Abstract
Objective
The objective of this study was to determine inaccuracies of miscoded blood glucose (BG) meters and potential errors in insulin dose based on values from these meters.
Research Design
Fasting diabetic subjects at three clinical centers participated in a 2-hour meal tolerance test. At various times subjects' blood was tested on five BG meters and on a Yellow Springs Instruments laboratory glucose analyzer. Some meters were purposely miscoded. Using the BG values from these meters, along with three insulin dose algorithms, Monte Carlo simulations were conducted to generate ideal and simulated-meter glucose values and subsequent probability of insulin dose errors based on normal and empirical distribution assumptions.
Results
Maximal median percentage biases of miscoded meters were +29% and −37%, while maximal median percentage biases of correctly coded meters were only +0.64% and −10.45% (p = 0.000, χ2 test, df = 1). Using the low-dose algorithm and the normal distribution assumption, the combined data showed that the probability of insulin error of ±1U, ±2, ±3, ±4, and ±5U for miscoded meters could be as high as 49.6, 50.0, 22.3, 1.4, and 0.04%, respectively. This is compared to manually, correctly coded meters where the probability of error of ±1, ±2, and ±3U could be as high as 44.6, 7.1, and 0.49%, respectively. There was no instance of a ±4 or ±5U insulin dose error with a manually, correctly coded meter. For autocoded meters, the probability of ±1 and ±2U could be as high as 35.4 and 1.4%, respectively. For autocoded meters there were no calculated insulin dose errors above ±2U. The probability of insulin misdosing with either manually, correctly coded or autocoded meters was significantly lower than that with miscoded meters. Results using empirical distributions showed similar trends of insulin dose errors.
Conclusions
Blood glucose meter coding errors may result in significant insulin dosing errors. To avoid error, patients should be instructed to code their meters correctly or be advised to use an autocoded meter that showed superior performance over manually, correctly coded meters in this study.
Keywords: autocode, autocoded blood glucose meter, blood glucose, blood glucose meter, insulin dose error, manual code, miscoded meter, Monte Carlo simulation, self-monitoring of blood glucose, user error
Introduction
Measurement of blood glucose is required for appropriate diabetes management, particularly for adjustment of insulin. Using specific insulin dose algorithms, some patients are taught to adjust their insulin based on their blood glucose (BG) values. If the BG results are inaccurate, there is a potential for serious consequences from therapeutic errors.1
Many people with diabetes do not have access to diabetes education or to a diabetes care team to assist them. Many who purchase a blood glucose meter at a pharmacy or through the mail are not carefully instructed in its use. Factors that contribute to error in the use of BG meters by patients include use of expired test strips, improper maintenance of meters, technique errors, and failure to properly code the meter to the lot of test strips.2–10 The magnitude of this problem may be larger than is generally appreciated. Studies have shown that approximately 16% of people did not code their BG meter properly.11,12 Moreover, it has been observed by the authors that many people with diabetes either do not understand what proper coding means or do not realize its importance.
The importance of BG control has been substantiated over the last two decades. Recent research has focused on tight glycemic control in the hospital setting for all patients and for patients with diabetes in particular. Tight glucose control has been shown to have a dramatic positive effect on morbidity, mortality, and costs.13,14 Meters used by patients for self-monitoring of blood glucose and those used by health care professionals at the point of care use similar technology; therefore issues of proper usage, including coding, apply to both.
Accuracy of BG results is especially crucial when the values are used to adjust insulin dose. Inaccuracies caused by incorrect BG testing procedures can lead to incorrect decisions on insulin dosing. The current study examines the accuracy of five popular blood glucose meters that were properly coded, as well as some that were purposely miscoded. Included were two types of autocoded BG meters that do not require coding by the user. Mathematical simulations (Monte Carlo) based on three insulin dose algorithms were then performed on the values to estimate the errors in insulin dosing that could result if these BG values were used to make changes in insulin dose.
Methods
Clinical Trial
A multicenter study was conducted to quantify the inaccuracies of various miscoded and properly coded meters during a glucose excursion. The study was approved by an IRB and all subjects completed the informed consent process.
The study was conducted at the Diabetes Control Center in Orangeburg, South Carolina, at the Outpatient Diabetes Education Program, Elkhart General Hospital in Elkhart, Indiana, and at the VA San Diego Healthcare System in San Diego, California. A total of 116 fasting subjects were given a 2-hour meal tolerance test with BOOST®. The subject population included 65 men and 51 women ranging in age from 20 to 82 years of age. Fourteen subjects had type 1 diabetes and 102 subjects had type 2 diabetes. At times 0, 60, and 120 minutes following ingestion of BOOST, subjects' fingerstick blood was tested on miscoded and properly coded meters and on a Yellow Springs Instruments (YSI) laboratory glucose analyzer (Yellow Springs, OH). Two types of autocoded meters (meters A and B) and three types of meters requiring manual coding (meters C, D, and E) were used in the study. The autocoded meters were always properly coded due to their inherent design. The manually coded meters were tested when properly coded and also when miscoded (with two different miscode combinations). The two miscode numbers for each brand of meter were chosen by screening for meter code number settings that gave results having a large difference from each other before the code numbers of the test strips were known. Test strips for each brand of meter were then purchased from an independent vendor (Diabetic Promotions, Willowick, OH), without specifying any particular code number. Whatever test strips received were then used in the study. Thus, the impact of each miscode on accuracy and on insulin dose errors was unknown prior to conducting the study.
Each subject tested an array of properly coded and miscoded meters according to a randomization schedule. All meter values were compared to YSI values (determined at each time point), and the mean, standard deviation, and median percentage errors were determined.
Monte Carlo Simulations and Insulin Dose Algorithms
Blood glucose measurements (ranging from 52 to 498 mg/dl) were used to determine the mean and standard deviation of percentage biases from the YSI values. With the assumption of a normal distribution of the percentage bias, these parameters were then used in a Monte Carlo simulation15 on a hypothetical population (n = 10,000) of patients with ideal glucose values uniformly distributed over the range of 150–450 mg/dl according to the insulin dose algorithms. The range of clinical glucose values (52–498 mg/dl) covers the simulation range (150–450 mg/dl). The ideal glucose values and the errors were added to generate the simulated-meter glucose values. These values were then used to predict insulin dosage errors using three insulin dose algorithms (low, medium, and high, Table 1). Table 2 uses results for the low-dose algorithm to show the probability of an error of ±N units of insulin for each meter condition.
Table 1.
Low, Medium, and High Insulin Dose Algorithms Used in Monte Carlo Simulations
| Premeal BG (mg/dl) (for Lispro, Apidra, Novolog) | Low-dose algorithm (<40 units insulin/day) | Medium-dose algorithm (40–80 units insulin/day) | High-dose algorithm (>80 units insulin/day) |
|---|---|---|---|
| Additional insulin | Additional insulin | Additional insulin | |
| 150–199 | 1 unit | 1 unit | 2 units |
| 200–249 | 2 units | 3 units | 4 units |
| 250–299 | 3 units | 5 units | 7 units |
| 300–349 | 4 units | 7 units | 10 units |
| >349 | 5 units | 8 units | 12 units |
Table 2.
Probability of Insulin Dose Errora
| Meter | Blood sample number | Coding | % probability of occurrence | ||||
|---|---|---|---|---|---|---|---|
| ± 1 unit | ± 2 units | ± 3 units | ± 4 units | ± 5 units | |||
| A | 139 | Correctly coded (autocoding) | 27.65 | 0.96 | |||
| B | 140 | Correctly coded (autocoding) | 35.42 | 1.41 | |||
| C | 143 | Correctly coded (manual coding) | 43.07 | 6.57 | 0.48 | ||
| C | 143 | Miscoded 1 | 40.20 | 40.66 | 14.00 | 1.4 | 0.04 |
| C | 143 | Miscoded 2 | 36.98 | 4.22 | 0.22 | ||
| D | 136 | Correctly coded (manual coding) | 32.55 | 1.77 | 0.04 | ||
| D | 136 | Miscoded 1 | 49.61 | 28.29 | 5.92 | 0.78 | |
| D | 135 | Miscoded 2 | 41.85 | 15.64 | 0.52 | ||
| E | 137 | Correctly coded (manual coding) | 44.64 | 7.06 | 0.49 | ||
| E | 138 | Miscoded 1 | 33.99 | 50.00 | 15.14 | 0.40 | |
| E | 137 | Miscoded 2 | 26.7 | 49.7 | 22.25 | 1.21 | |
Calculated insulin dose error for all meters across all time points based on the low-dose algorithm and on a normal distribution assumption.
The simulation was also conducted using the empirical distributions of percentage biases. Results from both empirical and normal distributions showed similar trends of insulin errors. The simulation was conducted using Matlab 6.5 R13.
Results
Sixty-five men and 51 women with type 1 or type 2 diabetes completed the study. Blood glucose concentrations of the subjects ranged from 52 to 498 mg/dl over the course of the meal tolerance test. Subjects' median BG values at 0, 60, and 120 minutes were 125, 201, and 169 mg/dl, respectively.
At each time point, subjects' fingerstick blood was assayed on five popular brands of BG meters under various coding conditions. Figures 1a, 1b, and 1c show median percentage biases from the YSI at times 0, 60, and 120 minutes, respectively, for all meters under all coding conditions tested. Glucose values from miscoded meters showed median percentage biases as great as +29 and −37%, whereas glucose values from properly coded meters showed significantly lower maximal median percentage biases ranging from +0.64 to −10.45% (p = 0.000, χ2 test, df = 1). Meter biases from YSI values were consistent over the three time points and hence over the glucose ranges.
Figure 1.
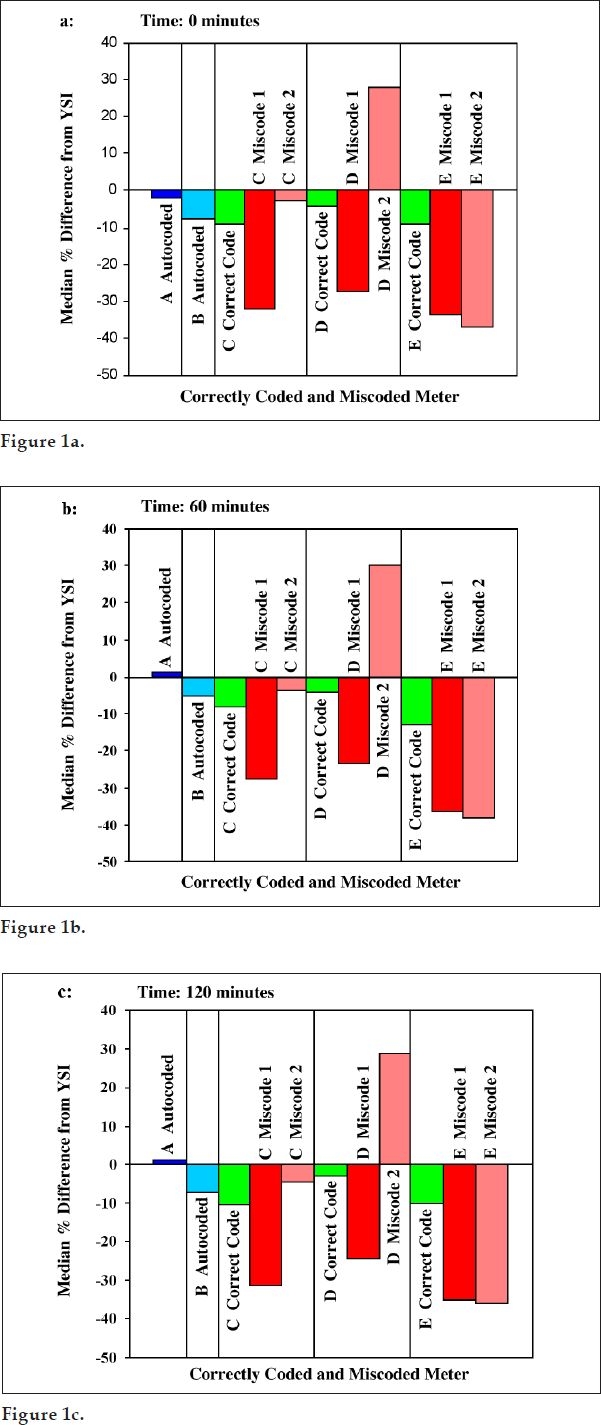
Median of percentage bias against YSI for correctly coded and miscoded meters at (a) 0 min, (b) 60 min, and (c) 120 min. Meters A and B are autocoded meters and thus are coded correctly; meters C, D, and E are three different brands of manually coded meters tested when coded correctly (green bars) and when tested with two different miscode combinations (red bars and pink bars).
Three insulin dose algorithms were then used in Monte Carlo simulations to determine the probability of insulin dose error that would occur if values from the properly coded and miscoded meters were used to make decisions about adjusting insulin dosages. Simulations were based on normal distributions and empirical distributions of the percentage bias.
Normal Distribution
Table 2 shows the calculated probability of insulin dose errors for all meter and strip combinations using the low-dose algorithm under normal distribution assumptions. Figure 2 illustrates the highest probability of insulin dose errors for miscoded meters (Figure 2a), for manually, correctly coded meters (Figure 2b), and for autocoded meters (Figure 2c).
Figure 2.
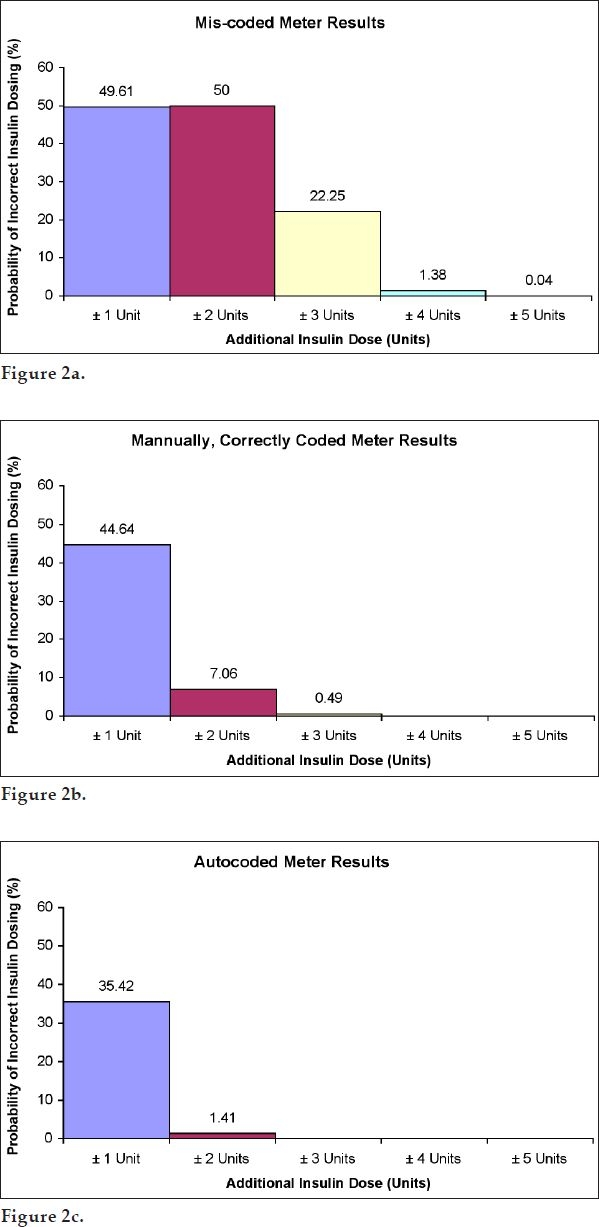
Maximal probability (%) of insulin dose errors based on a low-dose insulin algorithm and normal distribution assumptions: (a) miscoded meters, (b) manually, correctly coded meters, and (c) autocoded meters.
For the low-dose insulin algorithm (i.e., for patients taking less than 40 units of insulin per day), combined data across all time points showed that, for miscoded meters, the probability of insulin error of ±1, ±2, ±3, ±4, and ±5U could be as high as 49.6, 50.0, 22.3, 1.4, and 0.04%, respectively (Figure 2a). This is compared to correctly, manually coded meters where the probability of error of ±1, ±2, and ±3U could be as high as 44.6, 7.1, and 0.49%, respectively (Figure 2b). There was no instance of a ±4 or ±5U insulin dose error with a manually, correctly coded meter. For autocoded meters, the probability of ±1 and ±2U could be as high as 35.4 and 1.4%, respectively (Figure 2c). The probability of insulin misdosing with either manually, correctly coded or autocoded meters was significantly lower than that with miscoded meters. For autocoded meters there were no calculated insulin dose errors above ±2U. Thus, autocoded meters showed superior performance over manually, correctly coded meters.
For the medium-dose insulin algorithm (i.e., for patients taking between 40 and 80 units of insulin per day), combined data across all time points showed that, for miscoded meters, the probability of insulin error of ±1, ±2, ±3, ±4, ±5U, ±6, ±7, and ±8U could be as high as 23.5, 25.8, 26.9, 24.4, 15.5, 6.2, 1.5, and 0.03%, respectively (not shown). This is compared to manually, correctly coded meters where the probability of error of ±1, ±2, ±3, ±4, ±5, and ±6U could be as high as 18.4, 26.4, 3.8, 3.5, 0.3, and 0.08%, respectively (not shown). For autocoded meters, the probability of ±1, ±2, ±3, and ±4U could be as high as 14.0, 21.3, 0.98, and 0.63%, respectively (not shown). There was no calculated insulin dose error of more than ±4U for autocoded meters.
For the high-dose insulin algorithm (i.e., for patients taking more than 80 units of insulin per day), combined data across all time points showed that, for miscoded meters, the probability of insulin error of ±2, ±3, ±4, ±5, ±6, ±7, ±8, ±10, and ±12U could be as high as 35.0, 16.2, 11.6, 30.6, 11.5, 1.6, 19.3, 1.7, and 0.05%, respectively (not shown). This is compared to correctly coded meters where the probability of error of ±2, ±3, ±4, ±5, ±6, ±7, and ±8U could be as high as 26.8, 17.5, 0.4, 4.6, 1.8, 0.01, and 0.5%, respectively (not shown). For autocoded meters, the probability of ±2, ±3, ±4, ±5, and ±6U could be as high as 20.0, 15.3, 0.01, 1.3, and 0.4%, respectively (not shown). There was no calculated insulin dose error of more than ±6U for autocoded meters.
The absolute insulin dose errors are largest when the high-dose algorithm is used, followed by the medium-dose algorithm and smallest when using the low-dose algorithm. This is, in part, a function of a patient's weight and also the varying degree of insulin resistance among people with diabetes. Because the three insulin dose algorithms used here (low, medium, and high) correspond to increasing degrees of weight and insulin resistance, a given BG meter error evokes a larger absolute error in insulin dose for a larger, more insulin-resistant patient than for one who is less so, but the clinical effect is similar for both of them. For instance, a ±2 unit insulin dose error for a person on the low-dose algorithm should have a similar effect on blood glucose as a ±4 unit insulin dose error for a person using the high-dose algorithm. In essence, the algorithm prescribed has effectively equalized their susceptibility to BG meter errors. Thus, most of the data presented here are based on the low-dose algorithm only.
Empirical Distribution
The results were slightly different when the empirical distribution, generated directly from the clinical study, was used. However, the trend was similar to that generated by the normal distribution. For example, when using the empirical distribution, the following results were obtained. For the low-dose insulin algorithm, the probability of insulin error of ±1, ±2, ±3, ±4, and ±5U could be as high as 55.7, 51.6, 23.9, 1.3, and 0.1%, respectively. This is compared to correctly, manually coded meters where the probability of error of ±1, ±2, ±3, and ±4U could be as high as 45.4, 7.3, 0.52, and 0.04%, respectively. There was no instance of a ±5U insulin dose error with a manually, correctly coded meter. For autocoded meters, the probability of ±1, ±2, and ±3U could be as high as 34.7, 1.4, and 0.2%, respectively. Although the specific values of insulin errors are different from the normal assumption results, both results showed that the probability of insulin misdosing with either manually, correctly coded or autocoded meters was significantly lower than that with miscoded meters. For autocoded meters there were no calculated insulin dose errors above ±3U. Thus, autocoded meters also showed superior performance over manually, correctly coded meters when the empirical distribution was used.
Discussion
The values generated by self-monitoring of blood glucose are used by patients to make adjustments in meal planning, exercise, and insulin dosage. It is essential that BG meter data are accurate, especially for patients on insulin therapy who make adjustments to their insulin dose.
The appropriate procedure for blood glucose testing is not always well understood by patients who self-monitor blood glucose. Patients may use expired test strips and/or an incorrect technique. In addition, patients may sometimes fail to properly code their BG meter to the lot of test strips they are using. For persons who are not detail oriented, coding a meter may be perceived as an insignificant detail. Therefore, every patient should be informed of the possible wide variance in test results when a meter is not coded properly.
If a patient is using a meter requiring coding, providing this information will reinforce the importance of attention to detail in diabetes self-care. If a patient is selecting a new meter, having this information may help in making a decision as to what meter to choose.
For various types of meters that require manual coding, miscoding can occur in a number of different ways. Miscoding can occur, under certain circumstances, if a patient accidentally pushes the wrong button or if he or she enters an incorrect code number into the meter, forgets to change the code number in the meter, or forgets to insert a new code chip or code strip into the meter when using a new bottle of test strips.
As shown in this study, use of an incorrectly coded meter can result in significant errors in insulin dose. The calculated probability of the absolute value of insulin error is a function of a number of variables, including the assumptions used in the Monte Carlo simulation and the specific insulin dose algorithm used in the calculations.
Although most manually, corrected coded meters in this study gave accurate results, some did result in modest errors in insulin dose. The autocoded meters used in this study gave BG values that resulted in the lowest risk of insulin dose error.
Under certain circumstances, use of an incorrectly coded meter may contribute to an increased risk of hypoglycemia or hyperglycemia, which can lead to potentially serious adverse patient consequences and an unnecessary increase in health care costs. Accordingly, one cannot underestimate the potentially serious consequences of using an incorrectly coded hospital meter.
Every patient and health care professional should be informed of the potential for error in test results when a BG meter is not coded properly. Patients should be carefully instructed how to correctly code their meters or be advised to use an autocoded meter (i.e., one that automatically sets the correct code whenever a test strip or disk is inserted without the patient having to select the code or insert a code strip or code chip) that showed superior performance over manually, correctly coded meters in this study.
Abbreviations
- BG
blood glucose
- YSI
Yellow Springs Instruments
References
- 1.Winter WE. A Rosetta stone for insulin treatment: self-monitoring of blood glucose. Clin Chem. 2004 Jun;50(6):985–987. doi: 10.1373/clinchem.2004.033167. [DOI] [PubMed] [Google Scholar]
- 2.Skeie S, Thue G, Nerhus K, Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin Chem. 2002 Jul;48(7):994–1003. [PubMed] [Google Scholar]
- 3.Batki AD, Thomason HL, Holder R, Nayyar P, Thorpe GHG. 2002. May, TheraSense FreeStyle blood glucose meter: Medical Devices Agency Evaluation Report. MDA Report No. 02049. [Google Scholar]
- 4.Baum JM, Monhaut NM, Parker DR, Price CP. Improving the quality of self-monitoring blood glucose measurement: A study in reducing calibration errors. Diabetes Technol Ther. 2006 Jun;8(3):347–357. doi: 10.1089/dia.2006.8.347. [DOI] [PubMed] [Google Scholar]
- 5.Using blood glucose meters minimizing errors, maximizing accuracy [guidance article] Health Devices. 2004;33:251–256. [PubMed] [Google Scholar]
- 6.Alto WA, Meyer D, Schneid J, Bryson P, Kindig J. Assuring the accuracy of home glucose monitoring. J Am Board Fam Pract. 2002 Jan–Feb;15(1):1–6. [PubMed] [Google Scholar]
- 7.Bergenstal R, Cembrowski GS, Bina D, Davidson J, List S. Identifying variables associated with inaccurate self-monitoring of blood glucose: proposed guidelines to improve accuracy. Diabetes Educ. 2000 Nov–Dec;26(6):981–989. doi: 10.1177/014572170002600610. [DOI] [PubMed] [Google Scholar]
- 8.Steel LG. Identifying technique errors: self-monitoring of blood glucose in the home setting. J Gerontol Nurs. 1994 Feb;20(2):9–12. doi: 10.3928/0098-9134-19940201-04. [DOI] [PubMed] [Google Scholar]
- 9.Jones H. Common errors associated with capillary blood glucose monitoring. Br J Nurs. 1994;3(19):1021–1022. doi: 10.12968/bjon.1994.3.19.1021. Oct 27–Nov 9. [DOI] [PubMed] [Google Scholar]
- 10.Vincze G, Barner J, Lopez D. Factors associated with adherence to self-monitoring of blood glucose among persons with diabetes. Diabetes Educ. 2004 Jan–Feb;30(1):112–125. doi: 10.1177/014572170403000119. [DOI] [PubMed] [Google Scholar]
- 11.Raine CH. Self-monitored blood glucose: a common pitfall. Endocr Pract. 2003 Mar–Apr;9(2):137–139. doi: 10.4158/EP.9.2.137. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen G, Nerhus K, Thue G, Sandberg S. Standardization evaluation of instruments for self-monitoring of blood glucose by patients and a technologist. Clin Chem. 2004 Jun;50(6):1068–1071. doi: 10.1373/clinchem.2004.031575. [DOI] [PubMed] [Google Scholar]
- 13.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004 Feb;27(2):553–591. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 14.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 15.Boyd JC, Bruns DE. Quality specifications for glucose meters: assessment by simulation modeling of errors in insulin dose. Clin Chem. 2001 Feb;47(2):209–214. [PubMed] [Google Scholar]


