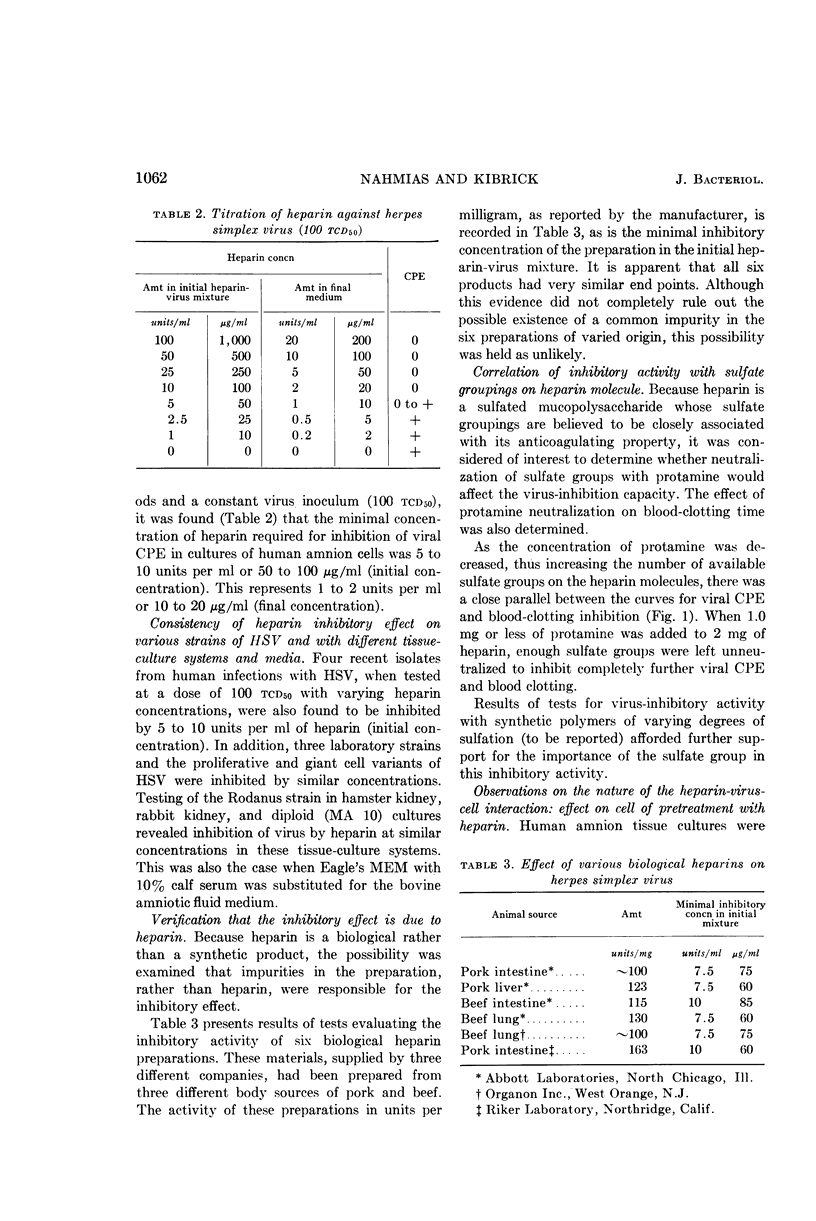
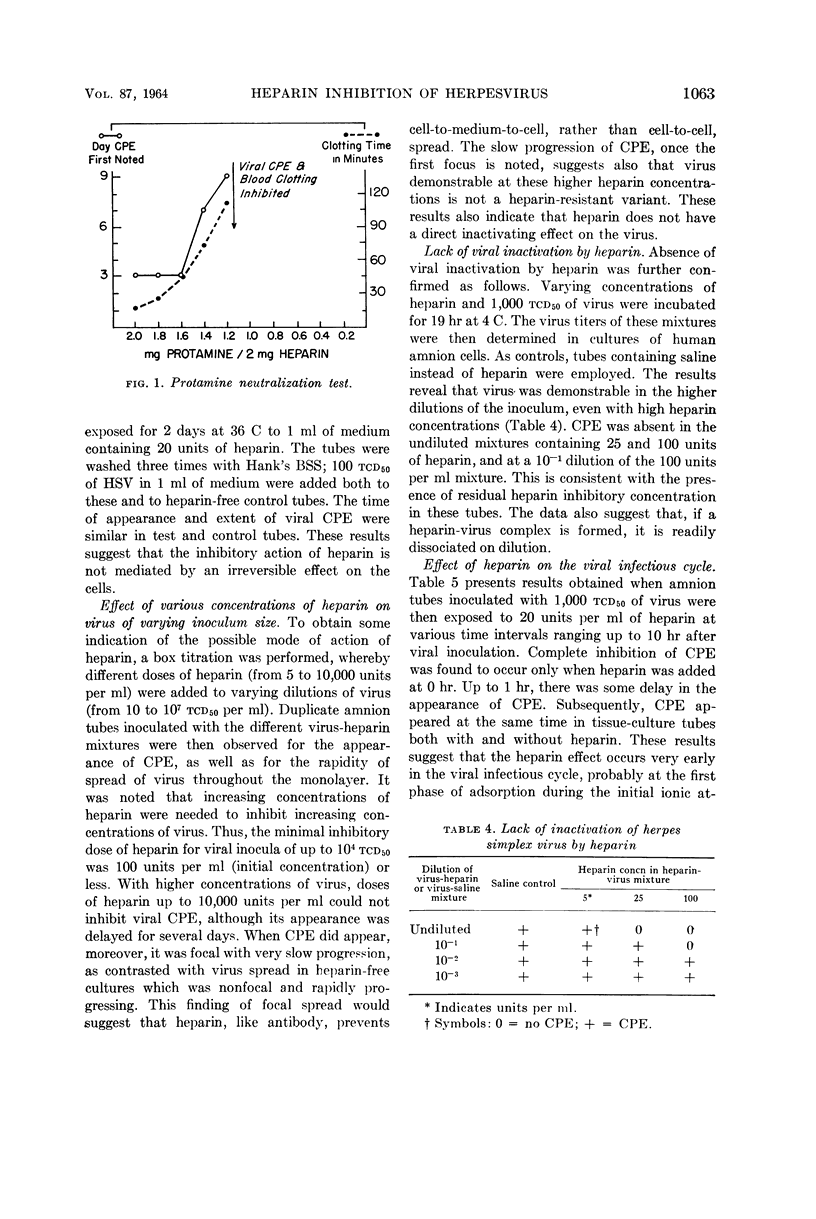
Abstract
Nahmias, André J. (Boston University School of Medicine, Boston, Mass.), and Sidney Kibrick. Inhibitory effect of heparin on herpes simplex virus. J. Bacteriol. 87:1060–1066. 1964.—A substance inhibitory to herpes simplex virus was observed during experiments with leukocyte cultures. The component in the cultures responsible for this inhibition was identified as heparin. The minimal inhibitory concentration required to inhibit 30 to 300 tcd50 of the virus in human amnion tissue culture was found to be 1 to 2 units per ml (10 to 20 μg/ml). This effect was confirmed with other strains of herpes simplex virus, other tissue-culture systems, and other media. The inhibitory activity of the heparin was found to be related to the sulfate groupings on the molecule. The effect of heparin appears to be on the virus, rather than on the cell. The virus is not inactivated, however, and the heparin-virus “complex” is readily dissociable on dilution. Heparin was shown to affect viral infection in its earliest phase, probably at the primary electrostatic attachment of virus to cell. The import of these and related observations on common virological laboratory procedures and the possible biological significance of our findings are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FELTZ E. T., REGELSON W. Ethylene maleic anhydride copolymers as viral inhibitors. Nature. 1962 Nov 17;196:642–645. doi: 10.1038/196642a0. [DOI] [PubMed] [Google Scholar]
- GELLER P., COLEMAN V. R., JAWETZ E. Studies on herpes simplex virus. V. The fate of viable herpes simplex virus administered intravenously to man. J Immunol. 1953 Dec;71(6):410–418. [PubMed] [Google Scholar]
- GLASGOW L. A., HABEL K. Role of polyoma virus and interferon in a herpes simplex virus infection in vitro. Virology. 1963 Mar;19:328–339. doi: 10.1016/0042-6822(63)90072-2. [DOI] [PubMed] [Google Scholar]
- GRESSER I. Production of interferon by suspensions of human leucocytes. Proc Soc Exp Biol Med. 1961 Dec;108:799–803. doi: 10.3181/00379727-108-27072. [DOI] [PubMed] [Google Scholar]
- GULICK Z. R., HEYMANN H., DE BOER C. J., DE STEVENS G., MAYER R. L. The inhibition of ribonuclease by acidic polymers and their use as possible antiviral agents. Arch Biochem Biophys. 1958 Feb;73(2):366–383. doi: 10.1016/0003-9861(58)90282-0. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. Alteration plaque morphology of EMC virus with polycations. Virology. 1961 Aug;14:502–504. doi: 10.1016/0042-6822(61)90349-x. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. THE BASIS FOR THE SIZE DIFFERENCES IN PLAQUES PRODUCED BY VARIANTS OF ENCEPHALOMYOCARDITIS (EMC) VIRUS. Virology. 1963 Aug;20:559–566. doi: 10.1016/0042-6822(63)90280-0. [DOI] [PubMed] [Google Scholar]
- MANDEL B. Inhibition of Theiler's encephalomyelitis virus (GDVII strain) of mice by an intestinal mucopolysaccharide. III. Studies on factors that influence the virus-inhibitor reaction. Virology. 1957 Jun;3(3):444–463. doi: 10.1016/0042-6822(57)90002-8. [DOI] [PubMed] [Google Scholar]
- MILOVANOVIC M. V., ENDERS J. F., MITUS A. Cultivation of measles virus in human amnion cells and in developing chick embryo. Proc Soc Exp Biol Med. 1957 May;95(1):120–127. doi: 10.3181/00379727-95-23140. [DOI] [PubMed] [Google Scholar]
- MOORHEAD P. S., NOWELL P. C., MELLMAN W. J., BATTIPS D. M., HUNGERFORD D. A. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960 Sep;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- RILEY J. F. Functional significance of histamine and heparin in tissue mast cells. Ann N Y Acad Sci. 1963 Feb 26;103:151–163. doi: 10.1111/j.1749-6632.1963.tb53695.x. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Demonstration of a surface difference between virions of two strains of herpes simplex virus. Virology. 1963 Feb;19:198–204. doi: 10.1016/0042-6822(63)90009-6. [DOI] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Inhibition of infectious and hemagglutinating properties of type 2 dengue virus by aqueous Agar extracts. Virology. 1963 Jan;19:49–57. doi: 10.1016/0042-6822(63)90023-0. [DOI] [PubMed] [Google Scholar]
- SCOTT T. F. M., BURGOON C. F., CORIELL L. L., BLANK H. The growth curve of the virus of herpes simplex in rabbit corneal cells grown in tissue culture with parallel observations on the development of the intranuclear inclusion body. J Immunol. 1953 Dec;71(6):385–396. [PubMed] [Google Scholar]
- SCOTT T. F., McLEOD D. L., TOKUMARU T. A biologic comparison of two strains of Herpesvirus hominis. J Immunol. 1961 Jan;86:1–12. [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. I. An agar polysaccharide determining plaque morphology of EMC virus. Virology. 1961 Aug;14:456–462. doi: 10.1016/0042-6822(61)90338-5. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. II. Enhancement of plague formation and the detection of variants of poliovirus with dextran sulfate. Virology. 1962 Jul;17:499–501. doi: 10.1016/0042-6822(62)90148-4. [DOI] [PubMed] [Google Scholar]
- TOLMACH L. J. Attachment and penetration of cells by viruses. Adv Virus Res. 1957;4:63–110. doi: 10.1016/s0065-3527(08)60596-5. [DOI] [PubMed] [Google Scholar]
- TYTELL A. A., NEUMAN R. E. A medium free of agar, serum and peptone for plaque assay of herpes simplex virus. Proc Soc Exp Biol Med. 1963 Jun;113:343–346. doi: 10.3181/00379727-113-28362. [DOI] [PubMed] [Google Scholar]
- VAHERI A., PENTINEN K. Effect of polyphloroglucinol-phosphate, an acid polymer, on herpes simplex virus. Ann Med Exp Biol Fenn. 1962;40:334–341. [PubMed] [Google Scholar]
- YOUNG B. G., MORA P. T. Viability of T2 bacteriophage after interaction with negatively charged macromolecules. Virology. 1960 Nov;12:493–495. doi: 10.1016/0042-6822(60)90171-9. [DOI] [PubMed] [Google Scholar]



