Abstract
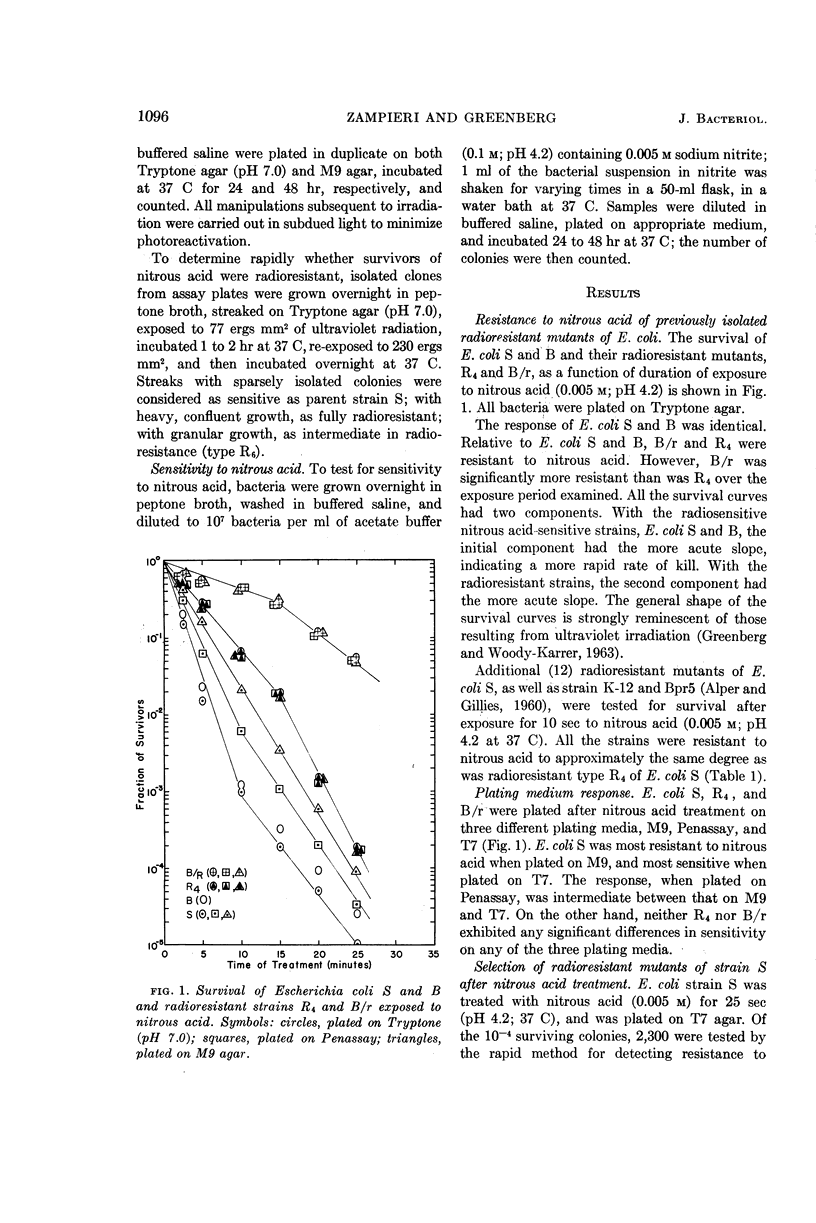
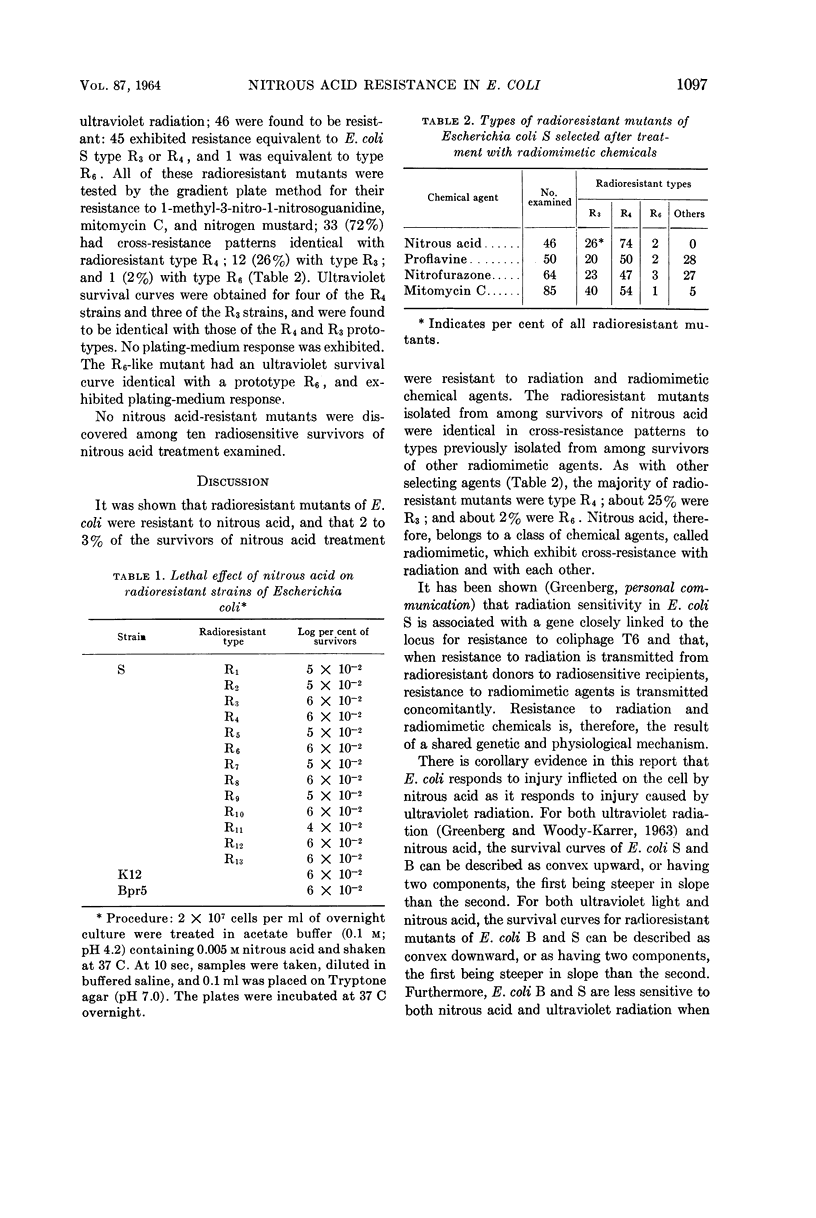
Zampieri, Antonio (Palo Alto Medical Research Foundation, Palo Alto, Calif.), and Joseph Greenberg. Cross-resistance relationships in Escherichia coli between ultraviolet radiation and nitrous acid. J. Bacteriol. 87:1094–1099. 1964.—A number of radiosensitive and radioresistant strains of Escherichia coli were tested for sensitivity to injury by nitrous acid. All the radioresistant strains, including 13 radioresistant mutants of strain S, B/r, Bpr5, and K-12, were found to be significantly more resistant to nitrous acid than were the radiosensitive strains S and B. The radioresistant mutants of strain S, Bpr5, and K-12 displayed similar responses to nitrous acid and were less resistant than was strain B/r. Strains B and S were indistinguishable on the basis of nitrous acid sensitivity. The survival curves of all strains examined were similar in shape to corresponding survival curves after ultraviolet radiation. The sensitivity to nitrous acid of the radiosensitive strains S and B, but not that of the radioresistant strains, was found to be greater on Tryptone medium than on Penassay medium, and greater on Penassay medium than on glucose-salts medium. Between 2 and 3% of the strain S survivors of nitrous acid treatment were radioresistant; 46 such radioresistant mutants were isolated and found to be identical in cross-resistance pattern with radioresistant types (R3, R4, or R6) previously described. The proportions in which these radioresistant types were found to occur were similar to those observed after selection by other radiomimetic agents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER T., GILLIES N. E. The relationship between growth and survival after irradiation of Escherichia coli strain B and two resistant mutants. J Gen Microbiol. 1960 Feb;22:113–128. doi: 10.1099/00221287-22-1-113. [DOI] [PubMed] [Google Scholar]
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLUM F. J., SETLOW R. B. Ultraviolet inactivation of DNA primer activity. I. Effects of different wavelengths and doses. Biochim Biophys Acta. 1963 Apr 30;68:599–607. doi: 10.1016/0006-3002(63)90189-6. [DOI] [PubMed] [Google Scholar]
- Brookes P., Lawley P. D. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem J. 1961 Sep;80(3):496–503. doi: 10.1042/bj0800496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P., Lawley P. D. The reaction of mustard gas with nucleic acids in vitro and in vivo. Biochem J. 1960 Dec;77(3):478–484. doi: 10.1042/bj0770478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- GIERER A., MUNDRY K. W. Production of mutants of tobacco mosaic virus by chemical alteration of its ribonucleic acid in vitro. Nature. 1958 Nov 22;182(4647):1457–1458. doi: 10.1038/1821457a0. [DOI] [PubMed] [Google Scholar]
- GREENBERG J., MANDELL J. D., WOODY P. L. Resistance and cross-resistance of Escherichia coli mutants to antitumour agent mitomycin C. J Gen Microbiol. 1961 Nov;26:509–520. doi: 10.1099/00221287-26-3-509. [DOI] [PubMed] [Google Scholar]
- GREENBERG J., WOODY-KARRER P. RADIORESISTANT MUTANTS OF ESCHERICHIA COLI B (ORNL). Radiat Res. 1963 Nov;20:350–363. [PubMed] [Google Scholar]
- KAUDEWITZ F. Production of bacterial mutants with nitrous acid. Nature. 1959 Jun 27;183:1829–1830. doi: 10.1038/1831829a0. [DOI] [PubMed] [Google Scholar]
- LATARJET R., MORENNE P., BERGER R. Un appareil simple pour le dosage des rayonnements ultraviolets émis par les lampes germicides. Ann Inst Pasteur (Paris) 1953 Aug;85(2):175–184. [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITMAN R. M., EPHRUSSI-TAYLOR H. [Inactivation and mutation of the genetic factors of the desoxyribonucleic acid of pneumococcus by ultraviolet light and by nitrous acid]. C R Hebd Seances Acad Sci. 1959 Aug 10;249:838–840. [PubMed] [Google Scholar]
- LUZZATI D. The action of nitrous acid on transforming desoxyribonucleic acids. Biochem Biophys Res Commun. 1962 Dec 19;9:508–516. doi: 10.1016/0006-291x(62)90117-1. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., WOODY P. L., GREENBERG J. Resistance and cross-resistance of Escherichia coli mutants to anticancer agents. 1-Methyl-3-nitro-1-nitrosoguanidine. J Bacteriol. 1961 Mar;81:419–424. doi: 10.1128/jb.81.3.419-424.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., van der KAMP, COHEN J. A. Phenotypic and genotypic characterization of radiation sensitivity in Escherichia coli B. Biochim Biophys Acta. 1962 Aug 20;61:278–289. doi: 10.1016/0926-6550(62)90090-7. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Aldous E. RECOVERY FROM ULTRAVIOLET IRRADIATION IN ESCHERICHIA COLI. J Bacteriol. 1949 Mar;57(3):363–375. doi: 10.1128/jb.57.3.363-375.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., SETLOW J. K. Evidence that ultraviolet-induced thymine dimers in DNA cause biological damage. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1250–1257. doi: 10.1073/pnas.48.7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J Bacteriol. 1952 Oct;64(4):489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIELMETTER W., SCHUSTER H. [Base specificity in the induction of mutations by nitrous acid in phage T-2]. Z Naturforsch B. 1960 May;15B:304–311. [PubMed] [Google Scholar]
- WOODY-KARRER P., GREENBERG J. RESISTANCE AND CROSS RESISTANCE OF ESCHERICHIA COLI S MUTANTS TO THE RADIOMIMETIC AGENT NITROFURAZONE. J Bacteriol. 1963 Jun;85:1208–1216. doi: 10.1128/jb.85.6.1208-1216.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODY P. L., MANDELL J. D., GREENBERG J. Resistance and cross-resistance of Escherichia coli mutants to anticancer agents: nitrogen mustard and nitromin. Radiat Res. 1961 Sep;15:290–297. [PubMed] [Google Scholar]
- Witkin E. M. Genetics of Resistance to Radiation in ESCHERICHIA COLI. Genetics. 1947 May;32(3):221–248. doi: 10.1093/genetics/32.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMENHOF S. Gene unstabilization induced by heat and by nitrous acid. J Bacteriol. 1961 Jan;81:111–117. doi: 10.1128/jb.81.1.111-117.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]



