Abstract
The prevalence of obesity is growing, is driving an increase in the prevalence of diabetes, and is creating a major public health crisis in the United States. Lifestyle and behavior therapy rarely give durable weight loss. There are few medications approved for the treatment of obesity. Those that exist are limited in efficacy and using them in combination does not result in greater weight loss. Surgical treatments for obesity are effective and give durable weight loss, but are accompanied by measurable morbidity and mortality. Several pacing approaches are being tried and are an outgrowth of pacing for gastroparesis. The Transcend® pacemaker blocks vagal efferents and delays gastric emptying, giving a 40% loss of excess body weight, if certain screening procedures are employed. The Tantulus™ pacemaker is still in development but increases antral muscular contractions and delays gastric emptying by stimulation during the absolute refractory period. Weight loss has been 30% of excess body weight, and glycohemoglobin decreased 1.6% in a trial of obese type 2 diabetes. Stimulation to the subdiaphragmatic sympathetics, vagal nerve stimulation with or without unilateral vagotomy, and intestinal pacing are other approaches that are still being evaluated preclinically. Clearly a safe, effective, and durable treatment for obesity is desperately needed. Electrical pacing of the gastrointestinal tract is promising therapeutically, and because pacemakers work through different mechanisms, combining pacemaker treatments may be possible. Rapid progress is being made in the field of electrical stimulation as a treatment for obesity and even greater progress can be expected in the foreseeable future.
Keywords: diabetes, electrical pacing, gastroparesis, nerve, obesity, stomach
Background
The prevalence of obesity is growing in epidemic proportions. The prevalence of obesity was 15% in 1980 and is now more than double that figure.1 The frequency of diabetes in the population follows the prevalence of obesity by about 10 years2 and, as expected, has grown from 7.3% of the population in 2000 to 7.9% in 2001, an increase of 8.2%.3 Obesity and diabetes both cost the U.S. economy over $100 billion a year.4,5
There have been three traditional treatments for obesity. Behavior modification with lifestyle change has been the basis for all obesity treatments. Although this treatment causes a 5–10% weight loss, 50% of the lost weight is regained in 1 year and essentially all is regained in 5 years.6 Obesity medications, the second treatment, has a disastrous safety record.7 Presently approved drugs appear safe, but long-term studies of obesity drugs are limited and weight gain appears to occur in both drug and placebo groups after 3 years of therapy.8 Surgery, the third form of obesity treatment, in the form of a gastric or biliopancreatic bypass, gives a durable loss of about one-third of the body's weight for at least 14 years.9,10 This form of treatment is invasive, expensive, and associated with significant morbidity.11
Despite advances that have occurred in gastric surgery with the introduction of less invasive procedures, such as lapraroscopic banding, there is clearly a need for minimally invasive obesity therapies with few adverse events and associated with sustained weight loss. Gastric pacing holds promise of being such a therapy. This review discusses the various forms of pacing being evaluated for the treatment of obesity and diabetes with emphasis on the physiology supporting their use, as well as the results of available clinical trials.
Gastric Pacing for Gastroparesis
The history of gastric pacing began as a therapy for gastroparesis unresponsive to medical treatment, a condition caused most commonly by either a viral infection or diabetes. Thus, although gastric pacing is now applied more frequently in the treatment of obesity, it was a complication of diabetes that resulted in its initial clinical application.
The first clinical use of gastric pacing in the early 1990s was preceded by an exploration of gastric electrical physiology in the 1980s.12 The electrical activity in the upper stomach is relatively silent. The pacemaker area from which phasic electrical activity propagates along the longitudinal muscle layer distally to the pyloris is located in the midbody of the stomach on the greater curvature.12The stomach acts as a pump. The upper stomach primarily empties liquids, and the lower stomach is mainly responsible for emptying solids. The liquids are emptied first by fundic pressure followed by antral motor activity for the emptying of solids.13 In addition to the nervous system, the stomach muscle is controlled by the cells of Cajal, a unique structure that controls the electromechanical functions of the stomach.13
The stomach has slow waves that normally occur at a frequency of three per minute. During fasting, these waves do not result in contractions. For approximately 2 to 3 hours after a meal, spikes are superimposed on the gastric slow waves, resulting in contractions that propagate distally with increasing velocity and amplitude up to a frequency of three cycles per minute.14 These slow waves are abnormal in gastroparesis. They can occur at an abnormally high frequency (tachygastria), an abnormally slow frequency (bradygastria), or in a disorganized fashion (gastric dysrythmia). Because abnormal slow waves are associated with gastroparesis, the first attempts at therapy for gastroparesis tried to normalize the electrical activity pattern.
In a study by McCallum et al.,15 one of the early studies, gastric pacing was attempted in nine patients dependent on jejunostomy feeding tubes for nutrition despite maximal medical therapy with prokinetic agents. A series of electrodes on the serosal surface of the greater curvature was able to entrain the slow waves and convert tachygastria to the normal three-cycle per minute frequency.15 Although this resulted in clinical improvement and improved gastric emptying, with 90% of the subjects being withdrawn from jejunostomy tube feeding, the study was criticized for the lack of a control group.
Two types of stimulation have been used in the treatment of gastroparesis. The first uses long pulse widths in the order of milliseconds and a stimulation frequency in the physiologic range of the gastric slow waves, as was done in the study by McCallum et al.15 The second type of stimulation uses a short pulse width of a few hundred microseconds with a frequency a few times higher than the physiologic frequency of the gastric slow waves. Chen et al.16 evaluated the two types of stimulation in dogs with gastric dysrhythmias induced by vasopressin. Only long pulse width stimulation was capable of preventing vasopressin-induced gastric dysrhythmias and gastric slow wave uncoupling, but only short pulse width stimulation prevented vomiting. It appears that the prevention of nausea is vagally mediated, probably through sensory afferent pathways, as vagotomy abolishes the improvement in nausea. The effect of long pulse width stimulation on gastric slow waves is not blocked by atropine and may not be vagally mediated.16
Abell et al.17 reviewed the experience with short pulse width, high-frequency gastric pacing for gastroparesis, which has humanitarian use approval in idiopathic and diabetic gastroparesis by the Food and Drug Administration. The majority of drug refractory gastroparesis patients experienced improved nutrition (serum albumin and weight improved) during pacing and decreased nausea and vomiting with improved quality of life that was sustained over 5 years of follow-up.17 It is now possible to place gastric electrodes endoscopically to determine if the subject will respond to permanent gastric pacing for gastroparesis,18 and gastric pacing for gastroparesis has also been shown to improve the impaired pancreatic function associated with gastroparesis.19
Gastric Pacing with Short Pulse Width and High Frequency for Obesity
The concept of gastric pacing for the treatment of obesity originated with the observation of a 6-month-old infant with vomiting and failure to thrive because of an abnormal endogenous antral pacemaker.20 In 1992, a single pig named Lucky lost weight in a cyclical manner and reduced food intake during gastric pacing.21 This led to antral stimulation in three groups of pigs. The stimulus parameters in group 2 were an amplitude of 10 V, a pulse width of 450 μs, and a frequency of 100 Hz, with an on time of 3.2 s and an off time of 5.1 s. This group decreased food intake by 12–16% and gained 10% less than group 1 controls over 35 weeks. Group 3 was similar to group 2 but the amplitude was 5 V, which was ineffective in causing weight loss.20
Human gastric pacing for obesity began in 1995. There have now been more than 500 subjects treated for obesity with gastric pacing. With proper screening, weight loss is now in the range of 40% excess body weight. Excess body weight is defined as the weight above the midpoint of the 1983 Metropolitan Life Insurance tables for a given height and gender. Excess body weight loss divided in half is approximately equivalent to the percentage loss of initial body weight. Most surgical studies report results in terms of excess body weight lost, whereas most medical studies report results in terms of percentage body weight loss.
Cigaina21 reported on four series of subjects. The first 5 subjects were operated upon in 1995 and maintained a 70% loss of excess body weight over 2 years. Ten additional subjects were operated upon in 1998 and maintained a 20% loss of excess body weight over a year. Ten more subjects operated upon in 2000 lost 30% of excess body weight over 1 year. The fourth group of 40 patients was operated upon beginning in 2002, and the entire group lost 20% of excess body weight over 1 year. A screening algorithm was developed through retrospective analysis of weight loss in all prior trials. Subjects enrolled into the fourth group were screened with this algorithm beginning in 2003. Subjects screened in this way lost an average of 40% of excess body weight over 1 year.21 Subjects in these trials decreased their mean blood pressure 12/12 mm Hg, normalized bowel habits despite decreased food intake, were relieved of gastric reflux symptoms, and increased metabolic rate 10%. Insulin sensitivity was increased in proportion to weight loss as measured by the homeostatis model.21
These and other studies with the gastric stimulator for obesity are summarized in Table 1. These studies have been performed with the Transcend® stimulator manufactured by Transneuronix (Mt. Arlington, NJ) (Figure 1). The pacemaker is implanted laparoscopically below the pes anserinus 3 cm from the edge of the lesser gastric curvature and 6 cm from the pyloris. The lead is fixed within a tunnel in the gastric muscular wall by a suture, and gastroscopy is performed to ensure that the lead does not perforate the stomach wall. The operative time is less than an hour, and the stimulator is activated 30 days after implantation. Stimulus parameters are an amplitude of 10 mA, a pulse width of 208 μs, and a frequency of 40 Hz with 2 s on time and 3 s off time.22
Table 1.
Clinical Studies with the Transcend Gastric Pacemaker
| Ref. | N | % EWLa | Time |
|---|---|---|---|
| 22 | 20 | 24 | 10 months |
| 21 | 65 | 20-40 | 1–2 years |
| 25 | 69 | 21 | 15 months |
| 39 | 12 | 30 | 9 months |
| 27 | 11 | 20 | 6 months |
| 23 | 103 | 1.3/2.4 | 6.5 months |
| 23 | 30 | 23 | 16 months |
Percentage excess body weight lost.
Figure 1.
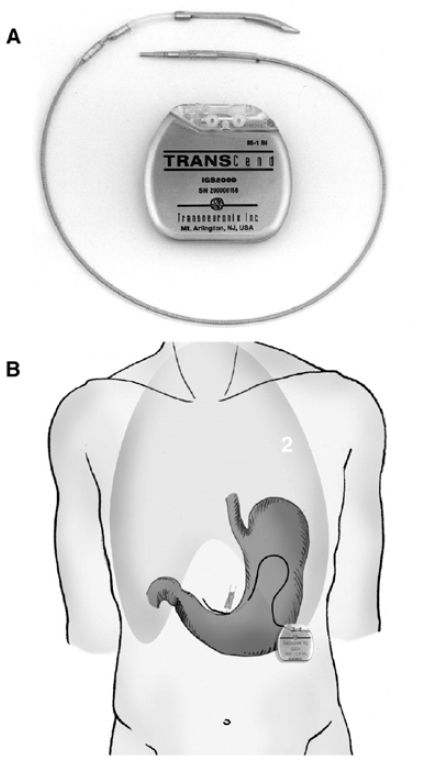
(A) Transcend pacemaker device. (B) Position of device. Reprinted with permission from Obesity Surgery Journal 2004;14:S14-S22.
All the clinical trials are open label studies with the exception of one study done in the United States. This study enrolled 103 patients who lost minimal weight over the blinded period. This study randomized patients to pacemaker on or pacemaker off in a 1:1 fashion for the first 6 months. Because this trial was done prior to the appreciation that the lead needed to be sutured in place, there were problems with lead displacement. There were 34 patients remaining in the trial at 29 months who lost 20% of excess body weight.23 Because proper patient selection is important in optimizing weight loss after pacemaker implantation, a retrospective analysis of the elements of success in prior trials was conducted and a predictive algorithm was created. Use of this algorithm has allowed selection of patients who lose an average of 40% of excess body weight.21
Since the trials to date have shown placement of the gastric pacemaker to be the safest and simplest operation for morbid obesity, there has been considerable interest in defining the mechanism by which it works.24 Several lessons have been learned through experience. The improvement in quality of life is proportional to the weight loss. In contrast to the original studies, the screening algorithm, the elimination of binge eaters, and a behavior modification program have been added to the more recent Screened Health Assessment and Pacer Evaluation trial, which is presently in progress.23 Nineteen subjects in the study reported by DeLuca and colleagues25 experienced increased satiety without the increase in ghrelin anticipated to accompany weight loss. Cigaina evaluated leptin, cholecystokinin (CCK), somatostatin, and glucagon-like peptide 1 (GLP-1) at baseline, 1 month, and 6 months following pacemaker implantation, as administration of these hormones is associated with weight loss.26 Subjects in this trial lost 10.4 kg, but the meal-related rise in all four hormones was reduced paradoxically. Although leptin is expected to fall with weight loss, the release of other hormones is dependent on the vagus nerve. Thus, these results are consistent with the pacemaker blocking efferent vagal nerve transmission.27
Other investigations into the mechanism by which the gastric pacemaker induces weight loss have focused on gastric myoelectrical activity. Obese patients have accelerated gastric emptying compared to nonobese patients.28 Two methods have been used to delay gastric emptying and increase satiety—long pulse width, low-frequency stimulation and short pulse width, high-frequency stimulation. The Transcend gastric pacemaker has been the most extensively studied and uses the latter. Its mechanism of action will be discussed first.
Short pulse width (a few hundred milliseconds) and high-frequency (a few times higher than physiological frequency of the gastric slow wave) stimulation increases afferent vagal activity as measured by spectral analysis, and vagotomy abolishes this effect, something that does not occur with long pulse width, low-frequency stimulation.29 This vagal effect is mediated by the afferent vagus through the nucleus of the tractus solitarius and may involve central circuits.30 Vagal afferents have been demonstrated to synapse with glucose-sensitive neurons in the ventromedial hypothalamus, and input from the cerebellar nucleus interpositus augments this vagal stimulation through an integrated pathway.31 Studies with positron emission tomography demonstrated higher metabolism in the right hippocampus with lower emotional eating scores when the Transcend pacemaker was in the “on” position compared to the “off” position.32 Although short pulse, high-frequency pacing increases vagal afferent activity, it blocks vagal efferent activity. The decrease in vagally controlled satiety hormones (CCK, GLP-1, and somatostatin) and an absence of the expected rise in ghrelin with fasting during stimulation are examples of efferent vagal inhibition.27,33 Ascribing the mechanism of short pulse width and high-frequency stimulation to blocking of the efferent vagus while activating the afferent vagus is based on the aforementioned studies and should be regarded as speculative until confirmed by further investigations.
Short pulse width, high-frequency stimulation induces gastric distention, inhibits postprandial antral contractions, and slows gastric emptying, which leads to early satiety and reduced food intake with weight loss.34 Cholinergic vagal efferents increase gastric tone, whereas nitric oxide pathways decrease it.35 Gastric stimulation blocks the efferent vagal pathway and releases nitric oxide pathways from inhibition, resulting in gastric dilatation.14,36,37 The gastric slow waves are inhibited postprandially, which may also contribute to the delay of gastric emptying with a promotion of satiety.38
Gastric Pacing with Long Pulse Width and Low Frequency for Obesity
Studies with pacing for gastroparesis in dogs using a pulse width in milliseconds at a frequency of six cycles per minute gave gastric relaxation when the pacing occurred in the proximal stomach, but not in the distal stomach.40 Another study in dogs demonstrated that one can entrain the gastric slow wave and vary their frequencies between two and nine cycles per minute from the normal five per minute normally seen in dogs.41 Based on these animal studies demonstrating inhibition of gastric contractions and delayed gastric emptying, temporary pacing was evaluated in normal human volunteers. Retrograde pacing with a mucosal electrode placed endoscopically on the greater curvature of the stomach 5 cm above the pyolorus was performed in 12 normal volunteers. The gastric slow waves were entrained at nine cycles per minute, and the symptoms of satiety, bloating, discomfort, and nausea were linearly correlated with the stimulation energy in milliamps. There was a great deal of variability regarding the stimulation energy needed to illicit symptoms of dyspepsia in individual subjects, and it was postulated that those with a lower threshold might be more sensitive to this type of retrograde stimulation.42
Encouraged by these initial physiologic studies, 12 normal volunteers were studied for 3 days using temporary electrodes placed endoscopically in the stomach 5 cm above the pylorus on the greater curvature (Figure 2). Retrograde gastric pacing at nine cycles per minute resulted in retrograde propagation of electrical waves from the antrum. These waves fought against the normal electrical waves that propagate distally and cause gastric hypomotility. During retrograde pacing, water consumption was decreased by 13%, food intake was decreased by 16%, and gastric retention of solids was increased by 15%. These changes were accompanied by tolerable dyspeptic symptoms.43 The same experiment was repeated using 50% of the energy needed to induce the first gastric sensations. These lower stimulus parameters slowed gastric emptying and reduced water intake. Subjects who were more viscerally sensitive had a greater response to the retrograde pacing.44 Another study in 12 normal volunteers using a stimulus intensity below the threshold to induce dyspepsia delayed gastric emptying, decreased food intake, and decreased water intake compared to the sham control, further suggesting a potential of longer stimulation for the treatment of obesity.45
Figure 2.
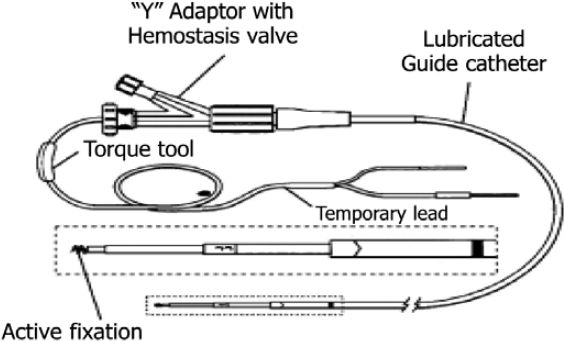
Temporary endoscopically placed pacemaker. From World Journal of Gastroenterology 2005; 11(29); 4541-4546.
Sympathetic Stimulation for Weight Loss
Obesity induced by hypothalamic damage is associated with increased vagal tone, increased insulin secretion, increased food intake, and weight gain.46 Performing a vagotomy below the diaphragm reverses the obesity from hypothalamic damage in rodents, and if the pancreatic islet cells are isolated from vagal enervation by transplantation beneath the kidney capsule, ventromedial hypothalamic injury no longer causes obesity.47 Thus, obesity seems to be associated with efferent vagal stimulation, something that is inhibited by the Transcend pacemaker presently in use.
Obesity is associated with a decrease in sympathetic tone,48 whereas an increase in sympathetic activity is associated with weight loss.49 Caffeine and ephedrine stimulate sympathetic activity systemically and were used successfully for more than a decade as a prescription obesity drug in Denmark.50 Caffeine and ephedrine are no longer available without a prescription as a dietary herbal supplement due to central nervous system stimulation and cardiovascular side effects associated with systemic sympathetic activation.51 It is possible, however, that selective stimulation of the sympathetic innervation to the gastrointestinal tract may decrease food intake without systemic side effects on the cardiovascular or central nervous systems. In fact, splanchnic nerve stimulation has been reported to decrease food intake, to cause weight loss, and to blunt ghrelin release in dogs over a 28-day period.52 Studies in humans have not been reported.
Vagal Nerve Stimulation for Weight Loss
Studies with the Transcend gastric pacemaker suggest that blocking efferent vagal impulses can reduce gastric tone accompanied by slow gastric emptying, decreased food intake, and induce weight loss. This suggests that vagal pacing may have a role in the treatment of obesity. To test this possibility, rabbits were implanted with a microchip at laparotomy on the posterior vagus. Over a 4-week period, body weight was decreased 12%, food intake was decreased 40%, and pulse rate was decreased. There were no observed adverse events, but the change in heart rate suggested stimulation of the afferent vagus in addition to blocking of the efferent vagus.53 In another study, pigs were implanted with microchips on both vagal nerves at laparotomy for stimulation. Food intake was decreased and body weight gain was reduced with no observable side effects at stimulus parameters of amplitude 170 mV, frequency 1 Hz, and 170 ms impulse duration. Normogastria was reduced and tachygastria was increased consistent with reduced efferent vagal transmission.54 A second pig study extended the observations by noting that the metabolic rate was not affected.55 The mechanism appears to be through afferent vagal pathways.
The only human studies on weight loss with vagal nerve stimulation come from trials of a vagal nerve stimulator for the treatment of epilepsy. These patients had cervical vagal stimulation, which gave hoarseness, cough, throat pain, and dyspnea. Thirty-two patients were evaluated and 17 lost weight. Eight patients lost more than 5% of body weight, 5 patients lost more than 10% of body weight, and one subject lost 36 kg.56 Because blocking the efferent vagus and stimulating the afferent vagus cause weight loss, studies were initiated in rats to evaluate the differential effects of vagal stimulation and vagotomy. Subdiphragmatic stimulation by microchip of the left vagal nerve gave weight loss and reduction in food intake. Weight loss and reduction in food intake were greater when both vagal nerves were stimulated, but were greatest when the left vagus nerve was stimulated and the right vagal nerve was sectioned. In fact, the combination of unilateral vagal stimulation and contralateral vagal section gave a synergistic increase in weight loss and reduction in food intake but vagotomy alone had no effect. Thus, unilateral vagotomy seemed to break a compensatory neural loop.57 This loop may involve the hypothalamus, as stimulation in the ventromedial hypothalamus reduces food intake and could be the mechanism of weight loss.58
Stimulating in the Electrical Refractory Period to Treat Obesity
A new form of pacing for obesity was an outgrowth of a cardiovascular application. In congestive heart failure there is a deficit in cardiac contractility. An approach called paired pacing stimulated a premature beat, which released calcium from the sarcoplasmic reticulum, enhancing the contractile strength of the subsequent beat. This older approach had the disadvantage that it could trigger adverse ventricular arrhythmias. Therefore, stimulation in the absolute refractory period was attempted in dogs whose coronary arteries had been embolized to create congestive heart failure. This nonstimulatory pacing increased cardiac contractility and improved congestive heart failure over 7 days.59 Using cardiomyopathic muscle, it was shown that stimulation during the absolute refractory period causes release of calcium into the myocytes by L-type channels. This increased calcium became available from the sarcoplasmic reticulum on the next cardiac beat, increasing contractility. Thus, stimulation during the absolute refractory period increased contractility in diseased cardiac muscle without inducing arrhythmias and maintained its effectiveness in the presence of a beta blocker.60
Pacing during the cardiac absolute refractory period was attempted in 15 subjects with New York Heart Association (NYHA) class 2 heart failure with an ejection fraction below 35%. Pacing increased cardiac contractility and increased the ejection fraction by 16% without any adverse events.61 Following that initial safety study, 25 patients with NYHA class 3 heart failure were paced during the absolute refractory period 3 hours per day for 8 weeks. These subjects improved from class 3 to class 2, improved their ejection fraction by 25%, and increased their 6-minute walk time significantly.62
Stimulation of the gastric antrum rats during the absolute refractory period increases the strength of gastric contractions and increases vagal afferent firing, as does stomach distension.63 Pyloric stimulation in dogs decreased food intake, decreased antral contractions, and delayed gastric emptying.64 These observations led to the Tantulus™ device, which is implanted laparoscopically (Figure 3). It has a pulse generator and three bipolar leads. Two pairs of electrodes are implanted in the gastric antrum and the other two pairs are implanted in the gastric fundus. The electrodes in the gastric fundus sense the beginning of a meal and signal the pulse generator to stimulate the antral electrodes in the absolute antral refractory period, enhancing spontaneous gastric contractions and sending a signal through the afferent vagus that the stomach is distended. A trial in 12 obese subjects with a mean weight of 129 kg demonstrated that the fundal electrodes were able to sense the start of a meal >75% of the time and that stimulation of the antral electrodes increased the strength of antral contractions by 43%. Subjects lost 10 kg over 20 weeks and 9 subjects who continued to week 52 lost an additional 7 kg for a 30.5% excess body weight loss. Blood pressure decreased 17/8 mm Hg, as did hunger with an increase in the intermeal interval.65 A second study was performed in 24 type 2 diabetic subjects with an average body mass index of 42 kg/m2. Weight loss in this study was 4.6 and 8.6% of initial body weight at 5 and 9 months after implantation. Glycohemoglobin in the same study decreased from 8.0% at baseline to 7.3% at 5 months and to 6.4% at 9 months.66
Figure 3.
Tantulus™ pacemaker device. Reprinted with permission from Obesity Surgery Journal 2006;16:627-634.
Intestinal Electrical Pacing for Obesity
Various parts of the intestinal tract have been stimulated in the search for an effective obesity treatment. Duodenal stimulation in 12 healthy human volunteers did not induce dyspepsia, but did reduce water intake and slowed gastric emptying.67 Bypass operations have accelerated intestinal transit, and fat in the distal small intestine slows intestinal transit through a mechanism referred to as the ileal brake. Intestinal stimulation using seven sequential electrodes in dogs with a frequency of 24 cycles per minute, a pulse duration of 50 ms, and a pulse amplitude of 1–3mA entrained the intestinal pacesetter. This was effective in stimulating intestinal transit, even in the face of fat in the distal small intestine.68 Intestinal stimulation in rats accelerated intestinal transit and reduced fat absorption.69 Stimulation of the intestine in rats also decreased gastric motility and activated neurons in the tractus solitarius through the vagus.70 Clinical trials of intestinal pacing have yet to be reported.
Future Potential for Pacing in the Treatment of Obesity
Obesity is a chronic disease that, like diabetes, has multiple redundant control systems. Diabetes is usually treated with medications that act on different aspects of the diverse mechanisms controlling carbohydrate metabolism. Using a sulfonylurea with a biguanide is just one example of this approach. It is likely that the same strategy will be used with obesity drugs, and combinations such as bupropion and naltrexone presently in drug development attest to this assertion.71 Different pacing strategies impact obesity through different physiologic mechanisms, and there is no apparent reason that different pacing mechanisms could not be combined for greater efficacy in weight loss.72 For example, the Transcend pacemaker appears to block efferent vagal activity and slow gastric emptying. Splanchnic nerve stimulation appears to work by sympathetic stimulation, a different mechanism entirely. Because the sympathetic to parasympathetic ratio of autonomic activation is directly proportional to obesity, one could envision these two approaches being used together and giving additive weight loss. To date, almost all of the studies reviewed in this article have been observational. It is clear that there is a need for randomized controlled trials in the field of gastrointestinal pacing and it is hoped that future trials will fulfill this need.
Conclusions
Obesity is an epidemic disease that is increasing in prevalence and is associated with a rising incidence of diabetes. Behavior and lifestyle therapy are safe and effective treatments for obesity in the short term, but the durability of the weight loss is limited, as can be appreciated from the rising obesity burden. Although promising obesity drugs may be in the developmental pipeline, presently available drugs are lacking in efficacy and combining them to improve efficacy has been, so far, disappointing. Surgery gives durable weight loss, but comes with higher morbidity and even mortality. A safe and effective method of inducing significant and durable weight loss is desperately needed. Electrical pacing holds promise of being such a treatment. One pacing approach is presently available and others with different physiologic mechanisms are in various stages of development. The field of electrical pacing as a treatment for obesity should produce exciting new developments in the foreseeable future.
Abbreviations
- CCK
cholecystokinin
- GLP-1
glucagon-like peptide 1
- NYHA
New York Heart Association
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004 Jun 16;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA. Obesity: a time bomb to be defused. Lancet. 1998 Jul 18;352(9123):160–161. doi: 10.1016/S0140-6736(98)22029-0. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003 Jan 1;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998 Mar;6(2):97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 5. www.diabetes.org. Statistics: economic costs of diabetes in the United States in 2002. American Diabetes Association, 2006.
- 6.Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes. 1989;13(Suppl 2):39–46. [PubMed] [Google Scholar]
- 7.Greenway FL, Caruso MK. Safety of obesity drugs. Expert Opin Drug Saf. 2005 Nov;4(6):1083–1095. doi: 10.1517/14740338.4.6.1083. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub M, Sundaresan PR, Cox C. Long-term weight control study. VI. Individual participant response patterns. Clin Pharmacol Ther. 1992 May;51(5):619–633. doi: 10.1038/clpt.1992.74. [DOI] [PubMed] [Google Scholar]
- 9.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. etal Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995 Sep;222(3):339–350. doi: 10.1097/00000658-199509000-00011. discussion 350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinari GM, Murelli F, Camerini G, Papadia F, Carlini F, Stabilini C, Adami GF, Scopinaro N. A 15-year evaluation of biliopancreatic diversion according to the Bariatric Analysis Reporting Outcome System (BAROS) Obes Surg. 2004 Mar;14(3):325–328. doi: 10.1381/096089204322917828. [DOI] [PubMed] [Google Scholar]
- 11.Greenway FL. Surgery for obesity. Endocrinol Metab Clin North Am. 1996 Dec;25(4):1005–1027. doi: 10.1016/s0889-8529(05)70367-4. [DOI] [PubMed] [Google Scholar]
- 12.Abell TL, Malagelada JR. Electrogastrography. Current assessment and future perspectives. Dig Dis Sci. 1988 Aug;33(8):982–992. doi: 10.1007/BF01535995. [DOI] [PubMed] [Google Scholar]
- 13.Abell TL, Minocha A. Gastroparesis and the gastric pacemaker: a revolutionary treatment for an old disease. J Miss State Med Assoc. 2002 Dec;43(12):369–375. [PubMed] [Google Scholar]
- 14.Chen J. Mechanisms of action of the implantable gastric stimulator for obesity. Obes Surg. 2004 Sep;14(Suppl 1):S28–32. doi: 10.1007/BF03342135. [DOI] [PubMed] [Google Scholar]
- 15.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998 Mar;114(3):456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 16.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003 Feb;124(2):401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 17.Abell T, Lou J, Tabbaa M, Batista O, Malinowski S, Al-Juburi A. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003 Jul–Aug;27(4):277–281. doi: 10.1177/0148607103027004277. [DOI] [PubMed] [Google Scholar]
- 18.Ayinala S, Batista O, Goyal A, Al-Juburi A, Abidi N, Familoni B, Abell T. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005 Mar;61(3):455–461. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 19.Luo J, Al-Juburi A, Rashed H, O'Dorisio T, Marchal B, Starkebaum W, Abell TL. Gastric electrical stimulation is associated with improvement in pancreatic exocrine function in humans. Pancreas. 2004 Aug;29(2):e41–4. doi: 10.1097/00006676-200408000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Cigaina VV, Saggioro A, Rigo VV, Pinato G, Ischai S. Long-term effects of gastric pacing to reduce feed intake in swine. Obes Surg. 1996 Jun;6(3):250–253. doi: 10.1381/096089296765556854. [DOI] [PubMed] [Google Scholar]
- 21.Cigaina V. Long-term follow-up of gastric stimulation for obesity: the Mestre 8-year experience. Obes Surg. 2004 Sep;14(Suppl 1):S14–22. doi: 10.1007/BF03342133. [DOI] [PubMed] [Google Scholar]
- 22.Favretti F, De Luca M, Segato G, Busetto L, Ceoloni A, Magon A, Enzi G. Treatment of morbid obesity with the Transcend Implantable Gastric Stimulator (IGS): a prospective survey. Obes Surg. 2004 May;14(5):666–670. doi: 10.1381/096089204323093462. [DOI] [PubMed] [Google Scholar]
- 23.Shikora SA. “What are the yanks doing?” the U.S. experience with implantable gastric stimulation (IGS) for the treatment of obesity—update on the ongoing clinical trials. Obes Surg. 2004 Sep;14(Suppl 1):S40–8. doi: 10.1007/BF03342137. [DOI] [PubMed] [Google Scholar]
- 24.Shikora SA. Implantable gastric stimulation—the surgical procedure: combining safety with simplicity. Obes Surg. 2004 Sep;14(Suppl 1):S9–13. doi: 10.1007/BF03342132. [DOI] [PubMed] [Google Scholar]
- 25.De Luca M, Segato G, Busetto L, Favretti F, Aigner F, Weiss H, de Gheldere C, Gaggiotti G, Himpens J, Limao J, Scheyer M, Toppino M, Zurmeyer EL, Bottani G, Penthaler H. Progress in implantable gastric stimulation: summary of results of the European multi-center study. Obes Surg. 2004 Sep;14(Suppl 1):S33–9. doi: 10.1007/BF03342136. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Z, Lewis MW, Travagli RA. In vitro analysis of the effects of cholecystokinin on rat brain stem motoneurons. Am J Physiol Gastrointest Liver Physiol. 2005 May;288(5):G1066–73. doi: 10.1152/ajpgi.00497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res. 2003 Dec;11(12):1456–1462. doi: 10.1038/oby.2003.195. [DOI] [PubMed] [Google Scholar]
- 28.Shikora SA. Implantable gastric stimulation for weight loss. J Gastrointest Surg. 2004 May–Jun;8(4):408–412. doi: 10.1016/j.gassur.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Qiao X, Chen JD. Vagal afferent is involved in short-pulse gastric electrical stimulation in rats. Dig Dis Sci. 2004 May;49(5):729–737. doi: 10.1023/b:ddas.0000030081.91006.86. [DOI] [PubMed] [Google Scholar]
- 30.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu JN, Zhang YP, Song YN, Wang JJ. Cerebellar interpositus nuclear and gastric vagal afferent inputs reach and converge onto glycemia-sensitive neurons of the ventromedial hypothalamic nucleus in rats. Neurosci Res. 2004 Apr;48(4):405–417. doi: 10.1016/j.neures.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, Wong CT, Tomasi D, Thanos PK, Fowler JS. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003 Dec;144(12):5184–5187. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 34.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999 Jan 15;514(Pt 2):369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermann GE, Travagli RA, Rogers RC. Esophageal-gastric relaxation reflex in rat: dual control of peripheral nitrergic and cholinergic transmission. Am J Physiol Regul Integr Comp Physiol. 2006 Jun;290(6):R1570–6. doi: 10.1152/ajpregu.00717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei Y, Xing J, Chen JD. Effects and mechanisms of implantable gastric stimulation on gastric distention in conscious dogs. Obes Surg. 2005 Apr;15(4):528–533. doi: 10.1381/0960892053723358. [DOI] [PubMed] [Google Scholar]
- 37.Zheng ZL, Rogers RC, Travagli RA. Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience. 1999 May;90(2):685–694. doi: 10.1016/s0306-4522(98)00586-7. [DOI] [PubMed] [Google Scholar]
- 38.Ouyang H, Yin J, Chen JD. Therapeutic potential of gastric electrical stimulation for obesity and its possible mechanisms: a preliminary canine study. Dig Dis Sci. 2003 Apr;48(4):698–705. doi: 10.1023/a:1022824406648. [DOI] [PubMed] [Google Scholar]
- 39.D'Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002 Apr;12(Suppl 1):21S–25S. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]
- 40.Xing JH, Brody F, Brodsky J, Larive B, Ponsky J, Soffer E. Gastric electrical stimulation at proximal stomach induces gastric relaxation in dogs. Neurogastroenterol Motil. 2003 Feb;15(1):15–23. doi: 10.1046/j.1365-2982.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 41.Familoni BO, Abell TL, Gan Z, Voeller G. Driving gastric electrical activity with electrical stimulation. Ann Biomed Eng. 2005 Mar;33(3):356–364. doi: 10.1007/s10439-005-1738-6. [DOI] [PubMed] [Google Scholar]
- 42.Yao SK, Ke MY, Wang ZF, Xu DB, Zhang YL. Visceral response to acute retrograde gastric electrical stimulation in healthy human. World J Gastroenterol. 2005 Aug 7;11(29):4541–4546. doi: 10.3748/wjg.v11.i29.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao S, Ke M, Wang Z, Xu D, Zhang Y, Chen JD. Retrograde gastric pacing reduces food intake and delays gastric emptying in humans: a potential therapy for obesity? Dig Dis Sci. 2005 Sep;50(9):1569–1575. doi: 10.1007/s10620-005-2899-8. [DOI] [PubMed] [Google Scholar]
- 44.Yao S, Ke M, Wang Z, Xu D, Zhang Y, Chen JD. Visceral sensitivity to gastric stimulation and its correlation with alterations in gastric emptying and accommodation in humans. Obes Surg. 2005 Feb;15(2):247–253. doi: 10.1381/0960892053268363. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Hou X, Song G, Cha H, Yang B, Chen JD. Gastric electrical stimulation using endoscopically placed mucosal electrodes reduces food intake in humans. Am J Gastroenterol. 2006 Apr;101(4):798–803. doi: 10.1111/j.1572-0241.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 46.Bray GA, Gallagher TF., Jr Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a review of the literature. Medicine (Baltimore) 1975 Jul;54(4):301–330. doi: 10.1097/00005792-197507000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Bray GA. Hypothalamic and genetic obesity: an appraisal of the autonomic hypothesis and the endocrine hypothesis. Int J Obes. 1984;8(Suppl 1):119–137. [PubMed] [Google Scholar]
- 48.Bray GA. Obesity, a disorder of nutrient partitioning: the MONA LISA hypothesis. J Nutr. 1991 Aug;121(8):1146–1162. doi: 10.1093/jn/121.8.1146. [DOI] [PubMed] [Google Scholar]
- 49.Bray GA. Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int J Obes Relat Metab Disord. 2000 Jun;24(Suppl 2):S8–17. doi: 10.1038/sj.ijo.0801269. [DOI] [PubMed] [Google Scholar]
- 50.Greenway FL. The safety and efficacy of pharmaceutical and herbal caffeine and ephedrine use as a weight loss agent. Obes Rev. 2001 Aug;2(3):199–211. doi: 10.1046/j.1467-789x.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 51.Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L, Gagne J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003 Mar 26;289(12):1537–1545. doi: 10.1001/jama.289.12.1537. [DOI] [PubMed] [Google Scholar]
- 52.Dobak JD. Dynamic nerve stimulation for treatment of disorders. 2005. p. 37. In: Office USPT, ed. U.S. [Google Scholar]
- 53.Sobocki J, Thor PJ, Uson J, Diaz-Guemes I, Lipinski M, Calles C, Pascual S. Microchip vagal pacing reduces food intake and body mass. Hepatogastroenterology. 2001 Nov–Dec;48(42):1783–1787. [PubMed] [Google Scholar]
- 54.Matyja A, Thor PJ, Sobocki J, Laskiewicz J, Kekus J, Tuz R, Koczanowski J, Zaraska W. Effects of vagal pacing on food intake and body mass in pigs. Folia Med Cracov. 2004;45(3-4):55–62. [PubMed] [Google Scholar]
- 55.Sobocki J, Fourtanier G, Estany J, Otal P. Does vagal nerve stimulation affect body composition and metabolism? Experimental study of a new potential technique in bariatric surgery. Surgery. 2006 Feb;139(2):209–216. doi: 10.1016/j.surg.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 56.Burneo JG, Faught E, Knowlton R, Morawetz R, Kuzniecky R. Weight loss associated with vagus nerve stimulation. Neurology. 2002 Aug 13;59(3):463–464. doi: 10.1212/wnl.59.3.463. [DOI] [PubMed] [Google Scholar]
- 57.Laskiewicz J, Krolczyk G, Zurowski G, Sobocki J, Matyja A, Thor PJ. Effects of vagal neuromodulation and vagotomy on control of food intake and body weight in rats. J Physiol Pharmacol. 2003 Dec;54(4):603–610. [PubMed] [Google Scholar]
- 58.Takaki A, Aou S, Oomura Y, Okada E, Hori T. Feeding suppression elicited by electrical and chemical stimulations of monkey hypothalamus. Am J Physiol. 1992 Apr;262(4 Pt 2):R586–94. doi: 10.1152/ajpregu.1992.262.4.R586. [DOI] [PubMed] [Google Scholar]
- 59.Sabbah HN, Haddad W, Mika Y, Nass O, Aviv R, Sharov VG, Maltsev V, Felzen B, Undrovinas AI, Goldstein S, Darvish N, Ben-Haim SA. Cardiac contractility modulation with the impulse dynamics signal: studies in dogs with chronic heart failure. Heart Fail Rev. 2001 Jan;6(1):45–53. doi: 10.1023/a:1009855208097. [DOI] [PubMed] [Google Scholar]
- 60.Brunckhorst CB, Shemer I, Mika Y, Ben-Haim SA, Burkhoff D. Cardiac contractility modulation by non-excitatory currents: studies in isolated cardiac muscle. Eur J Heart Fail. 2006 Jan;8(1):7–15. doi: 10.1016/j.ejheart.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Pappone C, Vicedomini G, Salvati A, Meloni C, Haddad W, Aviv R, Mika Y, Darvish N, Kimchy Y, Shemer I, Snir Y, Pruchi D, Ben-Haim SA, Kronzon I. Electrical modulation of cardiac contractility: clinical aspects in congestive heart failure. Heart Fail Rev. 2001 Jan;6(1):55–60. doi: 10.1023/a:1009807309006. [DOI] [PubMed] [Google Scholar]
- 62.Stix G, Borggrefe M, Wolpert C, Hindricks G, Kottkamp H, Bocker D, Wichter T, Mika Y, Ben-Haim S, Burkhoff D, Wolzt M, Schmidinger H. Chronic electrical stimulation during the absolute refractory period of the myocardium improves severe heart failure. Eur Heart J. 2004 Apr;25(8):650–655. doi: 10.1016/j.ehj.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 63.Peles S, Petersen J, Aviv R, Policker S, Abu-Hatoum O, Ben-Haim SA, Gutterman DD, Sengupta JN. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am J Physiol Gastrointest Liver Physiol. 2003 Sep;285(3):G577–85. doi: 10.1152/ajpgi.00109.2003. [DOI] [PubMed] [Google Scholar]
- 64.Xu X, Zhu H, Chen JD. Pyloric electrical stimulation reduces food intake by inhibiting gastric motility in dogs. Gastroenterology. 2005 Jan;128(1):43–50. doi: 10.1053/j.gastro.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 65.Bohdjalian A, Prager G, Aviv R, Policker S, Schindler K, Kretschmer S, Riener R, Zacherl J, Ludvik B. One-year experience with Tantalus: a new surgical approach to treat morbid obesity. Obes Surg. 2006 May;16(5):627–634. doi: 10.1381/096089206776945101. [DOI] [PubMed] [Google Scholar]
- 66.Ludvik B. Glucose improvement by gastric stimulation in morbidly obese type-2 diabetic patients—interim results. Diabetes. 2006;55:A396. [Google Scholar]
- 67.Liu S, Hou X, Chen JD. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterol. 2005 Apr;100(4):792–796. doi: 10.1111/j.1572-0241.2005.40511.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen JD, Lin HC. Electrical pacing accelerates intestinal transit slowed by fat-induced ileal brake. Dig Dis Sci. 2003 Feb;48(2):251–256. doi: 10.1023/a:1021911023155. [DOI] [PubMed] [Google Scholar]
- 69.Sun Y, Chen J. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obes Res. 2004 Aug;12(8):1235–1242. doi: 10.1038/oby.2004.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun Y, Qin C, Foreman RD, Chen JD. Intestinal electric stimulation modulates neuronal activity in the nucleus of the solitary tract in rats. Neurosci Lett. 2005 Sep 2;385(1):64–69. doi: 10.1016/j.neulet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 71.Greenway FL. Bupropion and naltrexone for the treatment of obesity. Diabetes. 2006;55:A394. [Google Scholar]
- 72.Travagli RA, Rogers RC. Receptors and transmission in the brain-gut axis: potential for novel therapies. V. Fast and slow extrinsic modulation of dorsal vagal complex circuits. Am J Physiol Gastrointest Liver Physiol. 2001 Sep;281(3):G595–601. doi: 10.1152/ajpgi.2001.281.3.G595. [DOI] [PMC free article] [PubMed] [Google Scholar]