Abstract
Background
There is increasing evidence that insulin resistance (IR) has an important implication in the pathogenesis of polycystic ovary syndrome (PCOS), a common endocrinopathy in women. This study was performed to investigate the impact of different treatments for IR on five currently discussed markers for insulin resistance: intact proinsulin, adiponectin, retinol-binding protein 4 (RBP4), resistin, and visfatin in patients with PCOS.
Methods
Thirty-five women with clinically confirmed PCOS diagnosis were included in the study [age (mean±SD): 24.7±4.8 years; body mass index: 27.4±6.0 kg/m2]. They were randomized to receive either metformin (850 mg twice a day) or rosiglitazone (4 mg once a day). Blood samples for measurement of the HOMAIR score, visfatin, RBP4, intact proinsulin, resisitin, and adiponectin were taken at baseline and after 6 months of treatment.
Results
Both drugs improved ovulation, and an increase in insulin sensitivity was observed, especially in the rosiglitazone arm. Adiponectin levels increased in both treatment arms (metformin: 8.6±3.3 to 16.7±7.2 mg/liter, p < 0.001; rosiglitazone: 8.2±3.5 to 26.2±9.5 mg/liter, p < 0.001), but the increase was more pronounced with rosiglitazone (p < 0.001). While no changes of visfatin concentrations were observed during rosiglitazone therapy (15.4±6.9 ng/ml vs 17.4±4.8 ng/ml, n.s.), there was an increase in the metformin treatment arm (11.9±4.0 to 21.8±8.3 ng/ml, p < 0.001). Significant increases demonstrated for RBP4 in both treatment arms were more pronounced in the metformin group (metformin: +66%, rosiglitazone: +33%). All patients were in stage I or II of ß-cell dysfunction and none of them showed increased intact proinsulin levels or changes in resisitin at baseline or end point.
Conclusions
Both drugs slightly improved ovulation in our PCOS patient population during 6 months of therapy, which was accompanied by improved insulin sensitivity and an increase in adiponectin levels. Metformin increased visfatin concentrations. Despite improved insulin resistance, an increase in RBP4 concentration was seen for both drugs. Rosiglitazone seems to be the more favorable drug under these circumstances. However, our results regarding visfatin and RBP4 contradict other reports and further research is required to clarify their value as diagnostic markers for the metabolic syndrome. In this study, adiponectin appeared to be the most promising indicator of both metabolic status and therapeutic success.
Keywords: adiponectin, insulin resistance, metformin, polycystic ovary syndrome, rosiglitazone
Introduction
Polycystic ovary syndrome (PCOS) has a complex and multifactorial etiology, where adipose tissue seems to have an important role in the development and maintenance of the condition. Weight gain in normal women is associated with an increase in insulin resistance (IR), but in women PCOS, this phenomenon is observed in a significantly larger extent than in age and body mass index (BMI)-matched control women.1–3 The use of insulin-sensitizing drugs significantly improves the metabolic state, ovulatory function, menstrual cyclicity, and fertility rates,4–8 and it is therefore assumed that insulin resistance plays an important role in the pathogenesis of the disorder.1 Several potential biomarkers of insulin resistance and metabolic syndrome have been discussed in the scientific literature, including adiponectin, visfatin, and retinal-binding protein 4 (RBP4).
Adiponectin is expressed by the adipose and connective tissue and exerts anti-inflammatory and antiatherogenic effects.9 Decreased plasma levels of adiponectin are correlated to high BMI and IR. Low adiponectin levels are associated with metabolic syndrome and a high risk of myocardial infarction.10 The recently reported new adipokine visfatin is also expressed predominantly in the visceral lipid tissue, the accumulation of which has been linked to the metabolic syndrome.11,12 Visfatin binds to and activates the insulin receptor. It is assumed that it induces the intracellular signaling cascade for insulin with tyrosine phosphorylation of the insulin receptor and its substrates (IRS1 and IRS2) and activation of protein kinase B activity. This activation, however, seems to be distinct from that of insulin,13 and visfatin has become a research target with the attempt to investigate novel approaches for antidiabetic treatments. Retinol-binding protein 4 is a specific transport protein for retinol (vitamin A) in the human circulation.14,15 RBP4 is secreted by adipocytes and the liver, and elevated RBP4 concentrations have been reported from patients with type 2 diabetes.16,17 Graham and co-workers18 demonstrated that RBP4 levels are elevated in the serum before development of overt type 2 diabetes. The levels were associated with the components of the metabolic syndrome and could be reduced by physical exercise training in correlation to insulin resistance improvement.18 It is currently suggested that insulin resistance in adipose tissue is associated with reduced levels of glucose transporter 4 molecules, which results in increased RBP4 production. The consecutively elevated circulating serum RBP4 proteins cause insulin resistance in muscle and induce hepatic gluconeogenesis. These factors lead to increased blood glucose concentrations and finally to impaired glucose tolerance and diabetes.19 Therefore, RBP4 has been described to be a suitable biomarker for the metabolic syndrome and increased cardiovascular risk and it was even suggested that antidiabetic therapies should aim at lowering serum RBP4 levels.18 Intact proinsulin was shown by us to be a marker of severe ß-cell dysfunction following exhaustion of the ß-cell proinsulin processing capacity and a highly specific indicator of clinically significant insulin resistance.20,21 The search for genes that are downregulated by thiazolidinediones in mouse adipocytes resulted in the detection of an adipose-specific protein called resistin by Steppan and co-workers.4 Formation of resistin is induced during adipogenesis, and the peptide is secreted by white and brown fat tissue of C57B1/6J mice into the serum.22 The clinical implications of resistin determinations in human serum are still a matter of debate, and new laboratory methods have become available for the assessment of resistin levels in human plasma.23
The purpose of this investigation was to explore whether treatment of patients with PCOS with two different insulin-sensitizing drugs has an impact on these newly described biomarkers for metabolic syndrome. We also wanted to identify the most suitable marker for this condition out of the hormone panel tested, which was composed of intact proinsulin, adiponectin, resisitin, visfatin, and retinol-binding protein 4.
Patients and Methods
The study was performed with 35 women with PCOS as classified according to National Institute of Child Health and Human Development criteria.24 All subjects gave their written informed consent before entering the study, which was conducted in accordance with the Declaration of Helsinki and approved by the national ethical committee. Baseline characteristics of the patients are presented in Table 1. Clinical hyperandrogenism was defined by the presence of hirsutism, represented by a modified Ferriman–Gallwey score of 7 or more, persistence of acne during the third decade of life or later, or the presence of androgenic alopecia. No attempts were made to grade the severity of acne or alopecia. Hyperandrogenemia was defined as a total of free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate level above the 95th percentile of control values. Menstrual dysfunction was defined by more than six cycles with a length of more than 35 days and/or when the patient had not had any menstrual bleeding for 3 consecutive months during the last year. All patients had normal serum prolactin concentrations and thyroid function tests. Possible Cushing's syndrome or congenital (nonclassic) adrenal hyperplasia was excluded.24 Exclusion criteria were type 1 or type 2 diabetes mellitus, significant cardiovascular or hepatic disease, and the use of medications known or suspected to affect reproductive or metabolic functions within 60 days of study entry. No patient had ever taken insulin-sensitizing drugs prior to the study.
Table 1.
Patients' Characteristics at Baseline
| Characteristic | Metformin | Rosiglitazone |
|---|---|---|
| N | 17 | 18 |
| Age (years) | 22.9±4.5 | 25.2±4.8 |
| BMI (kg/m2) | 29.3±6.5 | 27.9±3.0 |
| Systolic blood pressure (mmHg) | 121±11 | 115±11 |
| Diastolic blood pressure (mmHg) | 67±18 | 72±5 |
| Waist circumference (cm) | 88.5±14.4 | 84.2±7.5 |
| HOMAIR | 2.9±1.9 | 2.2±1.3 |
| Dehydroepiandrosterone (Einheit) | 6.8±2.8 | 6.4±2.4 |
| Androstendione (Einheit) | 9.4±3.8 | 8.8±3.9 |
| Total testosterone | 3.2±1.1 | 2.8±1.2 |
| Free testosterone | 9.9±4.4 | 8.1±5.4 |
| Menstrual bleedings/month | 0.45±0.31 | 0.61±0.27 |
The patients were randomly allocated to a 6-month treatment with either metformin 850 mg twice daily (n = 17) or rosiglitazone 4 mg/day (n = 18). At baseline and end point, blood samples were drawn after an overnight fast for determination of glucose, insulin, and other observation parameters. All the blood samples were centrifuged, and the separated serum was kept frozen at –70°C until the time of the assay. Glucose levels were determined using a standard laboratory reference method (glucose oxidase method; Roche Hitachi 917). Visfatin was measured with an ELISA method (Phoenix, La Jolla, CA; intra-assay variability: 2.2–2.6%; interassay variability: 4.0–10.7%), and adiponectin was measured by means of a radioimmunoassay (Linco, St. Charles, MO, intra-assay variability: 2.8–5.0%, interassay variability: 3.5–8.2%). Measurement RBP4 was also performed by a validated ELISA (Phoenix, Karlsruhe, Germany; intra-assay variability: 2.2–2.6%, interassay variability: 4.0–10.7%). Intact proinsulin was assessed by means of a specific ELISA method (Zentech, Liege, Belgium). Resistin values were measured by means of an ELISA method, as published previously.23
Homeostasis model assessment (HOMAIR) score calculation was applied as a measure for insulin resistance.25 The estimate of insulin resistance by HOMAIR score was calculated with the following formula: fasting serum insulin (μU/ml) x fasting plasma glucose (mmol/liter)/22.5. HOMAIR score values exceeding 2.0 were considered as insulin resistant, as published previously for a nondiabetic patient population.26
Statistical Analysis
Pretreatment differences between the two groups for the different biomarkers and changes in the women with PCOS, before and after treatment, with controls, were analyzed using the Student's independent two-tailed t test. Pretreatment and post-treatment data were expressed as mean±SD for continuous variables and as percentages for categorical data. Analysis of covariance (ANCOVA) was used to compare parameters between treatments. By using ANCOVA, we adjusted the final variables, or their change with treatment, for the baseline results to avoid possible baseline imbalances. The Wilcoxon test was used to determine the effect of treatment on chosen parameters in the 6-month time interval. Categorical variables were compared using Fisher's exact test. A value of p < 0.05 was considered significant. Data analysis was formed using the statistical software package SPSS for Windows (Version 12.0, SPSS Inc., Chicago, IL).
Results
After initial inclusion of 35 patients, 33 (94.3%) finished the trial according to the protocol. Two women, one from each group, were excluded due to protocol violations. The mean±SD values for the observation parameters at baseline and end point are represented in Table 2. Only minor and nonsignificant changes were observed in the BMI values in both groups. There were no significant differences at baseline in any of those parameters between the treatment groups. HOMAIR score values decreased in a similar direction in both groups. At baseline, 47% of the patients in the metformin group and 50% of the patients in the rosiglitazone group were insulin sensitive according to their HOMAIR score (<2). After 6 months of treatment, HOMAIR <2 was found again in 47% of the patients in the metformin group and in 56% of the patients in the rosiglitazone group. There were nonsignificant tendencies of HOMAIR improvement in both groups that were predominantly driven by a decrease in fasting insulin values. Fasting intact proinsulin levels were in the normal range at all times but were decreased further in the rosiglitazone group. Mean resistin concentrations were low at baseline, and no change was observed in any of the treatment groups at end point. RBP4 increased in both groups from baseline, whereas visfatin only increased in the metformin group and concentrations remained the same in the rosiglitazone group. The adiponectin levels increased in both groups also but the increase was more pronounced in the rosiglitazone group. Figure 1 presents the correlation between adiponectin and the HOMAIR score at baseline. The percentage changes in the laboratory markers from baseline to end point as shown in Figure 2 demonstrate the sensitivity of the adiponectin values to glitazone and metformin treatment.
Table 2.
Observation Parameters at Baseline and End Pointa
| Parameter | Metformin | Rosiglitazone | ||
|---|---|---|---|---|
| Baseline | End point | Baseline | End point | |
| Glucose (mmol/liter) | 4.43±0.38 | 4.26±0.30 | 4.31±0.36 | 4.33±0.27 |
| Insulin (µU/ml) | 14.7±9.4 | 12.1±6.9 | 11.1±5.8 | 9.2±3.9 |
| HOMAIR | 2.9±1.9 | 2.3±1.4 | 2.2±1.3 | 1.8±0.8 |
| Adiponectin (mg/liter) | 8.6±3.3 | 16.7±7.7*** | 8.2±3.5 | 26.2±9.5*** |
| Visfatin (ng/ml) | 11.9±4.0 | 21.8±8.3*** | 15.4±6.9 | 17.4±4.8 |
| Intact proinsulin (pmol/liter) | 2.7±0.7 | 3.1±1.0 | 3.2±0.8 | 1.5±0.6* |
| Resistin (ng/liter) | 71±5 | 68±6 | 74±3 | 69±8 |
| RBP4 (ng/m) | 14.2±6.6 | 21.8±8.3*** | 17.8±7.2 | 23.7±3.5** |
| Periods/month | 0.45±0.31 | 0.61±0.33 | 0.61±0.27 | 0.77±0.26 |
| BMI (kg/m2) | 29.3±6.5 | 28.6±7.2 | 27.0±3.9 | 27.2±3.9 |
p vs baseline: *p < 0.05; **p < 0.01 ***p < 0.001.
Figure 1.
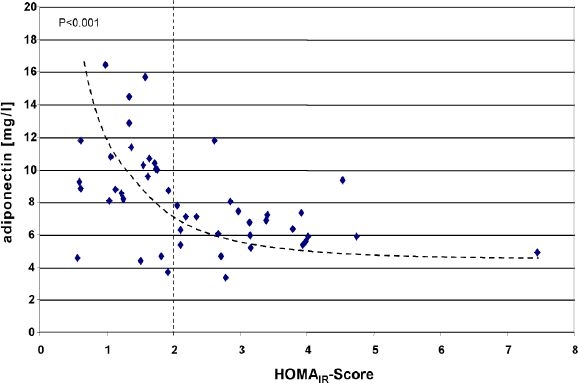
Correlation of adiponectin levels with the HOMAIR score at baseline. The vertical dotted line indicates the cutoff value of the HOMAIR score (>2) for insulin resistance in a nondiabetic population.26
Figure 2.
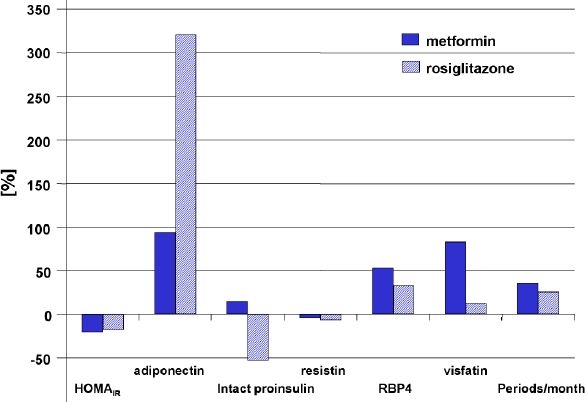
Percentage changes in the observed biomarkers from baseline to end point by treatment group.
There was an improvement of ovulation frequencies in both groups. However, this finding did not reach statistical significance because of a pronounced variability of this observation parameter.
Both drugs were well tolerated and no patient dropped out because of adverse reactions. In particular, no case of edema was reported in the rosiglitazone group.
Discussion
Polycystic ovary syndrome is associated with insulin resistance, and improvement of this condition by treatment with metformin or thiazolidinediones has the potential to normalize ovulation.7,8,27 From our trial we obtained confirmatory treatment data showing an overall positive effect of both treatment interventions. While PCOS normally shows progressive development of insulin resistance, both drugs not only blocked resistance progression, but resulted even in a slight decrease of the HOMAIR score. A study design problem in this context was that we had no placebo arm in the trial to show natural development. However, it was considered unethical to leave the patients potentially without treatment for 6 months. One aim of our study was to identify a suitable biomarker for this condition. PCOS in our patient population had not driven the ß-cell dysfunction into exhaustion of the proinsulin cleavage capacity, represented by stage III of our recently published classification.21 Therefore, no intact proinsulin elevation could be observed and proinsulin is not a good marker for insulin resistance in PCOS.
Resistin concentrations were very low at baseline and no changes could be observed at end point in any of the treatment groups. We conclude that resistin determination does not provide a valuable estimate for insulin resistance and metabolic syndrome in our patient population.
Retinol-binding protein 4 is a specific transport protein for retinol (vitamin A) in the human circulation.14,15 RBP4 is secreted by adipocytes and the liver, and serum levels have been shown to be increased in insulin-resistant states in mice, induced by selective genetic knockout of the GLUT4 glucose transporter gene.28 Graham and co-workers18 demonstrated that RBP4 levels are elevated in the serum before development of overt type 2 diabetes. The levels were associated with the components of the metabolic syndrome and could be reduced by physical exercise training in correlation to insulin resistance improvement.18
Despite different indications of improvement in insulin resistance induced by treatment with rosiglitazone or metformin for 6 months, we did not observe a decrease in RBP4 concentrations in our PCOS patients. In contrary, the levels of circulating RBP4 were in fact increased by metformin. One explanation of the different findings may be that we employed a different laboratory method than the one used by Graham et al.34 and no standardization procedure is available yet. Also the variability of RBP4 concentrations was quite high in some of their reported groups (e.g., 40–150 ng/ml in nine men with impaired glucose tolerance). In comparison to these findings, our patient population was a bit larger and appeared to be much more homogeneous in the RBP4 results. In any case, at this stage RBP4 does not really appear to us to be a useful biochemical marker for insulin resistance and metabolic syndrome in PCOS patients. Further research is required to elucidate the role of RBP4 in the regulation of glucose and lipid metabolism in this patient population.
Visfatin, a recently described new adipokine,13 is abundantly produced by adipocytes and is also considered to link obesity with diabetes and insulin resistance. It has been demonstrated in cultured cells and in animal experiments that visfatin binds to the insulin receptor and exerts insulin action, and it is assumed that it induces the intracellular signaling cascade for insulin with tyrosine phosphorylation of the insulin receptor and its substrates (IRS1 and IRS2) and activation of protein kinase B activity.13This activation seems to be distinct from that of insulin, and the mechanisms of cellular visfatin expression and secretion are still not fully understood. Contradictive data have been published about visfatin concentrations in patients with diabetes and insulin resistance. While one study reported elevated plasma levels of visfatin in patients with type 2 diabetes, which were independently associated with the waist-to-hip ratio, a clinical marker for obesity and insulin resistance,29 another report indicated a decreased visfatin plasma circulation in obese nondiabetic subjects in comparison to lean subjects.30 The authors also showed that the observed differences in plasma concentrations were due to increased expression of visfatin in the visceral but not the subcutaneous adipose tissue and that free fatty acid-induced insulin resistance did not lead to changes in the circulating plasma visfatin concentrations. They concluded that visfatin is not regulated by insulin resistance in human subjects.30 Our data support these latter findings. It needs to be pointed out that in all reports discussed here, visfatin levels were analyzed by means of the same ELISA method and should therefore be comparable. In conclusion, visfatin may also not be an easy to apply marker for insulin resistance and metabolic syndrome in PCOS patients.
The most promising biomarker in our PCOS patient population seems to be adiponectin. The adiponectin level increase in both treatment groups matches the observed clinical improvements, and the slightly better clinical outcome with rosiglitazone appears to be reflected in a more pronounced adiponectin increase. It needs to be pointed out, however, that activation of adipocyte peroxisome proliferator-activated receptor γ (PPARγ) receptors by thiazolidinediones leads to dose-dependent increases in circulating adiponectin concentrations, as shown previously.31 Adiponectin was negatively correlated to BMI in many trials.32 It was shown that the PPARγ-induced adiponectin increase was independent from the body weight changes occurring at the same time.33 These findings indicate a direct and independent stimulating effect of thiazolidinediones on adiponectin expression and secretion in adipocytes. Our findings are confirmed by Gulcelik et al.,34 who found that adiponectin and BMI were independent determinants of insulin resistance in PCOS patients. They concluded that adiponectin did not seem to be actively involved in the pathogenesis of PCOS, but that adiponectin levels were independently associated with insulin resistance in PCOS patients, suggesting that adiponectin might play a role in the complicated metabolic abnormalities of PCOS.34
In conclusion, treatment of women with PCOS with rosiglitazone and metformin led to slight improvements of ovulation and the metabolic situation. From the biomarkers tested, intact proinsulin, visfatin, and RBP4 failed to reflect these changes, resulting in partly contradictory data in the literature. In our clinical trial, adiponectin appeared to be the most promising marker to assess insulin resistance and the metabolic situation in the PCOS patients. However, larger prospective clinical trials are required to further understand the clinical value of adiponectin measurement in this patient population.
Abbreviations
- ANCOVA
analysis of covariance
- BMI
body mass index
- HOMA
homeostatic model assessment
- IR
insulin resistance
- PCOS
polycystic ovary syndrome
- PPARγ
peroxisome proliferator-activated receptor γ
- RBP4
retinol-binding protein 4
References
- 1.Barber TM, McCarthy MI, Wass JAH, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol. 2006;65(2):137–146. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- 2.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, indendent of obesity, in polycystic ovary syndrome. Diabetes. 1989 Sep;38(9):1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 3.Venkatesan AM, Dunaif A, Corbould A. Insulin resistance I polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res. 2001;56:295–308. doi: 10.1210/rp.56.1.295. [DOI] [PubMed] [Google Scholar]
- 4.Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995;80(9):2585–2593. doi: 10.1210/jcem.80.9.7673399. [DOI] [PubMed] [Google Scholar]
- 5.Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, Frank S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol. 1992 Jan;36(1):105–111. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 6.Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, O'Keefe M, Ghazzi MN PCOS/Troglitazone Study Group. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001 Apr;86(4):1626–1632. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metforma meta-analysis. Fertil Steril. 2006 Sep;86(3):658–663. doi: 10.1016/j.fertnstert.2006.02.098. [DOI] [PubMed] [Google Scholar]
- 8.Glintborg D, Hermann AP, Andersen M, Hagen C, Beck-Nielsen H, Veldhuis JD, Henriksen JE. Effect of pioglitazone on glucose metabolism and luteinizing hormone secretion in women with polycystic ovary syndrome. Fertil Steril. 2006;86(2):385–397. doi: 10.1016/j.fertnstert.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 9.Schöndorf T, Maiworm A, Emmission N, Forst T, Pfützner A. Biological background and role of adiponectin as marker for insulin resistance and cardiovascular risk. Clin Lab. 2005;51(9-10):489–494. [PubMed] [Google Scholar]
- 10.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infaction in men. JAMA. 2004 Apr 14;291(14):1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Ann NY Acad Sci. 1993 Mar 15;676:270–278. doi: 10.1111/j.1749-6632.1993.tb38740.x. [DOI] [PubMed] [Google Scholar]
- 12.Kissebah AH, Peiris AN. Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1989 Mar;5(2):83–109. doi: 10.1002/dmr.5610050202. [DOI] [PubMed] [Google Scholar]
- 13.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfata protein secreted by visceral fat that mimics the effects of insulin. Science. 2005 Jan 21;307(5708):426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 14.Blaner WS. Retinol binding protethe serum transport protein for vitamin A. Endocr. 1989 Aug;10(3):308–316. doi: 10.1210/edrv-10-3-308. [DOI] [PubMed] [Google Scholar]
- 15.Newcomer ME, Ong DE. Plasma retinol binding protestructure and function of the prototypic lipocalin. Biochim Biophys Acta. 2000 Oct 18;1482(1-2):57–64. doi: 10.1016/s0167-4838(00)00150-3. [DOI] [PubMed] [Google Scholar]
- 16.Basualdo CG, Wein EE, Basu TK. Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr. 1997 Feb;16(1):39–45. doi: 10.1080/07315724.1997.10718647. [DOI] [PubMed] [Google Scholar]
- 17.Abahusain MA, Wright J, Dickerson JW, de Vol EB. Retinol, alpha-tocopherol and carotenoids in diabetes. Eur J Clin Nutr. 1999 Aug;53(8):630–635. doi: 10.1038/sj.ejcn.1600825. [DOI] [PubMed] [Google Scholar]
- 18.Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese and diabetic subjects. N Engl J Med. 2006 Jun 15;354(24):2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 19.Polonsky KS. Retinol-binding protein 4, insulin resistance, and type 2 diabetes. N Engl J Med. 2006 Jun 15;354(24):2596–2598. doi: 10.1056/NEJMe068091. [DOI] [PubMed] [Google Scholar]
- 20.Pfützner A, Kunt T, Mondok A, Pahler S, Konrad T, Lübben G, Forst T. Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care. 2004 Mar;27(3):682–687. doi: 10.2337/diacare.27.3.682. [DOI] [PubMed] [Google Scholar]
- 21.Pfützner A, Pfützner AH, Larbig M, Forst T. Role of intact proinsulin in diagnosis and treatment of type 2 diabetes mellitus. Diab Technol Ther. 2004 Jun;6(3):405–412. doi: 10.1089/152091504774198124. [DOI] [PubMed] [Google Scholar]
- 22.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001 Jan 18;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 23.Pfützner A, Langenfeld M, Kunt T, Löbig M, Forst T. Evaluation of human resistin assays with serum from patients with type 2 diabetes and different degrees of insulin resistance. Clin Lab. 2003;49(11-12):571–576. [PubMed] [Google Scholar]
- 24.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam GR, editors. Polycystic ovary syndrome. Boston: Blackwell; 1992. pp. 377–384. [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and ß-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabetic Med. 2000 Apr;17(4):299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma ST, Nestler JE. Prevention of diabetes and cardiovascular disease in women with PCOS: treatment with insulin sensitizers. Best Pract Res Clin Endocrinol Metab. 2006 Jun;20(2):245–260. doi: 10.1016/j.beem.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005 Jul 21;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 29.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ. Elevated plasma level of visfatin/pre-B cell colony enhancement factor in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006 Jan;91(1):295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 30.Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, Serra R, Milan G, Rossato M, Federspil G, Vettor R. Reduced plasma visfatin/pre-B cell colony enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab. 2006 Aug;91(8):3165–3170. doi: 10.1210/jc.2006-0361. [DOI] [PubMed] [Google Scholar]
- 31.Pfützner A, Schöndorf T, Seidel D, Winkler K, Matthaei S, Hamann A, Forst Tl. Impact of rosiglitazone on ß-cell function, insulin resistance and adiponektin concentrations: results from a double blind oral combination study with glimepiride. Metabolism. 2006 Jan;55(1):20–25. doi: 10.1016/j.metabol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Martos-Moreno GA, Barrios V, Soriano-Guillen L, Argente J. Relationship between adiponectin levels, acylated ghrelin levels, and short-term body mass index changes in children with diabetes mellitus type 1 at diagnosis and after insulin therapy. Eur J Endocrinol. 2006 Nov;155(5):757–761. doi: 10.1530/eje.1.02273. [DOI] [PubMed] [Google Scholar]
- 33.Forst T, Lübben G, Karagiannis E, Roth W, Pfützner A. PPARγ activation increases plasma adiponectin levels. Diabetes Stoffw. Herz. 2006;15(5):17–19. [Google Scholar]
- 34.Gulcelik NE, Aral Y, Serter R, Demir Y, Culha C. Adiponectin is an independent determinant of insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2006 Sep;22(9):511–515. doi: 10.1080/09513590600917943. [DOI] [PubMed] [Google Scholar]


