Abstract
Infection with the parasite Toxoplasma gondii stimulates an innate immune response in the host. T. gondii also induces alterations in infected monocytes and dendritic cells that probably contribute to its ability to disseminate and ultimately to establish persistent infection. Recent progress has linked specific parasite molecules to immune stimulation or the parasite’s ability to subvert intracellular signaling pathways in infected cells to evade immunity.
Toxoplasma gondii and innate immunity
Immunity to T. gondii is dependent on interferon-gamma (IFN-γ) induced by interleukin (IL)-12 production by a variety of cell types and cell-mediated immunity. IL-10 is also important to regulate the immune response. As an intracellular pathogen, T. gondii has mechanisms to interfere with host signaling pathways to subvert innate immunity [1, 2]. T. gondii has proved to be a model system to study host immunity to intracellular parasites; however, parasite molecules that stimulate or manipulate immune responses are largely unknown. Only recently have studies begun to link specific T. gondii molecules to effects on host cell function (Table 1; Figure 1).
Table 1.
Summary of T. gondii molecules identified to date that regulate the innate immune response a
| T. gondii molecule | Host target | Effect in host | References |
|---|---|---|---|
| Profilin | TLR11 | IL-12 production through MyD88 | Reviewed in Ref. [2] |
| GPI | TLR2 & TLR4 | Induction of TNF-α through NF-kB and upregulation of MHC class I & II | Reviewed in Ref. [2] |
| Cyclophilin | CCR5 | IL-12 production through CCR5 | Reviewed in Ref. [7] |
| Lipoxygenase | unknown | Downregulation of CCR5; decease in IL-12 | Reviewed in Ref. [7] |
| ROP16 | unknown | Type I & II strains induce STAT 3/6; decrease in IL- 12 | [21] |
| ROP18 | unknown | Infection by strain carrying type I or II allele results in increased mortality | [20, 22, 23] |
| HSP70 | unknown | May inhibit NF-kB nuclear translocation and iNOS | [26] |
The T. gondii molecule (first column) is followed by its host target (second column). The primary effect seen in the host (in vivo and/or in vitro) due to the T. gondii molecule is summarized in column 3 and the references for the corresponding study are given in column 4.
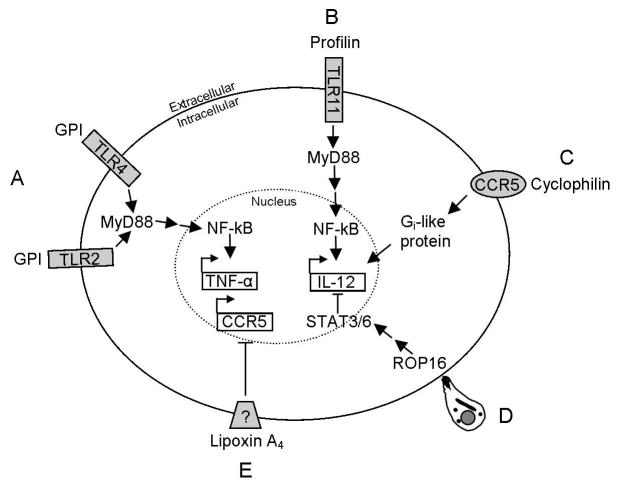
Figure 1.
Representation of the interactions between T. gondii molecules and host innate immunity signaling. (A) Free GPI of T. gondii binds with TLR2 or TLR4, resulting in transcription TNF-α through NF-kB. (B) T. gondii profilin induces IL-12 via TLR11/MyD88. (C) T. gondii cyclophilin interacts with CCR5 that signals through a Gi-like protein to increase IL-12 transcription. (D) ROP16 is released into the host cell upon T. gondii penetration causing activation of STAT3/6 which results in a decrease of IL-12. (E) T. gondii has lipoxygenase activity. A decrease in IL-12 is seen in response to the downregulation of CCR5 by lipoxin A4 generated by soybean lipoxygenase.
Cytokine production via Toll-like receptors
Innate immunity is elicited by diverse pathogen-associated molecular patterns (PAMPs) stimulating host pattern recognition receptors (PRR) including the family of Toll-like receptors (TLRs). TLR engagement triggers intracellular signaling cascades via the common adapter myeloid differentiation factor 88 (MyD88) with the exception of TLR3. The discovery that MyD88-deficient mice, but not IL-1 or IL-18 that also signal through MyD88, are unable to control T. gondii infection due to an impaired IL-12 and IFN-γ response indicates that TLRs must recognize T. gondii molecules to initiate effective immunity. Mice deficient in individual TLRs, including TLR2, 4, and 11, are only modestly more susceptible to T. gondii infection when compared to wild-type mice [2]. This indicates that a number of diverse TLRs and PAMPs likely contribute to the importance of MyD88-dependent immune stimulation. T. gondii profilin promotes parasite actin assembly, contributes to gliding motility, invasion, and egress, and is a PAMP for TLR11 [2,3]. Mice that lack TLR11 have a decreased IL-12 response to purified profilin and are slightly more susceptible to T. gondii. Profilin is relatively conserved in Apicomplexa, but Plasmodium profilin is, at most, a weak inducer of TLR11. It is important to note that TLR11 is not functionally expressed in humans [2]. Glycosylphosphatidylinositol (GPI)-anchored proteins on the surface of T. gondii trigger TNF-α production from macrophages in a TLR2/TLR4/MyD88-dependent manner [4] similar to GPIs from Trypanosoma cruzi and Plasmodium falciparum [2].
MyD88 signaling is clearly important to induce a rapid anti-Toxoplasma response required to survive parasite challenge, but a strong Th1-based immunity to the parasite can be induced by a vaccine strain of the parasite independent of MyD88 [5]. MyD88 dependency may vary, based on the parasite genotype and the cell type infected [2]. Parasite molecules that are responsible for stimulation of the host immune system during infection may depend on a multitude of factors [5, 6]. Different responses may be elicited by the parasite molecules based on the type of inflammatory cells recruited, degree of parasite invasion and replication, and number of parasites degraded or phagocytosed.
Cytokine production via Cys-Cys chemokine receptor CCR5
T. gondii also stimulates IL-12 production from dendritic cells (DC) via host CCR5, a transmembrane receptor expressed by multiple cell types including DC that transmits signals through Gi-like proteins [1, 7]. CCR5-deficient mice produce less IL-12 and IFN-γ and have an increased susceptibility to T. gondii infection. However, the impaired immune response of CCR5-deficient mice is likely a combination of impaired production of DC IL-12 and loss of CCR5-mediated recruitment of natural killer (NK) cells to sites of T. gondii infection independent of CCR5 effects on IL-12 [8]. T. gondii cyclophilin C-18, is a secreted peptidyl-prolyl isomerase that binds CCR5 and stimulates DC IL-12 production. Purified C-18 causes a partial IL-12 response in DCs, indicating additional factors may be required for optimal IL-12 production. Host lipoxin A4 (LXA4) acts via CCR5 to suppress IL-12 production by DC during T. gondii infection to dampen inflammation. T. gondii lacks an endogenous substrate for lipoxygenase, but it produces a lipoxygenase that generates LXA4 in the presence of exogenous arachidonic acid suggesting the primary purpose of this molecule may be immune regulation [7].
Inhibition of host cell signaling cascades
T. gondii has developed numerous techniques to modulate its host cell [9,10]. It blocks apoptosis of infected cells, interferes with the function of host cell transcription factors including STAT1 and NFkB, and inhibits production of inflammatory cytokines including TNF-α and IL-12. Conversely, T. gondii increases the activity of other transcription factors including STAT3/6, hypoxia inducible factor (HIF-1) and early growth response 2 (EGR2). Activation of STAT3 and EGR2 require viable parasites, whereas induction of HIF-1 is due to a short lived diffusible product, suggesting multiple mechanisms operate to alter host cell transcription. T. gondii interferes with phosphorylation and acetylation of histone H3 to prevent chromatin remodeling surrounding the TNF-α promoter following TLR stimulation to inhibit TNF-α production from infected cells [11]. Parasite molecules that directly or indirectly target histone modification at inducible promoters may provide an explanation for the widespread suppressive effects of the parasite on proinflammatory gene transcription in response to LPS and other stimuli.
Parasite inhibition of STAT1 underlies the parasite’s ability to downregulate surface major histocompatibility complex (MHC) class II on infected cells [12]. IFN-γ regulates many downstream effector proteins through the transcription factor STAT1 including increased expression of p47 GTPases, inducible nitric oxide synthase (iNOS), and surface MHC class I and II [13, 14]. Consequently, inhibition of STAT1-signaling might contribute to T. gondii’s ability to survive in host cells during infection. The parasite products responsible for inhibition of STAT1 are unknown. Viable parasites and lysates, but not heat-killed parasites, induce downregulation of MHC class II, but their mechanisms might differ as they affect different IFN-γ responsive promoters downstream of STAT1 [15]. Debierre-Grockiego et al. [16] tested the hypothesis that parasite GPI-anchored proteins contribute to T. gondii downregulation of surface MHC class II. Co-culture of bone marrow-derived macrophages with GPIs slightly increased expression of MHC class I and class II as well as enhancing antigen presentation to CD8 T cells [19]. These results suggest T. gondii downregulation of surface MHC class I and II is not dependent on parasite GPIs, unless their activity on the intact parasite is markedly different than the purified molecules.
Effect of parasite genotype on infection and host cell signaling
T. gondii has a largely clonal population structure which in Europe and North America consists largely of three genotypic lineages, each with distinct properties in terms of virulence and effects on host cell signaling [17]. Type I is distinct in its pronounced virulence in mice during the acute stage of infection. Types II and III are relatively less virulent and readily establish chronic infections [18]. Type II, but not type I or III, strains induce murine macrophages to produce high levels of IL-12 [19].
Genetic crosses to map loci crucial for strain specific differences in T. gondii virulence and host cell signaling identified two rhoptry kinases, ROP16 and 18. The proteins are secreted during parasite invasion and mediate key functional differences between the genotypes [20–23]. Whereas types I, II and III parasites all induce rapid phosphorylation of STAT3/6 (1 hour post-infection) [24], only types I and III maintain STAT3/6 activation 18 hours later [21]. The strain-specific difference in maintaining STAT3/6 activation is dependent on T. gondii ROP16, which traffics to the host cell nucleus after secretion by the parasite rhoptries during invasion. Type I and III, but not type II, isoforms of the ROP16 kinase induce activation of STAT 3/6 and suppress IL-12 production from macrophages. Expression of type II ROP16 in a type I strain resulted in a decrease in STAT 3/6 activation and an increase in IL-12 production. Although the key difference between the two ROP16 alleles and the target are unknown, their expression level, secretion and nuclear localization are similar [21]. ROP18 is expressed in type I and most type II strains, but is poorly expressed in type III strains. Increased expression of ROP18 correlates strongly with virulence [20, 22, 23]. Expression of the type I or type II ROP18 allele in a type III strain increased virulence in mice [20, 23]. ROP18 is a confirmed serine threonine kinase that localizes to the parasitophorous vacuole membrane (PVM), where it could interact with proteins in the host cell cytosol [23, 25].
Concluding remarks and future directions
T. gondii extensively modifies its host cell environment, including affecting diverse signaling cascades in order to disseminate and establish a chronic infection. Secreted products from specialized secretory organelles of T. gondii (micronemes, rhoptries and dense granules) probably mediate many effects on host cell signaling pathways. To date, parasite products identified that affect host cell function and traffic to the host cell nucleus or the PVM are largely rhoptry proteins secreted during parasite invasion. It is possible that other rhoptry secreted kinases and kinase-like proteins, of which more than 70 are predicted in the genome, also have effects on host cell functions. It will be interesting to determine whether other parasite molecules are required post-invasion to maintain host cell changes, or whether the affects are permanently established during parasite invasion. As parasite molecules are identified that interface with the host cell, a key challenge will be dissecting their function, as many of the parasite’s predicted genes have little homology to guide investigators. In this respect, forward genetics may continue to be a fruitful avenue for linking parasite effects on host cells to parasite molecules.
Outstanding questions
What T. gondii molecules are recognized as PAMPS by human cells?
Does profilin act as a PAMP in humans, which lack TLR11?
Are CCR5 or identified TLRs the primary signaling mechanism for T. gondii immunity?
How do the effects of parasite molecules on host cells in vitro translate to their role in toxoplasmosis?
Does a T. gondii lipoxygenase suppress host cell IL-12 production?
What parasite molecules are involved in altering host cell transcriptional responses through STAT1 or other transcription factors?
What are the host cell targets of ROP16 and 18?
Are additional molecules necessary for maintaining host cell control post parasite invasion?
Acknowledgments
We thank our colleagues in the T. gondii field for their pioneering work, and regret that space restraints for this update limited the number of manuscripts that we could reference. We also thank the American Cancer Society (RSG-07-202-01-MBC to LJK), and the NIH (AI072817 to AMP and AI072028 to DGM) for their financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Denkers EY. From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii. FEMS Immunol and Med Micro. 2003;39:193–203. doi: 10.1016/S0928-8244(03)00279-7. [DOI] [PubMed] [Google Scholar]
- 2.Egan CE, et al. Functional aspects of Toll-like receptor/MyD88 signaling during protozoan infection: focus on Toxoplasma gondii. Clin Exp Immunol. 2009;156:17–24. doi: 10.1111/j.1365-2249.2009.03876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plattner F, et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Debierre-Grockiego F, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 5.Sukhumavasi W, et al. TLR adaptor MyD88 is essential for pathogen control during oral Toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol. 2008;181:3464–3473. doi: 10.4049/jimmunol.181.5.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim L, et al. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J Immunol. 2006;177:2584–2591. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- 7.Aliberti J. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat Rev Immunol. 2005;5:162–170. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- 8.Khan IA, et al. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2006;2:484–500. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blader IJ, Saeij JP. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. Acta Pathol Microbiol Immunol Scandinavica. 2009;117:458–476. doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laliberte J, Carruthers VB. Host cell manipulation by the human pathogen Toxoplasma gondii. Cell Mol Life Sci. 2008;65:1900–1915. doi: 10.1007/s00018-008-7556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng J, et al. Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation. J Immunol. 2009;182:489–497. doi: 10.4049/jimmunol.182.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luder CG, et al. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1alpha. Eur J Immunol. 2001;31:1475–1484. doi: 10.1002/1521-4141(200105)31:5<1475::AID-IMMU1475>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Boehm U, et al. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 14.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signaling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 15.Lang C, et al. Diverse mechanisms employed by Toxoplasma gondii to inhibit IFN-gamma-induced major histocompatibility complex class II gene expression. Microbes Infect. 2006;8:1994–2005. doi: 10.1016/j.micinf.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Debierre-Grockiego F, et al. Toxoplasma gondii glycosylphosphatidylinositols up-regulate major histocompatibility complex (MHC) molecule expression on primary murine macrophages. Innate Immun. 2009;15:25–32. doi: 10.1177/1753425908099936. [DOI] [PubMed] [Google Scholar]
- 17.Sibley LD, Ajioka JW. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol. 2008;62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- 18.Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 19.Robben PM, et al. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on parasite genotype. J Immunol. 2004;172:3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 20.Saeij JPJ, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeij JPJ, et al. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan A, et al. Selection at a single locus leads to widespread expansion of Toxoplasma gondii lineages that are virulent in mice. PLoS Genet. 2009;5:1–14. doi: 10.1371/journal.pgen.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor S, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;15:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 24.Butcher BA, et al. IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. J Immunol. 2005;174:3148–3152. doi: 10.4049/jimmunol.174.6.3148. [DOI] [PubMed] [Google Scholar]
- 25.El Hajj H, et al. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 2007;3:200–211. doi: 10.1371/journal.ppat.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbin CA, et al. Heat shock protein 70 is a potential virulence factor in murine Toxoplasma infection via immunomodulation of host NF-kB and nitric oxide. J Immunol. 2002;169:958–965. doi: 10.4049/jimmunol.169.2.958. [DOI] [PubMed] [Google Scholar]