Introduction
Cure of type 1 diabetes mellitus (T1DM) will require one or more interventions, which both prevent the autoimmune destruction of β cells and restore most of the near-total loss of β-cell mass and function that occurs by the time the disease is diagnosed. An intervention that only controls islet autoimmunity can still provide benefit, but only if it is applied very soon after the onset of T1DM. Several therapies aimed at controlling islet autoimmunity are under development. They appear to induce tolerance by shifting the balance of the activated T lymphocytes from attack mode to a more tolerant state. This shift can be induced by presenting specific islet autoantigens to T lymphocytes. An example of this immunomodulatory approach is glutamic acid decarboxylase (GAD), a major autoantigen in T1DM patients and a therapy in phase 3 development. Important results have been recently published elsewhere describing the details of treating adolescents with recent-onset T1DM with the 65-kDa isoform of this enzyme.1 In this edition of the Journal of Diabetes Science and Technology, the lead investigator of this trial, Johnny Ludvigsson, provides a helpful review of the field of T1DM immunomodulatory approaches and details about the study he led.2 GAD therapy is an attractive approach because the pharmacologic use of this antigen is associated with easy administration, a very favorable safety profile, and a relatively well-understood mechanism of action.
Clinical Trial of GAD Therapy
Ludvigsson and colleagues1 reported results from a trial of GAD therapy in the New England Journal of Medicine on October 8, 2008. This randomized controlled trial assessed the ability of alum-formulated GAD (Diamyd®, Diamyd Medical AB, Stockholm) compared with placebo to preserve residual insulin secretion and reverse recent-onset type 1 diabetes.
The 70 subjects in this study were 10 to 18 years of age and had developed T1DM within the previous 18 months. The study duration included a 15-month period of treatment followed by a 15-month period of further observation. The study was intended to determine whether treatment with this autoantigen would reduce or halt the loss of residual insulin secretion.
The prespecified primary efficacy end point was the change between baseline and month 15 in the fasting C-peptide level. The prespecified secondary efficacy end points were changes between baseline and various prespecified time points, up to month 30, in fasting and stimulated C-peptide levels and glycated hemoglobin values. Other end points prespecified for formal analysis were insulin requirement, fasting plasma glucose level, fasting C-peptide, plasma glucose ratio, and GAD autoantibody titer.
Results
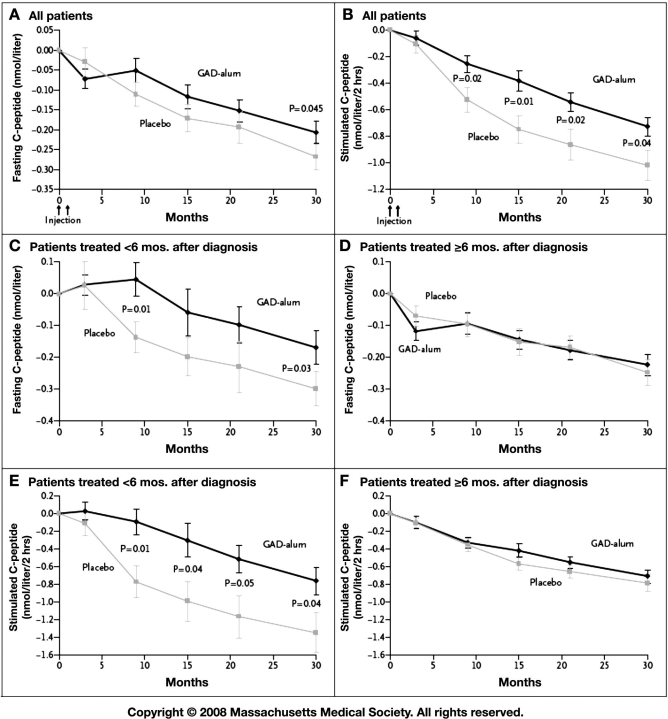
Glutamic acid decarboxylase–alum therapy resulted in no significant effect on fasting C-peptide levels after 15 months (the primary end point), although this inter-vention did have a favorable effect on stimulated C-peptide levels. After 30 months, both fasting and stimulated C-peptide levels declined significantly less in the GAD–alum group than in the placebo group. Over the course of the study, in both study groups there was an increase in insulin requirements, glycated hemoglobin levels, and plasma glucose levels. Glycated hemoglobin values did not differ significantly between the two study groups.
Among patients treated less than 6 months after diagnosis, both fasting and stimulated C-peptide secretion decreased significantly less in the GAD–alum group than in the placebo group by month 30, whereas no significant difference was observed between the two groups for patients treated 6 months or more after diagnosis. Therefore, the apparent protective effect of the GAD–alum treatment on C-peptide secretion was seen only in subjects treated less than 6 months after diagnosis.
Good News about the Results
Glutamic acid decarboxylase–alum therapy was associated with a small but statistically significant preservation of C-peptide (endogenous insulin secretion) in the subset of patients with more recent-onset T1DM. GAD–alum appears likely to have a very good safety profile, although a much larger exposure will be required to confirm this expectation and support use in the pediatric population. The trial reports very valuable data for purposes of designing future trials. An editorial by Denise Faustman, which accompanied the trial results, nicely presented some of the relevant science and important questions for T1DM therapeutic development.3
Not So Good News about These Results
The study failed to demonstrate its primary efficacy end point and therefore care should be taken in interpreting secondary and exploratory analyses. The therapeutic effect of GAD–alum, even in the subgroup of newer-onset patients, is modest and of uncertain clinical meaningfulness. Results reemphasize the hard reality of developing immunotherapies aimed at people with new-onset T1DM.
This report also underscores that the duration of trials for treating T1DM must be long (in excess of 24 or more months) to allow interpretation of clinical meaningfulness for the kind of treatment effects that have been observed in this and a few other T1DM trials. Patient selection is very important—probably only intervening within 3 months of diagnosis will allow the opportunity to achieve clear efficacy. Ironically, limiting clinical trials to people with less than 3 months' duration could actually increase the necessary sample size over trials that accept greater disease duration. This is because of the greater variability in baseline C-peptide among just-diagnosed patients compared to those with greater duration. Data from the GAD–alum trial actually illustrate this point. By inspecting Figure 1, it can be seen that standard errors for group mean fasting C-peptide values in the <6-month diabetes duration group are about twice those of the >6-month group. Variability would likely increase even further for diabetes duration <3 months because of the wider range of endogenous insulin secretion observed near the time of diagnosis. Conversely, with increasing duration of diabetes the mean C-peptide approaches near zero and the variability around that average becomes progressively smaller. The sample size to achieve adequate statistical power is, in part, determined by the variability of the outcome measure.
Figure 1.
Mean changes from baseline levels of fasting and stimulated C-peptide, according to treatment group and time of treatment relative to diagnosis. Mean changes from baseline in fasting (A) and stimulated (B) C-peptide levels are given for all patients included in intention-to-treat analyses in the group receiving the recombinant human 65-kDa isoform of glutamic acid decarboxylase in a standard vaccine formulation with alum (GAD–alum, 35 patients) and in the group receiving placebo (34 patients). Mean changes from baseline in fasting (C) and stimulated (E) C-peptide levels are also shown for patients treated less than 6 months after receiving the diagnosis of diabetes (11 patients in the GAD–alum group and 14 patients in the placebo group). Finally, mean changes from baseline in fasting (D) and stimulated (F) C-peptide levels are shown for those treated 6 months or more after diagnosis (24 patients in the GAD–alum group and 20 patients in the placebo group). Stimulated C-peptide level was measured on the basis of areas under the curve in response to the mixed-meal tolerance test. I bars indicate standard errors. To convert values for C-peptide to nanograms per milliliter, divide by 0.33. Reproduced from Reference 1 with permission from New England Journal of Medicine.
Even though T1DM affects well over 1.2 million people in North America, it is very difficult to find people with new-onset T1DM and recruit them into studies soon enough after diagnosis. Therapies targeted at this population actually qualify for orphan drug status by Food and Drug Administration criteria. These challenges and other uncertainties will continue to discourage the kinds of investments necessary for phase 3 studies to be completed.
Going Forward
A cure for T1DM will require that we develop therapies to control the underlying cause, which is autoimmunity. However, T1DM autoimmunity is a very difficult drug development target as a stand-alone indication and—unless used for prevention—will provide only modest benefits to a very small population. In her editorial, which accompanied the article, Denise Faustman asked rhetorically whether T1DM therapeutic efforts should include pancreatic regeneration as well as pancreatic preservation. This is a key question because combining these two approaches would presumably increase efficacy, shorten the time necessary to demonstrate efficacy, and extend the target population to include most, if not all, people with T1DM. The next questions are whether regeneration can be induced pharmacologically in people and, if so, what is the evidence for regeneration candidates.
The work of Johnny Ludvigsson and colleagues is important but also a reminder that, in the quest to cure T1DM, the control of autoimmunity is necessary but not sufficient. As is the case for type 2 diabetes mellitus, T1DM will likely require multiple therapeutic approaches to manage the disease effectively. We believe that β-cell regeneration may be necessary to cure both forms of diabetes.
Abbreviations
- GAD
glutamic acid decarboxylase
- T1DM
type 1 diabetes mellitus
References
- 1.Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359(18):1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 2.Ludvigsson J. The role of immunomodulation therapy in autoimmune diabetes. J Diabetes Sci Technol. 2009;3(2):320–330. doi: 10.1177/193229680900300213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faustman DL. Immunotherapy on trial for new-onset type 1 diabetes. N Engl J Med. 2008;359(18):1956–1958. doi: 10.1056/NEJMe0807425. [DOI] [PubMed] [Google Scholar]