Abstract
Insulin-like growth factor (IGF)-I is a ubiquitous peptide hormone involved in a host of critical physiological processes (e.g., protein synthesis and glucose homeostasis) and has been suggested to be a biomarker reflecting health and metabolic status. In most cases (muscle, bone, tendon, body composition, and cognitive function), elevated IGF-I concentrations are considered beneficial; however, cancer remains a notable exception. While the fact that both increased and decreased IGF-I can be considered reflective of favorable and beneficial health outcomes may appear as a paradox, it is important to emphasize that, in both cases, measured IGF-I concentrations do offer important insight into physiological processes. The effects of military operational field training on the circulating IGF-I system are discussed within the context of novel measurement technologies that (1) are field expedient and (2) provide more meaningful information. Prospective experimental approaches involving physical activity that sample and measure IGF-I in the body's various biocompartments will provide greater insight into the complex role that IGF-I possesses. Minimally invasive technologies that are field expedient, cost-effective, and allow for continuous and real-time feedback will have the greatest likelihood of being adapted and used in military environments.
Keywords: filter paper, interstitial fluid, microdialysis, transdermal body fluid
Introduction
Insulin-like growth factor (IGF)-I is a ubiquitous growth and metabolic factor that lends itself to being sampled and monitored in a number of different biological fluids. The liver is the primary source of circulating IGF-I, but local release from cells and tissues in an autocrine/paracrine fashion also contributes to the whole-body physiological effects that IGF-I exerts.1–4 The fact that IGF-I resides in a number of different biocompartments [e.g., blood, transdermal body fluid (TDF), interstitial fluid, muscle, bone, and nerve tissue] has warranted the investigation of different sampling methodologies in order to glean the most biologically relevant information.
Enhanced monitoring and measurement technologies for IGF-I should provide for major scientific breakthroughs, as IGF-I has truly emerged as an important biomarker with many different applications.5,6 For example, elevated IGF-I concentrations are associated with many positive health outcomes (e.g., aerobic fitness, bone mineral density, muscle hypertrophy, neurocognition, and longevity).2,5,7–11 Conversely, elevated IGF-I concentrations have also been associated with increased risk for several types of cancer and decreased longevity.12–14 Alterations in the IGF-I axis have also been associated with a number of pathological conditions such as cancer, obesity, and type II diabetes.15 While the fact that both increased and decreased IGF-I can be considered reflective of both favorable and beneficial health outcomes may appear as a paradox, it is important to emphasize that, in both cases, measured IGF-I concentrations do offer important insight into physiological processes.6
With regard to physically active populations,16,17 such as those found in the military, IGF-I has been reported to be a biomarker reflecting physiological status.18–21 The Military Performance Division at the United States Army Research Institute of Environmental Medicine in Natick, MA, has an active line of research that has studied the utility of IGF-I as a relevant biomarker as well as novel and expedient monitoring technologies. These technologies have included filter paper blots, TDF harvesting via laser microporation and vacuum pressure, microdialysis sampling of interstitial fluid, muscle biopsy, and in vitro cell-culture-based bioassays. The primary objective of this line of research is to provide insight into how IGF-I should be both sampled and measured in order to best provide meaningful information for soldier physiological and metabolic status. This commentary highlights the importance of IGF-I as a biomarker and describes the various experimental approaches that are being pursued.
Insulin-Like Growth Factor-I Physiology and Regulatory Complexity
Insulin-like growth factor-I has many metabolic and anabolic effects that are primarily associated with cell metabolism and growth.7 Insulin-like growth factor-I is known to promote amino acid uptake, enhance protein synthesis, and attenuate protein degradation. IGF-I shares structural homology and downstream signaling pathways with insulin and can facilitate glucose and free fatty acid uptake. Additionally, IGF-I plays a role in stimulating cell growth and differentiation.1,2 Insulin-like growth factor-I itself is a 7.6 kDa polypeptide consisting of 70 amino acids with three intrachain disulfide bonds. Only a small amount (<2%) of IGF-I, however, circulates in free form. Most circulates in either a binary (∼20–25%) or ternary (∼75%) complex. When circulating in the binary form, IGF-I is complexed with one of six binding proteins (BPs-1–6), ranging in mass from 22.8 to 31.4 kDa, although posttranslational modifications can result in masses ∼45 kDa. The ternary complex consists of IGF-I, IGF BP-3, and an 80–86 kDa protein called the acid labile subunit (ALS). An IGF-I-specific protease is responsible for breaking the bonds holding the ternary complex together and for making the IGF-I available for receptor binding.3,4 The IGF-I complexes are thought to regulate the availability of IGF-I to target tissues, as only the free and binary complexes can pass from the vascular compartment into the interstitial space. The different forms of BP are also thought to play a role in transporting the IGF-I to the target tissue (refer to Figure 1). Insulin-like growth factor-I signal transduction involves binding to the extracellular domain of a transmembrane receptor, activation of a receptor kinase leading to autophosphorylation, and tyrosine phosphorylation of multiple substrates, including the insulin receptor substrate and Src homology 2 domain-containing proteins. Through a cascade series of bifurcating intracellular events, IGF-I is responsible for a number of pleiotropic somatotrophic influences.1,2,4 The regulatory complexity of IGF-I underscores the importance of being able to measure and monitor IGF-I using the most relevant, reliable, and valid methods currently available.
Figure 1.
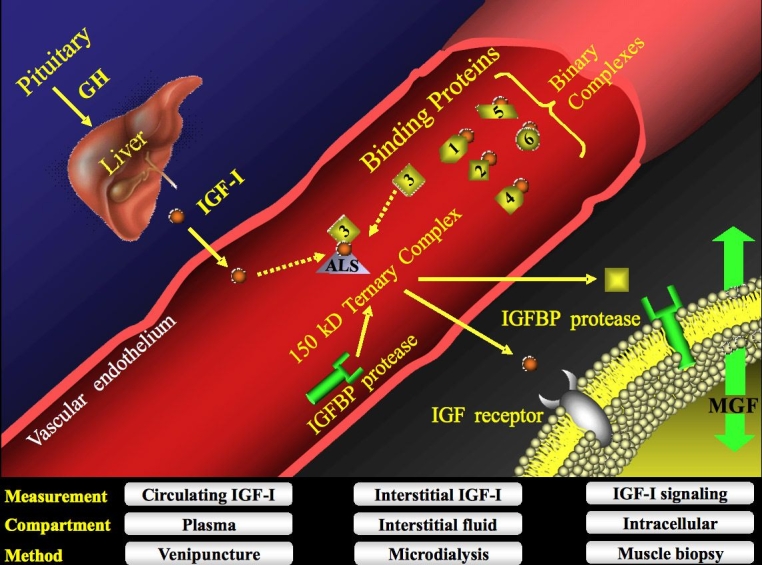
Schematic illustrating the regulatory complexity of the IGF-I system. Insulin-like growth factor-I bioavailability is governed by a family of six different BPs that serve to either inhibit or potentiate its physiological actions. Insulin-like growth factor-I is ubiquitous and can reside in a number of different biocompartments (e.g., blood, interstitial fluid, and muscle tissue), which can be sampled by various methods (e.g., venipuncture, microdialysis, and muscle biopsy). Most IGF-I is sequestered in a ternary complex (∼75–90%) comprised mainly of IGF-I, IGF BP-3, and ALS, although IGF BP-5 has also been demonstrated to form a ternary complex. GH, growth hormone; MGF, mechano growth factor.
Military Relevance of Monitoring Insulin-Like Growth Factor-I
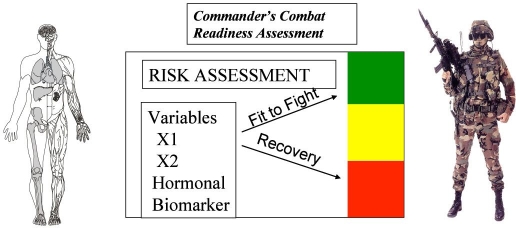
Soldiers are exposed to challenging conditions under austere environmental conditions, and the resultant physiological strain produced by these operational stressors can have deleterious effects on muscle mass, bone health, endocrine and metabolic function, as well as physical and mental performance.18,22–25 Hormones serve as the body's chemical messengers that rapidly respond to homeostatic challenges and can serve as biomarkers or “early warning signals” of physiological maladaptations. We propose that IGF-I is one candidate hormonal biomarker that, because of its influences on the human body's biological matrix, could potentially be used as a tool for military commanders and military advisors in term of mitigating injury-risk potential (i.e., composite risk management) (refer to Figure 2).
Figure 2.
Conceptual construct in which a biomarker (e.g., IGF-I) can be used to provide prognostic information with regard to the physiological status of soldiers. Optimal resolution of such a predictive model will likely be multifactorial and will include demographic, psychological, physical, and biochemistry information. Minimally invasive technologies that are field expedient, cost-effective, and allow for continuous and real-time feedback will have the greatest likelihood of being adapted and used in military environments.
The utility of IGF-I as a metabolic biomarker with military relevance was first demonstrated by Friedl and colleagues18,23 in U.S. Army ranger training. The U.S. Army ranger training course is 62 days in length and is designed to teach and evaluate individual leadership and small-unit tactics under physically and mentally challenging conditions. The course is characterized by near-continuous physical activity, energy restriction, and sleep deprivation. Friedl and colleagues18 demonstrated that average energy expenditures were ∼4000 kcal/day, resulting in average energy deficits of ∼1000 kcal/day over the entire course. At the end of the course, the participants had lost 13–16% of their initial body mass, ∼65% of their fat mass, and 7% of their initial lean body mass. Measured every 2 weeks during the ranger course, IGF-I progressively declined through the first 6 weeks, with no further reduction over the final 2 weeks of the course. At the end of the course, IGF-I values had fallen 62% [198 ± 54 ng ml-1(pre) versus 75 ± 25 ng ml-1 (post)]. The potential of IGF-I as a discriminating variable for assessing nutritional and/or metabolic stress was the separate observation that the soldiers who had the greatest decline in IGF-I were also the soldiers who lost the most weight (r = − 0.38, P < .01).
Shorter term (3–8 days) of military operational stress can also significantly perturb the IGF-I system. It has been reported that total (−24%) and free (−42%) IGF-I and IGF BP-1 (+256%) and IGF BP-3 (−6%) are altered after only 3 days of military sustained operations involving energy expenditure of ∼4500 kcal/day with intake limited to ∼1600 kcal/day.19 The striking rise in IGF BP-1 was thought to serve as a protective mechanism by neutralizing any potential insulin-like hypoglycemic activity of IGF-I and by serving to mobilize energy from fat and protein. This finding also demonstrated that IGF BPs can also reflect the magnitude and severity of metabolically challenging situations. It is important to note that the observed alterations of the IGF-I system in military operational stress paradigms are the exact opposite of what is observed in cross-sectional studies for type II diabetes patients (i.e., increased free IGF-I and IGF BP-3 and reduced IGF BP-1). It is likely that, for both situations, changes in IGF-I represent either regulatory or compensatory mechanisms relating to insulin sensitivity and resistance.15
Further, we have also demonstrated the utility of circulating IGF-I as a biomarker for assessing body composition changes in men during periods of high physical activity superimposed upon energy and sleep restriction.8 In a study involving 35 Marines who underwent 8 days of military operational stress, IGF-I was reported to be superior when compared to other conventional nutritional biomarkers (e.g., transferrin, ferritin, and retinol BP) in directionally tracking the resultant energy deficit. Free IGF-I was the only biomarker assessed that was significantly, albeit modestly, correlated with losses in fat-free mass (r = 0.43, r2 = 0.18). Also, receiver operator characteristic curve analysis revealed that a molar volume ratio of IGF-I to the ALS with a baseline value of <1.67 had an area under the curve of 0.745 and was a significant discriminator for those Marines losing >5% of their body mass. In other words, Marines with a baseline IGF-I/ALS molar volume ratio of <1.67 were 3.25 times more likely to lose 5% or more of their body mass than those with large ratios, while those Marines possessing a value of >1.67 were only approximately one-third as likely to lose >5% of their body mass.
Preliminary data from our laboratory also demonstrate that the relationship between IGF-I and body composition is a repeatable finding, as increases in fat-free mass are reflected by increased IGF-I concentrations in female soldiers during 9 weeks of basic combat training. Thus the IGF-I system does appear to be an important adjunct in the overall assessment of stress adaptation in military paradigms involving energy deficits and does offer prognostic information for body composition changes.8,9
Measurement Methodologies
Our laboratory's first attempt at exploring novel methods to sample and measure IGF-I in physically active military populations was to determine whether extraction of IGF-I from a dried blood spot from filter paper could reliably track the known decline in IGF-I during an 8-day energy deficit.20,26 Measuring blood analytes in field environments presents unique logistical challenges in terms of biohazards to research volunteers and technicians, separation and refrigeration, and transport and storage of samples. Minimally invasive methods such as finger sticks and the collection of small volumes of blood onto filter paper offer more feasible alternatives for research conducted in field environments. Results from this study demonstrated that measurement of IGF-I measured in serum and IGF-I measured from blood spots were highly correlated (r = 0.92) and that the filter paper blood spot method for IGF-I detected reductions accompanying an energy deficit. It was concluded that the filter paper blood spot method may be of potential value in characterizing the IGF-I response when conventional blood sampling methods are not feasible. Collection of blood spots from filter paper is relatively inexpensive, requiring only a lancet and filter paper, with the main cost coming from the laboratory assay costs for measuring IGF-I.
While IGF-I is typically measured in the blood, interstitial fluid remains a relatively unexplored biocompartment in which IGF-I also resides and may be more biologically relevant due to its proximity to tissues and cells. There are several technologies currently available in which TDF (i.e., fluid contained in the stratum corneum layer of the skin) can be sampled and obtained. We have collaborated with SpectRx, Inc., who has developed a patented and proprietary method for obtaining TDF that uses minimally invasive vacuum pressure in combination with a laser microporation process (creating micropores <100 μM in diameter) in the stratum corneum. Transdermal body fluid collected in this manner can be collected in a small reservoir and later measured for determination of selected analytes. This technology was originally developed for continuous monitoring of glucose. Our study results indicated that IGF-I measured in TDF was ∼76% less than IGF-I measured in serum and that there was only a low to moderate association of IGF-I in these two biocompartments, possibly suggesting an uncoupled, rather than a linked, regulation of IGF-I among the body's biocompartments.27 Current efforts in our laboratory include sampling IGF-I in blood, TDF, interstitial fluid, and muscle during exercise training using venipuncture, laser poration, microdialysis, and muscle biopsy, respectively, to more completely establish the relative relationship among the body's biocompartments for IGF-I. Microdialysis is a technique that can sample interstitial fluid and may allow for major advantages in our current knowledge of local growth factors.28 For example, recent cutting-edge work by Hansen and colleagues29,30 has provided mechanistic insight implicating a role of IGF-I as a potential etiologic mechanism, partially explaining greater injury rates in women versus men for collagen-rich tissues such as bone, ligament, muscle, and tendon. Additionally, the recent development of a cell-based in vitro bioassay for the measurement of IGF-I should provide more relevant information than conventional immunoassays.31
Future Directions
Continued scientific efforts should focus on further elucidating the link between alterations in the biological matrix (e.g., muscle, bone, adipose, immune, and neural cells) and ensuing influences on soldier physical performance as well as valid, reliable, expedient technologies to sample and measure biomarkers that mediate these responses. Because of its ubiquitous nature and pleiotropic role in a number of physiological functions, IGF-I does appear to be an ideal candidate biomarker that is highly relevant in military operational stress environments. While the fact that both increased and decreased IGF-I can be considered reflective of both favorable and beneficial health outcomes may appear as a paradox, it is important to emphasize that, in both cases, measured IGF-I concentrations do offer important insight into physiological processes. Prospective experimental approaches involving physical activity that can sample and measure IGF-I in the body's various biocompartments will provide greater insight into the complex role that IGF-I possesses.6 Minimally invasive technologies that are field expedient, cost-effective and allow for continuous and real-time feedback will have the greatest likelihood of being adapted and used in military environments.
Acknowledgements
Joe Alemany, Kevin Rarick, Alex Tuckow, Joe Pierce, and Jeff Staab are greatly acknowledged for completing the laboratory analytical work and COL Karl Friedl, Dr. Scott Montain, Dr. Edward Zambraski and Dr. Andrew Young are greatly acknowledged in their roles as collaborators and mentors.
Abbreviations
- ALS
acid labile subunit
- BP
binding protein
- IGF
insulin-like growth factor
- TDF
transdermal body fluid
References
- 1.Adams GR. Role of insulin-like growth factor-I in the regulation of skeletal muscle adaptation to increased loading. Exerc Sport Sci Rev. 1998;26:31–60. [PubMed] [Google Scholar]
- 2.Adams GR. Invited review: autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93(3):1159–1167. doi: 10.1152/japplphysiol.01264.2001. [DOI] [PubMed] [Google Scholar]
- 3.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 4.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endoc Rev. 1997;18(6):801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 5.Friedl KE. Insulin-like growth factor-I: a metabolic marker representing quality of life. Diabetes Technol Ther. 2003;5(3):463–465. doi: 10.1089/152091503765691983. [DOI] [PubMed] [Google Scholar]
- 6.Nindl BC, Pierce JR. Med Sci Sports Exerc. Insulin-like growth factor-I as a biomarker of health, fitness and training status. (In Review) [DOI] [PubMed] [Google Scholar]
- 7.Brugts MP, van den Beld AW, Hofland LJ, van der Wansem K, van Koetsveld PM, Frystyk J, Lamberts SW, Janssen JA. Low circulating insulin-like growth factor I bioactivity in elderly men is associated with increased mortality. J Clin Endocrinol Metab. 2008;93(7):2515–2522. doi: 10.1210/jc.2007-1633. [DOI] [PubMed] [Google Scholar]
- 8.Nindl BC, Alemany JA, Kellogg MD, Rood J, Allison SA, Young AJ, Montain SJ. Utility of circulating IGF-I as a biomarker for assessing body composition changes in men during periods of high physical activity superimposed upon energy and sleep restriction. J Appl Physiol. 2007;103(1):340–346. doi: 10.1152/japplphysiol.01321.2006. [DOI] [PubMed] [Google Scholar]
- 9.Nindl BC, Scoville CR, Sheehan KM, Leone CD, Mello RP. Gender differences in regional body composition and somatotrophic influences of IGF-I and leptin. J Appl Physiol. 2002;92(4):1611–1618. doi: 10.1152/japplphysiol.00892.2001. [DOI] [PubMed] [Google Scholar]
- 10.ALSPAC Study Team. Ong K, Kratzsch J, Kiess W, Dunger D. Circulating IGF-I levels in childhood are related to both current body composition and early postnatal growth rate. J Clin Endocrinol Metab. 2002;87(3):1041–1044. doi: 10.1210/jcem.87.3.8342. [DOI] [PubMed] [Google Scholar]
- 11.Poehlman ET, Copeland KC. Influence of physical activity on insulin-like growth factor-I in healthy younger and older men. J Clin Endocrinol Metab. 1990;71(6):1468–1473. doi: 10.1210/jcem-71-6-1468. [DOI] [PubMed] [Google Scholar]
- 12.Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LØ. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol. 2008;160(1):25–31. doi: 10.1530/EJE-08-0452. [DOI] [PubMed] [Google Scholar]
- 13.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 14.Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am J Pathology. 2002;160(6):2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajpathak SN, Gunter MJ, Wylie-Rosett J, Ho GY, Kaplan RC, Muzumdar R, Rohan TE, Strickler HD. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab Res Rev. 2009;25(1):3–12. doi: 10.1002/dmrr.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rarick KR, Pikosky MA, Grediagin A, Smith TJ, Glickman EL, Alemany JA, Staab JS, Young AJ, Nindl BC. Energy flux, more so than energy balance, protein intake, or fitness level, influences insulin-like growth factor-I system responses during 7 days of increased physical activity. J Appl Physiol. 2007;103(5):1613–1621. doi: 10.1152/japplphysiol.00179.2007. [DOI] [PubMed] [Google Scholar]
- 17.Rosendal L, Langberg H, Flyvbjerg A, Frystyk J, Ørskov H, Kjaer M. Physical capacity influences the response of insulin-like growth factor and its binding proteins to training. J Appl Physiol. 2002;93(5):1669–1675. doi: 10.1152/japplphysiol.00145.2002. [DOI] [PubMed] [Google Scholar]
- 18.Friedl KE, Moore RJ, Hoyt RW, Marchitelli LJ, Martinez-Lopez LE, Askew EW. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J Appl Physiol. 2000;88(5):1820–1830. doi: 10.1152/jappl.2000.88.5.1820. [DOI] [PubMed] [Google Scholar]
- 19.Nindl BC, Castellani JW, Young AJ, Patton JF, Khosravi MJ, Diamandi A, Montain SJ. Differential responses of IGF-I molecular complexes to military operational field training. J Appl Physiol. 2003;95(3):1083–1089. doi: 10.1152/japplphysiol.01148.2002. [DOI] [PubMed] [Google Scholar]
- 20.Nindl BC, Kellogg MD, Khosravi MJ, Diamandi A, Alemany JA, Pietila DM, Young AJ, Montain SJ. Measurement of insulin-like growth factor-I during military operational stress via a filter paper blood spot assay. Diabetes Technol Ther. 2003;5(3):455–461. doi: 10.1089/152091503765691974. [DOI] [PubMed] [Google Scholar]
- 21.Nindl BC, Kraemer WJ, Marx JO, Arciero PJ, Dohi K, Kellogg MD, Loomis GA. Overnight responses of the circulating IGF-I system after acute, heavy-resistance exercise. J Appl Physiol. 2001;90(4):1319–1326. doi: 10.1152/jappl.2001.90.4.1319. [DOI] [PubMed] [Google Scholar]
- 22.Alemany JA, Nindl BC, Kellogg MD, Tharion WJ, Young AJ, Montain SJ. Effects of dietary protein content on IGF-I, testosterone, and body composition during 8 days of severe energy deficit and arduous physical activity. J Appl Physiol. 2008;105(1):58–64. doi: 10.1152/japplphysiol.00005.2008. [DOI] [PubMed] [Google Scholar]
- 23.Friedl KE, Moore RJ, Martinez-Lopez LE, Vogel JA, Askew EW, Marchitelli LJ, Hoyt RW, Gordon CC. Lower limit of body fat in healthy active men. J Appl Physiol. 1994;77(2):933–940. doi: 10.1152/jappl.1994.77.2.933. [DOI] [PubMed] [Google Scholar]
- 24.Nindl BC, Friedl KE, Frykman PN, Marchitelli LJ, Shippee RL, Patton JF. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med. 1997;18(5):317–324. doi: 10.1055/s-2007-972640. [DOI] [PubMed] [Google Scholar]
- 25.Nindl BC, Leone CD, Tharion WJ, Johnson RF, Castellani JW, Patton JF, Montain SJ. Physical performance responses during 72 h of military operational stress. Med Sci Sports Exerc. 2002;34(11):1814–1822. doi: 10.1097/00005768-200211000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Diamandi A, Khosravi MJ, Mistry J, Martinez V, Guevara-Aguirre J. Filter paper blood spot assay of human insulin-like growth factor I (IGF-I) and IGF-binding protein-3 and preliminary application in the evaluation of growth hormone status. J Clin Endocrinol Metab. 1998;83(7):2296–2301. doi: 10.1210/jcem.83.7.4923. [DOI] [PubMed] [Google Scholar]
- 27.Nindl BC, Tuckow AP, Alemany JA, Harman EA, Rarick KR, Staab JS, Faupel ML, Khosravi MJ. Minimally invasive sampling of transdermal body fluid for the purpose of measuring insulin-like growth factor-I during exercise training. Diabetes Technol Ther. 2006;8(2):244–252. doi: 10.1089/dia.2006.8.244. [DOI] [PubMed] [Google Scholar]
- 28.Berg U, Gustafsson T, Sundberg CJ, Kaijser L, Carlsson-Skwirut C, Bang P. Interstitial IGF-I in exercising skeletal muscle in women. Eur J Endocrinol. 2007;157(4):427–435. doi: 10.1530/EJE-07-0141. [DOI] [PubMed] [Google Scholar]
- 29.Hansen M, Koskinen SO, Petersen SG, Doessing S, Frystyk J, Flyvbjerg A, Westh E, Magnusson SP, Kjaer M, Langberg H. Ethinyl oestradiol administration in women suppresses synthesis of collagen in tendon in response to exercise. J Physiol. 2008;586(12):3005–3016. doi: 10.1113/jphysiol.2007.147348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen M, Miller BF, Holm L, Doessing S, Petersen SG, Skovgaard D, Frystyk J, Flyvbjerg A, Koskinen S, Pingel J, Kjaer M, Langberg H. J Appl Physiol. 2008. Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Chen JW, Ledet T, Orskov H, Jessen N, Lund S, Whittaker J, De Meyts P, Larsen MB, Christiansen JS, Frystyk J. A highly sensitive and specific assay for determination of IGF-I bioactivity in human serum. Am J Physiol Endocrinol Metab. 2003;284(6):E1149–E1155. doi: 10.1152/ajpendo.00410.2002. [DOI] [PubMed] [Google Scholar]