Abstract
Background
Cardiovascular and metabolic risk factors are gaining attention as potential indicators for intervention in the prevention and treatment of cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM). Blood spot technology offers an efficient, convenient method to measure these risk factors in an at-risk population. Simple and convenient methods to assess cardiometabolic risk factors will allow clinicians to formulate treatment strategies for effective prevention and management of these conditions.
Method
Insulin and high sensitivity C-reactive protein (hs-CRP) were measured in dried blood spot and corresponding serum samples using conventional commercial serum kits based on a direct sandwich ELISA technique. The triglyceride assay involved enzymatic hydrolysis of triglycerides by lipase to glycerol and free fatty acids. The glycerol produced was then measured by coupled enzyme reactions. Blood spot assays were modified from previously published methods to give improved accuracy and turnaround time.
Results
Blood spot levels correlated well with serum levels of hs-CRP (r = 0.99), triglycerides (fasting, r = 0.95; nonfasting, r = 0.94), and insulin (fasting, r = 0.93; nonfasting, r = 0.97).
Conclusion
Blood spot testing for insulin, hs-CRP, and triglycerides may be helpful in the cardiometabolic screening or monitoring of patients at risk of developing CVD, T2DM, or metabolic syndrome.
Keywords: assay method, automated, blood spot, cardiometabolic panel, cardiovascular disease, CRP, C-reactive protein, dried blood spot, hs-CRP, high sensitivity CRP, insulin, metabolic syndrome, risk factors, screening, triglycerides, type 2 diabetes
Inroduction
Recent studies support emergence of the term “cardiometabolic risk,” defined by Watson1 as “the cluster of modifiable risk factors and markers that identify individuals at increased risk for cardiovascular disease (myocardial infarction, stroke, peripheral arterial disease) and type 2 diabetes.” Cardiometabolic risk is attributed to a combination of conditions associated with metabolic syndrome, cardiovascular disease (CVD), and type 2 diabetes mellitus (T2DM). Markers of metabolic syndrome, such as elevated blood pressure, abdominal adiposity, low high-density lipoprotein cholesterol, elevated triglycerides, elevated blood glucose, smoking, elevated low-density lipoprotein cholesterol, inflammatory markers such as C-reactive protein (CRP), and insulin resistance, have all been combined to define the cardiometabolic risk.2,3 Screening patients to identify these risk factors can help prevent CVD and T2DM by providing information for early intervention and treatment.1
This article presents simple and convenient methods for testing insulin, high sensitivity CRP (hs-CRP), and triglycerides in dried blood spots collected by finger stick. These methods were modified from previously developed blood spot tests.4–11
Dried blood spot technology offers several advantages over conventional serum assays: it does not require any phlebotomy or separation of blood by centrifugation, it is less invasive, and it allows for convenient shipment of samples. Drying of blood also destroys infectious viruses, such as human immunodeficiency virus, and ensures sample stability for weeks at room temperature, allowing for shipment without a cold pack or special precautions. Blood spot assays therefore allow a quick and convenient assessment of patients presenting cardiometabolic health risks. Measurement of these components may add to the predictive value of other clinical characteristics of overall cardiometabolic risk.
Materials and Methods
Ultrasensitive insulin and hs-CRP kits were purchased from DRG International, Inc. Triglyceride kits were obtained from Randox Laboratories Ltd. Bio-Rad Lypocheck Immunoassay Plus control levels 1, 2, and 3 were used for establishing controls and calibrators for the blood spot insulin. Lipid control levels 2 and 3 from Randox were used for triglycerides, and wide-range calibrators from Core Laboratory Supplies, Inc. were used for hs-CRP. The Wallac Victor2 1420 Multilabel Counter was from Perkin Elmer. Unistik 2 lancets were purchased from Fisher Scientific. Blood spot collection cards (Protein Saver 903) were purchased from Whatman. Phosphate-buffered saline (PBS) and casein buffer concentrates were purchased from Sigma.
Preparation of Reagents
Dried blood spots were extracted from the filter paper with PBS containing 0.25% Tween 20 detergent and 0.05% Proclin antimicrobial. Casein blocking buffer (1%) was prepared in PBS.
Preparation of Blood Spot Standards, Controls, and Blanks
The most concentrated standards from the insulin, hs-CRP, and triglyceride kits; Bio-Rad Controls 1, 2, and 3; and 5% casein (blank) were mixed 1:1 with washed red blood cells (RBCs), and 75-μl aliquots of each were spotted onto Whatman 903 filter paper cards. Blood spots were allowed to dry overnight and were stored in a labeled zip-lock plastic bag with desiccant packets at –20°C.
Clinical Blood Spot Sample Collection
Fasting and nonfasting healthy volunteers contributed finger stick blood spots as well as conventional venipuncture blood samples for serum. The procedures followed were in accordance with the current revision of the Helsinki Declaration and all subjects gave their informed consent. Blood spots obtained from finger sticks were air-dried at room temperature for at least 1 hour after collection and were stored at –20°C. Serum was stored frozen at –20°C.
Blood Spot Insulin Assay Procedure
Two 6.0-mm blood spot disks from each of the casein–RBC blanks, standards, controls, and subject blood spot samples were punched into deep 96-well plates using an automated blood spot puncher (Wallac MultiPuncher). The dried blood spot samples were extracted with 350 μl of extraction buffer for 90 minutes on a microtiter plate shaker at room temperature. The high insulin standard extract was serially diluted 1:2 in the casein–RBC extract from 75 to 0.6 μlU/ml. Serum and rehydrated blood spots were tested in parallel for insulin using commercial ELISA kits from DRG.
Assay plates were prewashed for 15 min with 1% casein before adding samples. Two hundred microliters of the standards, controls, blanks, and clinical samples was then added to the microtiter plates followed by 50 μl of the enzyme conjugate diluted 1:4. After incubating the plate at room temperature on a shaker for 2 hours, the wells were washed five times with the diluted wash buffer and 200 μl of substrate (tetramethylbenzidine) was added. The reaction was stopped by adding 50 μl of stop solution contained in the kit. Absorbance was read at 450 nm within 30 minutes with the Wallac Victor2 1420 Multilabel Counter.
Blood Spot hs-CRP Assay Procedure
One 6.0-mm blood spot disk from each of the blanks, standards, controls, and subject blood spot samples was punched into a 2-ml round-bottom 96-well plate and was extracted with 200 μl of extraction buffer for 2 hours on a microtiter plate shaker at room temperature. The high concentration standard (10 mg/liter) was serially diluted as described earlier to prepare the standard curve. A 20-μl aliquot of each extracted standard, blank, control, and sample was added to the 96-well assay plate followed by 100 μl of the CRP enzyme conjugate from the assay kit. The assay plate was incubated at room temperature for 45 minutes and was washed five times with distilled water, substrate solution (100 μl) was added, and the plate was incubated in the dark for 20 minutes. The reaction was stopped by adding 100 μl of stop solution, and the plate was read at 450 nm within 15 minutes.
Blood Spot Triglyceride Assay Procedure
Two 6.0-mm blood spot disks from each of the blanks, standards, controls, and subject blood spot samples were punched into deep 96-well plates as described earlier. Triglycerides (lipids) were extracted from the dried blood spot samples with 200 μl of methanol for 2 hours at 37°C.
The high standard (200 mg/dl) was serially diluted to get a standard curve. Triglycerides were measured by modifying the method of Qurashi and co-workers.4 Methanol extracts (50 μl) of clinical samples, standards, controls, or blanks were added to 250 μl of the triglyceride reagent and were incubated at 37°C for 10 minutes. The absorbance was read at 490 nm. Serum triglyceride levels were determined using the same assay kit as described by the manufacturer.
Statistical Analysis
Linear regression analysis was used to analyze data.
Results
Blood Spot Insulin: Accuracy, Precision, Recovery, and Linearity
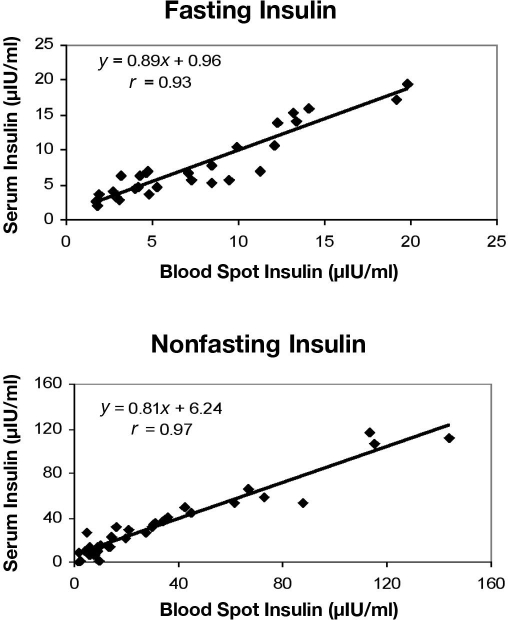
A positive correlation was found between insulin levels in blood spot and serum samples from fasting (r = 0.93) and nonfasting (r = 0.97) subjects (Figure 1). Mean fasting insulin levels were 7.69 μIU/ml (range 1.76–19.84) for serum and 7.79 μIU/ml (range 2.59–19.41) for blood spots. Mean nonfasting insulin levels were 29.9 μIU/ml (range 1.8–116.19) for serum and 31.6 μIU/ml (range 1.5–143.9) for blood spots.
Figure 1.
Correlation between serum and blood spot levels of insulin in samples from fasting and nonfasting subjects. Linear regression analysis showed a correlation coefficient of 0.93 for fasting and 0.97 for nonfasting subjects.
To check precision, six different samples of known concentrations were run in four separate sets of experiments. Each value represents the mean of two measurements. The observed interassay coefficient of variation (CV) ranged between 10.51 and 21.93%. Within-assay variability was estimated by running six replicates of each blood spot sample, which ranged between 3.25 and 11.46%. Five sample pairs with known concentrations of insulin were mixed and assayed for total recovery. The mean recovery was 98.83%. Three samples of known insulin concentrations were serially diluted and assayed for recovered quantity to test for assay linearity. The mean recovery was 108.13% (Table 1).
Table 1.
Precision and Accuracy of hs-CRP, Triglycerides, and Insulin Determinations in Dried Blood Spots
| Analyte | Interassay CV (%) | Intraassay CV (%) | Recovery (%) | Lower limit of detection |
|---|---|---|---|---|
| hs-CRP | 7.91 ± 2.39 | 7.26 ± 1.24 | 105.6 ± 8.5 | 0.114 mg/liter |
| Triglycerides | 8.93 ± 2.24 | 4.89 ± 1.41 | 110.02 ± 1.28 | 15.4 mg/dl |
| Insulin | 14.39 ± 3.61 | 7.57 ± 2.74 | 98.83 ± 14.3 | 1.5 µIU/ml |
Blood Spot hs-CRP: Accuracy, Precision, Recovery, and Linearity
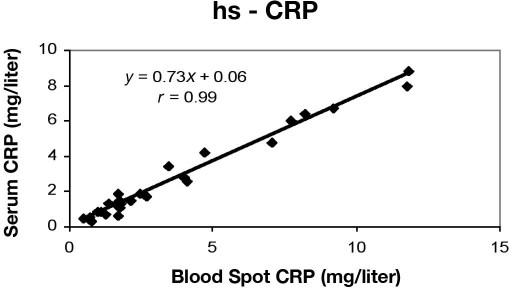
Blood spot hs-CRP levels were plotted against corresponding serum hs-CRP values from the same subjects. The correlation coefficient was 0.99, the slope was 0.7317, and the intercept was 0.031 (Figure 2). Mean hs-CRP levels were 3.31 mg/liter (range 0.30–8.80) for serum and 4.07 mg/liter (range 0.42–11.81) for blood spots. The minimum detectable concentration, obtained by determining two standard deviations from the mean of a zero standard, was estimated to be 0.114 mg/liter (Table 1). Additionally, the functional sensitivity was determined to be 0.114 mg/liter (as determined from the lowest concentration standard, giving an interassay CV <20%). The upper limit of the hs-CRP ELISA was found to be 15 mg/liter.
Figure 2.
Correlation between blood spot and serum hs-CRP concentrations in samples from volunteers.
Within-run precision was determined by replicate determinations of five different blood spot samples. Within-assay and between-assay CVs are shown in Table 1. Various blood spot samples of known hs-CRP levels were combined and assayed in duplicate. The mean recovery was 105.6% (Table 1). Three blood spot samples were serially diluted to determine linearity, which resulted in a mean recovery of 103.99%.
Blood Spot Triglycerides: Accuracy, Precision, Recovery, and Linearity
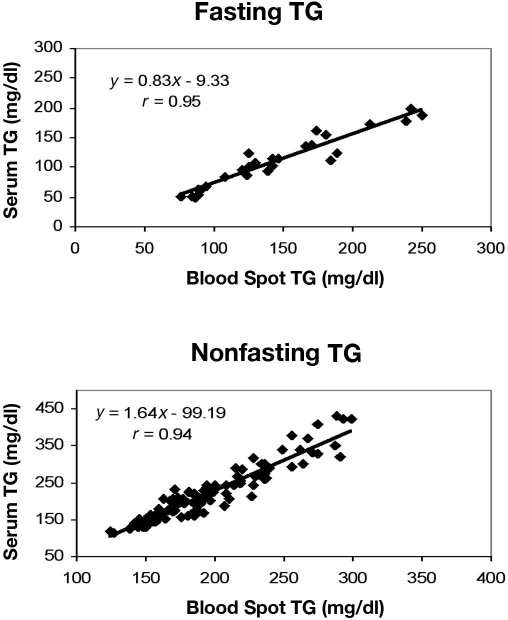
A positive correlation was observed when serum and blood spot triglyceride concentrations were compared in both fasting (r = 0.95) and nonfasting (r = 0.94) subjects (Figure 3). The minimum detectable concentration was 15.4 mg/dl. This was used as a cutoff based on the total recovery from linearity data. The percentage recovery at 15.4 mg/dl was 94%, and at 11.06 mg/dl the overall recovery was reduced to 87% (data not shown), which compromises the accuracy of data as a result.
Figure 3.
Correlation between serum and blood spot triglyceride concentrations in samples from fasting and nonfasting subjects.
Mean fasting triglyceride levels were 110.66 mg/dl (range 49.23–199.09) for serum and 145.08 mg/dl (range 76.15–249.80) for blood spots. Mean nonfasting triglyceride levels were 219.46 mg/dl (range 113.65–431.23) for serum and 194.82 mg/dl (range 124.15–298.62) for blood spots. Eight different samples of known concentrations were run in six separate sets of experiments that resulted in an interassay CV of 5.3 to 12.66%. Within-assay variability was estimated by running six replicates of each blood spot sample, which ranged between 2.3 and 6.23%. Five sample pairs with known concentrations of triglycerides were mixed and assayed for total recovery. The mean recovery was 110.02%. Linearity studies showed a mean recovery of 100.54%.
Discussion
This study presented simple and convenient methods for measuring insulin, hs-CRP, and triglycerides in dried blood spots derived from a finger stick. These methods allow for efficient and patient-friendly screening for cardiometabolic risk factors. Patients and clinicians often prefer finger stick blood spot over serum collection methods because of ease of sample collection and transport, lower overall cost, smaller sample requirements, minimal invasiveness, and stability of the test analyte in dried blood spots Blood spot testing also allows researchers to expand the scope of endocrinological studies to previously unreachable subjects and study sites. The design of the assay and the nature of the samples also make this method suitable for screening programs that employ the use of self-sampling. A few other studies have reported laboratory protocols for insulin,6,8,11 hs-CRP,5,7,9,10 and triglycerides4 in dried blood spots. The protocols in our laboratory have shown improved precision, accuracy, and ease of high-throughput screening of these biomarkers when extracted from dried blood spots. However, an increased number of samples must be tested in order to completely evaluate and validate the method to follow the commonly accepted Clinical and Laboratory Standards Institute guidelines. Work is under progress in our laboratory to study more patient samples as they become available for further precision and correlation studies.
We compared insulin concentrations in blood spot and serum samples from fasting and nonfasting patients and observed a linear relationship with a high correlation. Dried blood spot insulin concentrations have been reported to be stable for more than 2 months at –20°C,8 and we have found that insulin in dried blood spot clinical samples did not degrade after four freeze–thaw cycles. The intra- and interassay CVs (<10%) were also improved over those (14–25%) reported by Dowlati and colleagues11 using a radioimmunoassay method after the extraction of blood spots.
CRP has been known to be associated with infection and inflammation, but reports have shown that elevated levels of CRP as detected by sensitive assays, referred to as hs-CRP, could predict CVD in patients with type 1 diabetes,12 metabolic syndrome,12,13 or the onset of type 2 diabetes.14 Measurement of hs-CRP has been recommended by the American Heart Association and the Centers for Disease Control, but more research in a larger population is required before the clinical utility in epidemiological- and community-based studies is established.15 McDade and colleagues developed a highly sensitive enzyme immunoassay for CRP in dried blood spots5 and have underscored the difficulties involved in measuring hs-CRP in population-based studies, especially in terms of blood collection through venipuncture.
The stability of CRP in dried blood spots is not an issue in sample storage and transport, as it has been shown to be stable for 14 days at room temperature; up to five freeze–thaw cycles do not affect the recovery of CRP in samples.5 Our fast and improved assay for hs-CRP in blood spots may add significant value in assessing the overall cardiometabolic risk when combined with measurements of insulin and triglycerides.
High serum triglycerides have been established as a risk factor in cardiovascular and coronary artery disease.16,17 The National Cholesterol Education Program has recommended the need for accurate and precise determination of triglyceride levels in patients at risk for coronary artery disease. Several methods for the quantification of triglycerides in serum or whole blood have been reported in the literature, but there is a need for the development of methods that can be used in nonclinical settings, especially for individuals living in remote areas without access to a laboratory or a phlebotomist. Quraishi et al.4 have reported a triglyceride determination method in dried blood spots and have demonstrated that levels were stable for 30 days at 16–28°C and for 90 days at 4°C. Therefore, blood dried on a filter paper is a useful medium for the quantification of triglyceride levels, especially in cases where sample collection, storage, and transport are major issues. We have modified the method of Quraishi and colleagues4 to make it more cost effective for large-scale epidemiological and population-based research studies. The assay resulted in high precision and accuracy, as evident from inter- and intraassay CVs and complete recovery of triglycerides from blood dried on filter paper. Our method utilized a significantly reduced amount of sample and reagents, allowing the use of microtiter plates instead of test tubes, and the potential for automation.
Conclusion
This study assessed the application/feasibility of dried blood spot technology to measure important cardiometabolic risk markers. Dried blood spot collection has advantages compared to conventional blood draws, such as minimal invasiveness, low sample volume, convenience of repeated measurements, and ease of sample storage and transport. The positive correlation between blood spot and serum assays presents the possibility of using the dried blood spot sample collection method as an alternative when conventional blood draw facilities are not available or accessible. However, precision and accuracy of the assays presented in this article can be improved further by testing an increased number of patient samples, which is being done in our laboratory.
The stability, efficient recovery, and excellent correlation with conventional serum tests make the dried blood spot assay a reliable and convenient tool for screening cardiometabolic risk factors. A blood spot panel consisting of fasting insulin, hs-CRP, and triglycerides, along with blood pressure and cholesterol level assessments, is recommended for a population at risk and also for those with diagnosed CVD or T2DM.
Acknowledgments
We are thankful to Dr. Pamela Jeanne and Dr. Alison McAllister for their critical comments during the course of the study and to Yun Wu and Danielle Moore for their technical help. Margaret Groves, Holly Tsur, and Sherri Hood Zava have been extremely helpful in proofreading and editing of the manuscript. We are also grateful to all the volunteers at ZRT Laboratory who donated blood for this study.
Abbreviations
- CRP
C-reactive protein
- CV
coefficient of variation
- CVD
cardiovascular disease
- hs-CRP
high sensitivity CRP
- PBS
phosphate-buffered saline
- RBC
red blood cell
- T2DM
type 2 diabetes mellitus
References
- 1.Watson K. Managing cardiometabolic risk: an evolving approach to patient care. Crit Pathw Cardiol. 2007;6(1):5–14. doi: 10.1097/01.hpc.0000255063.43838.6d. [DOI] [PubMed] [Google Scholar]
- 2.Pescatello LS, Vanheest JL. Physical activity mediates a healthier body weight in the presence of obesity. Br J Sports Med. 2000;34(2):86–93. doi: 10.1136/bjsm.34.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 4.Quraishi R, Lakshmy R, Prabhakaran D, Mukhopadhyay AK, Jailkhani B. Use of filter paper stored dried blood for measurement of triglycerides. Lipids Health Dis. 2006;5:20. doi: 10.1186/1476-511X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDade TW, Burhop J, Dohnal J. High sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50(3):652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- 6.Shields BM, Knight B, Shakespeare L, Babrah J, Powell RJ, Clark PM, Hattersley AT. Determinants of insulin concentrations in healthy 1-week-old babies in the community: applications of a bloodspot assay. Early Hum Dev. 2006;82(2):143–148. doi: 10.1016/j.earlhumdev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Cordon SM, Elborn JS, Hiller EJ, Shale DJ. C-reactive protein measured in dried blood spots from patients with cystic fibrosis. J Immunol Methods. 1991;143(1):69–72. doi: 10.1016/0022-1759(91)90273-i. [DOI] [PubMed] [Google Scholar]
- 8.Butter NL, Hattersley AT, Clark PM. Development of a bloodspot assay for insulin. Clin Chim Acta. 2001;310(2):141–150. doi: 10.1016/s0009-8981(01)00545-9. [DOI] [PubMed] [Google Scholar]
- 9.Beesley R, al Serouri A, Filteau SM. Measurement of C-reactive protein in dried blood sports on filter paper. Trans R Soc Trop Med Hyg. 2000;94(3):348–349. doi: 10.1016/s0035-9203(00)90350-x. [DOI] [PubMed] [Google Scholar]
- 10.Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51(10):1854–1866. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 11.Dowlati B, Dunhardt PA, Smith MM, Shaheb S, Stuart CA. Quantification of insulin in dried blood spots. J Lab Clin Med. 1998;131(4):370–374. doi: 10.1016/s0022-2143(98)90188-3. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome and the risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 13.Marques-Vidal P, Mazoyer E, Bongard V, Gourdy P, Ruidavets JB, Drouet L, Ferrieres J. Prevalence of insulin resistance syndrome in southwestern France and its relationship with inflammatory and hemostatic markers. Diabetes Care. 2002;25(8):1371–1377. doi: 10.2337/diacare.25.8.1371. [DOI] [PubMed] [Google Scholar]
- 14.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6 and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 15.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 16.Hokanson JE, Austin MA. Plasma triglyceride is a risk factor for cardiovascular disease independent of HDL cholesterol level A meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–219. [PubMed] [Google Scholar]
- 17.Dennis LS. Triglyceride as a risk factor for coronary artery disease. Am J Cardio. 1998;82:49U–59U. doi: 10.1016/s0002-9149(98)00770-x. [DOI] [PubMed] [Google Scholar]