, Marsal K. Pregnancy outcome in women perceiving decreased fetal movement. Eur J Obstet Gynecol Reprod Biol. 1987;24:23–32. doi: 10.1016/0028-2243(87)90033-5. [DOI] [PubMed] [Google Scholar]
- 85.Mor-Yosef S, Sadovsky E, Brzezinski A, Levinsky R, Ohel G. Fetal movements and intrauterine growth retardation. Int J Gynaecol Obstet. 1983;21:315–8. doi: 10.1016/0020-7292(83)90022-x. [DOI] [PubMed] [Google Scholar]
- 86.Valentin L, Marsal K, Wahlgren L. Subjective recording of fetal movements. III. Screening of a pregnant population; the clinical significance of decreased fetal movement counts. Acta Obstet Gynecol Scand. 1986;65:753–8. doi: 10.3109/00016348609161495. [DOI] [PubMed] [Google Scholar]
- 87.Navot D, Yaffe H, Sadovsky E. Diagnosis of fetal jeopardy by assessment of fetal movement and heart rate accelerations. J Perinat Med. 1983;11:175–8. doi: 10.1515/jpme.1983.11.3.175. [DOI] [PubMed] [Google Scholar]
- 88.Harrington K, Thompson O, Jordan L, Page J, Carpenter RG, Campbell S. Obstetric outcome in women who present with a reduction in fetal movements in the third trimester of pregnancy. J Perinat Med. 1998;26:77–82. doi: 10.1515/jpme.1998.26.2.77. [DOI] [PubMed] [Google Scholar]
- 89.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. AAP/ACOG. (Sixth) 2007 [Google Scholar]
- 90.Whitty JE, Garfinkel DA, Divon MY. Maternal perception of decreased fetal movement as an indication for antepartum testing in a low-risk population. Am J Obstet Gynecol. 1991;165:1084–8. doi: 10.1016/0002-9378(91)90476-8. [DOI] [PubMed] [Google Scholar]
- 91.Korszun P, Dubiel M, Kudla M, Gudmundsson S. Doppler velocimetry for predicting outcome of pregnancies with decreased fetal movements. Acta Obstet Gynecol Scand. 2002;81:926–30. doi: 10.1034/j.1600-0412.2002.811005.x. [DOI] [PubMed] [Google Scholar]
- 92.Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth throughout pregnancy in the United States. Am J Obstet Gynecol. 2006;195:764–70. doi: 10.1016/j.ajog.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 93.Bai J, Wong FW, Bauman A, Mohsin M. Parity and pregnancy outcomes. Am J Obstet Gynecol. 2002;186:274–8. doi: 10.1067/mob.2002.119639. [DOI] [PubMed] [Google Scholar]
- 94.Aliyu MH, Salihu HM, Keith LG, Ehiri JE, Islam MA, Jolly PE. Extreme parity and the risk of stillbirth. Obstet Gynecol. 2005;106:446–53. doi: 10.1097/01.AOG.0000165825.65203.69. [DOI] [PubMed] [Google Scholar]
- 95.Salihu HM, Dunlop AL, Hedayatzadeh M, Alio AP, Kirby RS, Alexander GR. Extreme obesity and risk of stillbirth among black and white gravidas. Obstet Gynecol. 2007;110:552–7. doi: 10.1097/01.AOG.0000270159.80607.10. [DOI] [PubMed] [Google Scholar]
- 96.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–63. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 97.Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6–14. doi: 10.1016/s0301-2115(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 98.Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial) Am J Obstet Gynecol. 2004;191:1446–51. doi: 10.1016/j.ajog.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 99.Dugoff L, Hobbins JC, Malone FD, Vidaver J, Sullivan L, Canick JA, et al. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260–7. doi: 10.1097/01.AOG.0000172419.37410.eb. [DOI] [PubMed] [Google Scholar]
- 100.Manning FA, Lange IR, Morrison I, Harman CR. Fetal biophysical profile score and the nonstress test: a comparative trial. Obstet Gynecol. 1984;64:326–31. [PubMed] [Google Scholar]
- 101.Lagrew DC., Jr The contraction stress test. Clin Obstet Gynecol. 1995;38:11–25. doi: 10.1097/00003081-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 102.Platt LD, Walla CA, Paul RH, Trujillo ME, Loesser CV, Jacobs ND, et al. A prospective trial of the fetal biophysical profile versus the nonstress test in the management of high-risk pregnancies. Am J Obstet Gynecol. 1985;153:624–33. [PubMed] [Google Scholar]
- 103.Lavery JP. Nonstress fetal heart rate testing. Clin Obstet Gynecol. 1982;25:689–705. doi: 10.1097/00003081-198212000-00007. [DOI] [PubMed] [Google Scholar]
- 104.Phelan JP, Cromartie AD, Smith CV. The nonstress test: the false negative test. Am J Obstet Gynecol. 1982;142:293–6. doi: 10.1016/0002-9378(82)90733-5. [DOI] [PubMed] [Google Scholar]
- 105.Manning FA, Morrison I, Lange IR, Harman CR, Chamberlain PF. Fetal assessment based on fetal biophysical profile scoring: experience in 12,620 referred high-risk pregnancies. I. Perinatal mortality by frequency and etiology. Am J Obstet Gynecol. 1985;151:343–50. doi: 10.1016/0002-9378(85)90301-1. [DOI] [PubMed] [Google Scholar]
- 106.Dayal AK, Manning FA, Berck DJ, Mussalli GM, Avila C, Harman CR, et al. Fetal death after normal biophysical profile score: An eighteen-year experience. Am J Obstet Gynecol. 1999;181:1231–6. doi: 10.1016/s0002-9378(99)70114-6. [DOI] [PubMed] [Google Scholar]
- 107.Nageotte MP, Towers CV, Asrat T, Freeman RK, Dorchester W. The value of a negative antepartum test: contraction stress test and modified biophysical profile. Obstet Gynecol. 1994;84:231–4. [PubMed] [Google Scholar]
- 108.Vintzileos AM, Knuppel RA. Multiple parameter biophysical testing in the prediction of fetal acid-base status. Clin Perinatol. 1994;21:823–48. [PubMed] [Google Scholar]
- 109.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–35. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 110.Landon MB, Gabbe SG. Fetal surveillance and timing of delivery in pregnancy complicated by diabetes mellitus. Obstet Gynecol Clin North Am. 1996;23:109–23. doi: 10.1016/s0889-8545(05)70247-3. [DOI] [PubMed] [Google Scholar]
- 111.Devoe LD, Ramos-Santos E. Antepartum fetal assessment in hypertensive pregnancies. Clin Perinatol. 1991;18:809–32. [PubMed] [Google Scholar]
- 112.Pircon RA, Lagrew DC, Towers CV, Dorchester WL, Gocke SE, Freeman RK. Antepartum testing in the hypertensive patient: when to begin. Am J Obstet Gynecol. 1991;164:1563–9. doi: 10.1016/0002-9378(91)91437-2. [DOI] [PubMed] [Google Scholar]
- 113.Spong CY. Assessment of fetal well being. 2002 [Google Scholar]
- 114.Fretts RC, Boyd ME, Usher RH, Usher HA. The changing pattern of fetal death, 1961-1988. Obstet Gynecol. 1992;79:35–9. [PubMed] [Google Scholar]
- 115.Smulian JC, Ananth CV, Vintzileos AM, Scorza WE, Knuppel RA. Fetal deaths in the United States. Influence of high-risk conditions and implications for management. Obstet Gynecol. 2002;100:1183–9. doi: 10.1016/s0029-7844(02)02389-x. [DOI] [PubMed] [Google Scholar]
- 116.Almstrom H, Axelsson O, Cnattingius S, Ekman G, Maesel A, Ulmsten U, et al. Comparison of umbilical-artery velocimetry and cardiotocography for surveillance of small-for-gestational-age fetuses. Lancet. 1992;340:936–40. doi: 10.1016/0140-6736(92)92818-z. [DOI] [PubMed] [Google Scholar]
- 117.Elliott JP, Finberg HJ. Biophysical profile testing as an indicator of fetal well-being in high-order multiple gestations. Am J Obstet Gynecol. 1995;172:508–12. doi: 10.1016/0002-9378(95)90564-2. [DOI] [PubMed] [Google Scholar]
- 118.Casey BM, McIntire DD, Bloom SL, Lucas MJ, Santos R, Twickler DM, et al. Pregnancy outcomes after antepartum diagnosis of oligohydramnios at or beyond 34 weeks' gestation. Am J Obstet Gynecol. 2000;182:909–12. doi: 10.1016/s0002-9378(00)70345-0. [DOI] [PubMed] [Google Scholar]
- 119.Lewis DF, Adair CD, Weeks JW, Barrilleaux PS, Edwards MS, Garite TJ. A randomized clinical trial of daily nonstress testing versus biophysical profile in the management of preterm premature rupture of membranes. Am J Obstet Gynecol. 1999;181:1495–9. doi: 10.1016/s0002-9378(99)70395-9. [DOI] [PubMed] [Google Scholar]
- 120.Hanley ML, Vintzileos AM. Biophysical testing in premature rupture of the membranes. Semin Perinatol. 1996;20:418–25. doi: 10.1016/s0146-0005(96)80009-7. [DOI] [PubMed] [Google Scholar]
- 121.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- 122.Johnson JM, Harman CR, Lange IR, Manning FA. Biophysical profile scoring in the management of the postterm pregnancy: an analysis of 307 patients. Am J Obstet Gynecol. 1986;154:269–73. doi: 10.1016/0002-9378(86)90653-8. [DOI] [PubMed] [Google Scholar]
- 123.Adams D, Druzin ML, Edersheim T, Bond A, Kogut E. Condition specific antepartum testing: systemic lupus erythematosus and associated serologic abnormalities. Am J Reprod Immunol. 1992;28:159–63. doi: 10.1111/j.1600-0897.1992.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 124.Ramin SM, Vidaeff AC, Yeomans ER, Gilstrap LC., III Chronic renal disease in pregnancy. Obstet Gynecol. 2006;108:1531–9. doi: 10.1097/01.AOG.0000246790.84218.44. [DOI] [PubMed] [Google Scholar]
- 125.Salihu HM, Sharma PP, Ekundayo OJ, Kristensen S, Badewa AP, Kirby RS, et al. Childhood pregnancy (10-14 years old) and risk of stillbirth in singletons and twins. J Pediatr. 2006;148:522–6. doi: 10.1016/j.jpeds.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 126.Raymond EG, Cnattingius S, Kiely JL. Effects of maternal age, parity, and smoking on the risk of stillbirth. Br J Obstet Gynaecol. 1994;101:301–6. doi: 10.1111/j.1471-0528.1994.tb13614.x. [DOI] [PubMed] [Google Scholar]
- 127.Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2007;109:967–77. doi: 10.1097/01.AOG.0000259316.04136.30. [DOI] [PubMed] [Google Scholar]
- 128.Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87:1762–7. doi: 10.1210/jcem.87.4.8430. [DOI] [PubMed] [Google Scholar]