Abstract
Background
The intuitiveness, instruction time, and handling of the Levemir® (insulin detemir) FlexPen® and the Lantus® OptiClik® pen (with insulin glargine) were investigated.
Methods
This randomized open-label crossover study involved two groups of insulin-device-naive Japanese patients with type 2 diabetes [mean (SD) age 61.9 ± 12.3 years, 57% male]. Patients were evaluated on the ease-of-use of each insulin pen without instruction [intuitiveness group (n = 32)], or with instruction [instruction time group (n = 29)]. Patient preferences for the respective devices were assessed by questionnaire.
Results and Discussion
FlexPen required significantly less instruction time (p < .001) and was objectively more intuitive to use (p < .001) than OptiClik. Nevertheless, few patients in the intuitiveness group felt confident injecting either pen prior to instruction (FlexPen, 31%; OptiClik, 16%). No patients in the instruction time group found FlexPen difficult to learn, whereas 45% of patients found OptiClik difficult or very difficult to learn. FlexPen was rated simpler to use (77% versus 12%; p < .001), easier to inject (67% versus 13%; p < .001), and more convenient (71% versus 12%; p < .001) compared with OptiClik. More patients would trust FlexPen to deliver insulin injections (p < .01) and would prefer to use FlexPen compared with OptiClik (82% versus 13%; p < .001).
Conclusions
FlexPen was faster to teach, simpler to use, and more trusted by patients compared with OptiClik. Mean injection time was significantly shorter for FlexPen than OptiClik, with or without instruction. This study highlights not only how easy it is for patients to learn to use FlexPen, but also how easily health care providers can teach patients to use it.
Keywords: diabetes mellitus, FlexPen, insulin pen, preference
Introduction
Improved glycemic control in patients with diabetes mellitus reduces the incidence of long-term complications, particularly microvascular disease.1–3 In patients with type 2 diabetes, the need for adequate metabolic control has led to the advocacy of more intensive treatment regimens, including basal–bolus insulin therapy.4,5 While several studies corroborate the rigorous basal–bolus regimen,6–10 many patients still resist insulin therapy in general and intensive insulin regimens in particular.11–16 Fear of injection, hypoglycemia, and weight gain hinder patient compliance, in addition to the inconvenience and embarrassment caused by frequent injections.14,15,17,18 Insulin injection pens provide a convenient, accurate, preferred alternative to vial and syringe systems, with improved compliance.16,18–22 However, not all insulin pens consistently deliver accurate doses and instead may over- or underdose.23,24 Glycemic variations may result, ultimately providing suboptimal glycemic control.
With the rigors of the basal–bolus treatment regimen in mind, the Levemir® (insulin detemir) FlexPen® (Novo Nordisk A/S, Copenhagen, Denmark) was designed to help patients conveniently and accurately adhere to treatment, supporting patient confidence in self-management of diabetes. This noninvasive study investigated the ease of use of two insulin pens: the Levemir FlexPen and the OptiClik® pen with Lantus® (insulin glargine) (Sanofi-Aventis, Paris, France). In this study the following were investigated: (1) intuitive use (without instruction) of the insulin pens by injection time measurement and questionnaire, (2) instruction time needed to complete an injection, and (3) ease of use; perceptions of safety, reliability, and durability; trust and confidence in the device; and overall pen preference.
Patients and Methods
Patients
Patients with type 2 diabetes were included if they were on oral antidiabetes drug (OAD) treatment for at least 2 years, aged ≥18 years, and had no prior experience with insulin injection devices. Patients were excluded if they did not meet these requirements, were unable to read newspaper body text, or had neuropathy, visual impairment, and/or motor disabilities.
Study Design
This randomized open-label crossover noninvasive superiority investigation was conducted according to all applicable regulatory requirements, and written consent was received from all patients in accordance with the Declaration of Helsinki. The study compared the FlexPen with OptiClik in insulin-device-naive Japanese patients with type 2 diabetes. Overall the test took approximately 1 to 1.5 h.
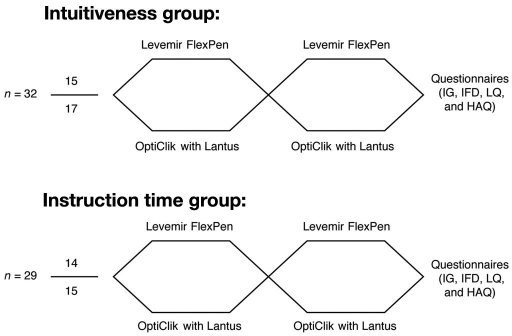
Sixty-one patients were randomized into either the intuitiveness group or the instruction time group (Figure 1). Patients were randomized again into either sequence: FlexPen followed by OptiClik or OptiClik followed by FlexPen.
Figure 1.
Study flow chart. Sixty-one patients were enrolled and randomized into either the intuitiveness group (n = 32) or the instruction time group (n = 29). In each group, patients were randomized to a treatment sequence: either Levemir FlexPen followed by OptiClik with Lantus or OptiClik with Lantus followed by Levemir FlexPen. Finally the patients were asked to answer several questionnaires.
FlexPen is a prefilled insulin pen that does not require replacement of insulin cartridges, whereas OptiClik is a reusable insulin pen requiring insulin cartridge replacement.
The intuitiveness group did not receive instructions on how to use the insulin pens. Patients were timed on how long it took to intuitively inject the pen into a needle cushion, with and without cartridge insertion (for OptiClik only). Patients in the instruction time group received instructions on how to operate the insulin pens, including cartridge insertion (for OptiClik only).
Only patients in the intuitiveness group completed a questionnaire regarding intuitiveness and device understanding (IG questionnaire) after both pens had been tested. Patients in both groups then completed the important features of the devices (IFD) questionnaire. Afterward, all patients were given the insulin pens again and received thorough instructions on how to use each pen. Patients completed questionnaires on ease of use [learning to use questionnaire (LQ)] and overall preference [handling and acceptance questionnaire (HAQ)].
Efficacy Assessments
The primary endpoint in the intuitiveness group was the injection time in minutes and seconds taken to deliver a specific dose (10 U) into a needle cushion, with and without cartridge insertion. Additional intuitive endpoints included determining (1) which insulin pen was the most intuitive to use without instruction and (2) confidence level in using either insulin pen without instruction (IG questionnaire). The primary endpoint in the instruction time group was the injection time with instruction needed to successfully inject a specific dose (10 U) into a needle cushion, with or without cartridge insertion. Secondary endpoints for both groups included assessing ease of use; perceptions of safety, reliability, and durability; trustfulness; confidence; and overall preference, using questionnaires.
Statistical Analysis
Injection times were analyzed using an analysis of variance, including device, period (1 or 2), and group, as main fixed effects, and an interaction effect between the group and device. Differences were considered significant if p < .05. Patient preferences were assessed by questionnaires. For preference variables, the frequencies of preference or nonpreference were calculated together with the associated 95% confidence interval (CI) and p value of no difference. All other nonpreference variables from the questionnaires involving pairwise comparison were analyzed using a Pearson test for independence of the patient evaluation.
Results
Demographic and Other Baseline Characteristics
Sixty-one patients were randomized and completed the study (Table 1). Mean (SD) age was 61.9 ± 12.3 years (age range, 22–80 years), 35 patients (57%) were male, and 26 (43%) were female.
Table 1.
Patient Demographics and Baseline Characteristics
| Intuitiveness Group | Instruction Time Group | Total | |
|---|---|---|---|
| Randomized n | 32 | 29 | 61 |
| Gender | |||
| Male | 14 (43.8) | 21 (72.4) | 35 (57.4) |
| Female | 18 (56.3) | 8 (27.6) | 26 (42.6) |
| Age (years) | |||
| n | 32 | 28 | 60 |
| Mean (SD) | 60.6 (13.4) | 63.3 (11.1) | 61.9 (12.3) |
| Duration of diabetes (years) | |||
| n | 32 | 29 | 61 |
| Mean (SD) | 8.66 (7.26) | 8.24 (8.27) | 8.46 (7.69) |
| Treatment with OAD (years) | |||
| n | 31 | 29 | 60 |
| Mean (SD) | 6.68 (6.05) | 6.00 (5.29) | 6.35 (5.66) |
Intuitiveness Group: Injection Time without Instruction
Mean injection time was significantly shorter with FlexPen than with OptiClik (excluding cartridge insertion) among patients in the intuitiveness group (2 min and 25 s versus 2 min and 50 s, p = .008) (Table 2). When cartridge insertion was included, the difference in the mean injection time was 2 min and 15 s longer with OptiClik (p < .0001) when compared with FlexPen. The mean total time for performing the injection with OptiClik was 4 min and 40 s.
Table 2.
Injection Time (min) with and without Cartridge Insertion (for the Intention-to-Treat Population)
| Intuitiveness Group | Instruction Time Group | Total | |
|---|---|---|---|
| Without Cartridge Insertion | |||
| OptiClik | |||
| n | 29 | 29 | 58 |
| Mean (SD) | 2.84 (0.89) | 3.99 (1.08) | 3.41 (1.14) |
| FlexPen | |||
| n | 30 | 29 | 59 |
| Mean (SD) | 2.42 (0.77) | 2.47 (0.86) | 2.44 (0.81) |
| With Cartridge Insertion | |||
| OptiClik | |||
| n | 29 | 29 | 58 |
| Mean (SD) | 4.66 (1.30) | 5.20 (1.54) | 4.93 (1.44) |
Additional Intuitiveness Endpoints
Sixty-nine percent (n = 22) of patients chose FlexPen as the most intuitive insulin pen, while 22% (n = 7) chose OptiClik (p = .001) (Table 3). Few patients were confident in using either pen without instructions (FlexPen, 31%, n = 10; OptiClik, 16%, n = 5; nonsignificant).
Table 3.
Intuitiveness and Device Understanding Questionnaire
| Patients (n) | 32 | ||
|---|---|---|---|
| Intuitiveness Group n (%) | |||
| IG Question 1: Which is the most intuitive insulin pen? | |||
| Equal | 3 (9.4) | ||
| FlexPen | 22 (68.8) | ||
| OptiClik | 7 (21.9) | ||
| Difference % (FlexPen-OptiClik) | 46.9 | ||
| 95% Cl for difference % | (18.2, 75.6) | ||
| p value | .001 | ||
| IG Question 2: Are you confident with FlexPen? | |||
| Yes | 10 (31.3) | ||
| No | 22 (68.8) | ||
| IG Question 3: Are you confident with OptiClik? | |||
| Yes | 5 (15.6) | ||
| No | 27 (84.4) | ||
Instruction Time Group: Injection Time with Instruction
Mean instruction and injection time was significantly shorter with FlexPen than with OptiClik among patients in the instruction time group (2 min and 28 s versus 3 min and 59 s, p < .001) (Table 2). When cartridge insertion was included, the difference in the mean total instruction and injection time was 2 min and 44 s longer for OptiClik (p < .001). The mean total instruction time for OptiClik was 5 min and 12 s.
Secondary Endpoints: Questionnaire Responses
Learning to Use Questionnaire
No patients in the instruction time group found FlexPen difficult to learn (0%), wheras 45% of patients found OptiClik difficult or very difficult to learn (Table 4). In both groups, FlexPen was considered the easiest pen to learn how to use compared with OptiClik (82% versus 7%, p < .001).
Table 4.
Learning to Use the Pen Questionnaire
| Patients (n) | 32 | 29 | 61 |
|---|---|---|---|
| Intuitiveness Group n (%) | Instruction Time Group n (%) | Total n (%) | |
| LQ Question 1: How easy/difficult is Levemir FlexPen to learn? | |||
| Very easy | 5 (15.6) | 7 (24.1) | 12 (19.7) |
| Easy | 17 (53.1) | 19 (65.5) | 36 (59.0) |
| Neither easy nor… | 5 (15.6) | 3 (10.3) | 8 (13.1) |
| Difficult | 5 (15.6) | 0 (0.0) | 5 (8.2) |
| Very difficult | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| LQ Question 2: How easy/difficult is OptiClik to learn? | |||
| Very easy | 1 (3.1) | 2 (6.9) | 3 (4.9) |
| Easy | 8 (25.0) | 8 (27.6) | 16 (26.2) |
| Neither easy nor… | 11 (34.4) | 6 (20.7) | 17 (27.9) |
| Difficult | 11 (34.4) | 12 (41.4) | 23 (37.7) |
| Very difficult | 1 (3.1) | 1 (3.4) | 2 (3.3) |
| LQ Question 3: Which pen is the easiest to learn? | |||
| Equal | 5 (15.6) | 2 (6.9) | 7 (11.5) |
| FlexPen | 23 (71.9) | 27 (93.1) | 50 (82.0) |
| OptiClik | 4 (12.5) | 0 (0.0) | 4 (6.6) |
| Difference % (FlexPen–OptiClik) | 59.4 | 93.1 | 75.4 |
| 95% CI for difference % | (35.1, 83.7) | (83.9, 102.3) | (61.3, 89.5) |
| p value | < .0001 | < .0001 | <.0001 |
Handling and Acceptance Questionnaire
More patients trusted FlexPen to deliver injections (39% versus 15%, p = .006) and to be the safest insulin pen to operate (36% versus 15%, p = .014) compared with OptiClik (Table 5). More patients also trusted the accuracy of FlexPen over OptiClik in terms of confidence in each pen to inject a full dose (61% versus 12%, p < .001). Overall more patients preferred FlexPen over OptiClik (82% versus 13%, p < .001).
Table 5.
Handling and Acceptance Questionnaire (Selected Questions)
| Patients (n) | 32 | 29 | 61 |
|---|---|---|---|
| Intuitiveness Group n (%) | Instruction Time Group n (%) | Total n (%) | |
| HAQ Question 11: Which pen has the highest quality? | |||
| Equal | 12 (37.5) | 4 (13.8) | 16 (26.2) |
| FlexPen | 4 (12.5) | 12 (41.4) | 16 (26.2) |
| OptiClik | 16 (50.0) | 13 (44.8) | 29 (47.5) |
| Difference % (FlexPen–OptiClik) | −37.5 | −3.4 | −21.3 |
| 95% CI for difference % | (−61.6,−13.4) | (−37.2, 30.3) | (−42.2, −0.4) |
| p value | .0023 | .8414 | .0454 |
| HAQ Question 13: Which pen is the most convenient? | |||
| Equal | 6 (18.8) | 4 (13.8) | 10 (16.4) |
| FlexPen | 21 (65.6) | 22 (75.9) | 43 (70.5) |
| OptiClik | 5 (15.6) | 2 (6.9) | 7 (11.5) |
| Difference % (FlexPen–OptiClik) | 50 | 69 | 59 |
| 95% CI for difference % | (24.0, 76.0) | (47.4, 90.6) | (41.8, 76.2) |
| p value | .0002 | < .0001 | < .0001 |
| HAQ Question 16: Which pen is the simplest to use? | |||
| Equal | 4 (12.5) | 3 (10.3) | 7 (11.5) |
| FlexPen | 23 (71.9) | 24 (82.8) | 47 (77.0) |
| OptiClik | 5 (15.6) | 2 (6.9) | 7 (11.5) |
| Difference % (FlexPen–OptiClik) | 56.3 | 75.9 | 65.6 |
| 95% CI for difference % | (30.4, 82.1) | (55.2, 96.5) | (48.6, 82.5) |
| p value | < .0001 | < .0001 | < .0001 |
| HAQ Question 19: With which pen is it easiest to inject the dose? | |||
| Equal | 5 (15.6) | 7 (24.1) | 12 (19.7) |
| FlexPen | 21 (65.6) | 20 (69.0) | 41 (67.2) |
| OptiClik | 6 (18.8) | 2 (6.9) | 8 (13.1) |
| Difference % (FlexPen–OptiClik) | 46.9 | 62.1 | 54.1 |
| 95% CI for difference % | (19.5, 74.2) | (39.8, 84.3) | (36.2, 72.0) |
| p value | .0008 | < .0001 | < .0001 |
| HAQ Question 24: With which pen do you feel most confident that the full dose has been injected? | |||
| Equal | 13 (40.6) | 4 (13.8) | 17 (27.9) |
| FlexPen | 17 (53.1) | 20 (69.0) | 37 (60.7) |
| OptiClik | 2 (6.3) | 5 (17.2) | 7 (11.5) |
| Difference % (FlexPen–OptiClik) | 46.9 | 51.7 | 49.2 |
| 95% CI for difference % | (25.7, 68.1) | (23.7, 79.8) | (31.8, 66.6) |
| p value | < .0001 | .0003 | < .0001 |
| HAQ Question 25: Which pen do you feel is the safest to operate? | |||
| Equal | 18 (56.3) | 12 (41.4) | 30 (49.2) |
| FlexPen | 9 (28.1) | 13 (44.8) | 22 (36.1) |
| OptiClik | 5 (15.6) | 4 (13.8) | 9 (14.8) |
| Difference % (FlexPen–OptiClik) | 12.5 | 31 | 21.3 |
| 95% CI for difference % | (−10.0, 35.0) | (5.6, 56.5) | (4.2, 38.4) |
| p value | .2763 | .0170 | .0144 |
| HAQ Question 31: Which pen would you trust the most for delivering your insulin injections? | |||
| Equal | 17 (53.1) | 11 (37.9) | 28 (45.9) |
| FlexPen | 9 (28.1) | 15 (51.7) | 24 (39.3) |
| OptiClik | 6 (18.8) | 3 (10.3) | 9 (14.8) |
| Difference % (FlexPen–OptiClik) | 9.4 | 41.4 | 24.6 |
| 95% CI for difference % | (−14.1, 32.9) | (17.0, 65.8) | (7.2, 42.0) |
| p value | .4342 | .0009 | .0056 |
| HAQ Question 34: Overall, which pen was the most simple to use? | |||
| Equal | 2 (6.3) | 2 (6.9) | 4 (6.6) |
| FlexPen | 25 (78.1) | 25 (86.2) | 50 (82.0) |
| OptiClik | 5 (15.6) | 2 (6.9) | 7 (11.5) |
| Difference % (FlexPen–OptiClik) | 62.5 | 79.3 | 70.5 |
| 95% CI for difference % | (36.9, 88.1) | (59.3, 99.3) | (53.9, 87.1) |
| p value | < .0001 | < .0001 | < .0001 |
| HAQ Question 35: Overall, which pen would you prefer to use every day (if necessary)? | |||
| Equal | 2 (6.3) | 1 (3.4) | 3 (4.9) |
| FlexPen | 26 (81.3) | 24 (82.8) | 50 (82.0) |
| OptiClik | 4 (12.5) | 4 (13.8) | 8 (13.1) |
| Difference % (FlexPen–OptiClik) | 68.8 | 69 | 68.9 |
| 95% CI for difference % | (45.1, 92.4) | (43.5, 94.4) | (51.5, 86.2) |
| p value | < .0001 | < .0001 | < .0001 |
The reusable OptiClik was rated the highest “quality” pen compared with FlexPen (48% versus 26%, p = .045), yet significantly more patients considered it easier to inject with FlexPen (67% versus 13%, p < .001) and easier to know if the injection push button was completely depressed compared with OptiClik (p < .001). In addition, significantly more patients rated FlexPen simpler to use overall (82% versus 12%, p < .001) and more convenient to use than OptiClik (71% versus 12%, p < .001).
Important Device Features Questionnaire
Patients rated confidence in injecting the correct dose as the most important feature of each insulin pen (Table 6). Likewise, confidence in setting the correct dose was “extremely important” for 44% and “pretty important” for 51% of patients. Appropriateness of the appearance of the pen was considered the least important feature.
Table 6.
Important Features of Devices Questionnaire (Selected Statements)
| Patients (n) | 32 | 29 | 61 |
|---|---|---|---|
| Intuitiveness Group n (%) | Instruction Time Group n (%) | Total n (%) | |
| IFD Statement 1: The pen is easy and intuitive to use. | |||
| Extremely important | 6 (18.8) | 9 (31.0) | 15 (24.6) |
| Pretty important | 22 (68.8) | 17 (58.6) | 39 (63.9) |
| Moderately important | 3 (9.4) | 1 (3.4) | 4 (6.6) |
| Slightly important | 1 (3.1) | 2 (6.9) | 3 (4.9) |
| Not at all important | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IFD Statement 4: I am confident that I am setting the correct insulin dose every time. | |||
| Extremely important | 11 (34.4) | 16 (55.2) | 27 (44.3) |
| Pretty important | 18 (56.3) | 13 (44.8) | 31 (50.8) |
| Moderately important | 1 (3.1) | 0 (0.0) | 1 (1.6) |
| Slightly important | 2 (6.3) | 0 (0.0) | 2 (3.3) |
| Not at all important | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IFD Statement 5: I am confident that I am injecting the correct amount of insulin every time. | |||
| Extremely important | 14 (43.8) | 15 (51.7) | 29 (47.5) |
| Pretty important | 16 (5.0) | 14 (48.3) | 30 (49.2) |
| Moderately important | 1 (3.1) | 0 (0.0) | 1 (1.6) |
| Slightly important | 1 (3.1) | 0 (0.0) | 1 (1.6) |
| Not at all important | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IFD Statement 6: The pen has an appropriate appearance. | |||
| Extremely important | 5 (15.6) | 1 (3.4) | 6 (9.8) |
| Pretty important | 11 (34.4) | 15 (51.7) | 26 (42.6) |
| Moderately important | 8 (25.0) | 5 (17.2) | 13 (21.3) |
| Slightly important | 7 (21.9) | 6 (20.7) | 13 (21.3) |
| Not at all important | 1 (3.1) | 2 (6.9) | 3 (4.9) |
| IFD Statement 7: The insulin pen is discreet to use in public. | |||
| Extremely important | 6 (18.8) | 5 (17.2) | 11 (18.0) |
| Pretty important | 13 (40.6) | 16 (55.2) | 29 (47.5) |
| Moderately important | 4 (12.5) | 3 (10.3) | 7 (11.5) |
| Slightly important | 8 (25.0) | 3 (10.3) | 11 (18.0) |
| Not at all important | 1 (3.1) | 2 (6.9) | 3 (4.9) |
| IFD Statement 12: It is easy to know if the push button has been pushed completely down. | |||
| Extremely important | 12 (37.5) | 13 (44.8) | 25 (41.0) |
| Pretty important | 16 (50.0) | 16 (55.2) | 32 (52.5) |
| Moderately important | 2 (6.3) | 0 (0.0) | 2 (3.3) |
| Slightly important | 2 (6.3) | 0 (0.0) | 2 (3.3) |
| Not at all important | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IFD Statement 13: It is easy to see the dose scale during the injection. | |||
| Extremely important | 9 (28.1) | 9 (31.0) | 18 (29.5) |
| Pretty important | 18 (56.3) | 18 (62.1) | 36 (59.0) |
| Moderately important | 3 (9.4) | 0 (0.0) | 3 (4.9) |
| Slightly important | 1 (3.1) | 2 (6.9) | 3 (4.9) |
| Not at all important | 1 (3.1) | 0 (0.0) | 1 (1.6) |
Discussion
In this study, mean injection time was significantly shorter with the FlexPen than with the OptiClik pen, with or without instruction. This difference was greater when cartridge insertion was included for OptiClik. OptiClik was rated as having the highest quality compared with FlexPen, which could possibly be due to OptiClik being a reusable device wheras FlexPen is disposable. As expected for a device designed to be reused for years, the OptiClik was rated higher on quality than the prefilled FlexPen. In this study, patients' rating of quality was based on subjective perception. As such, it may be speculated that “quality” refers to the durability of the device, which would be in line with OptiClik being reusable and the FlexPen being disposable. However, FlexPen was rated highest with regard to trust and accuracy, where significantly more patients were confident that FlexPen would inject the full dose and be the safest insulin pen to operate as compared with OptiClik. In general, among all patients, injecting the correct amount of insulin was rated the most important device feature. In addition, FlexPen earned significantly higher ratings than OptiClik on the operating quality of several mechanical features of the insulin pens. For example, significantly more patients rated FlexPen easiest to inject and simplest to use. Significantly more patients also considered it easier to know if the injection push button was completely depressed with FlexPen compared with OptiClik.
The results of this investigation are consistent with previous studies examining FlexPen versus other insulin pens or the conventional vial and syringe.19,22,24–27 For example, Dreyer25 observed a significantly higher preference for FlexPen compared with the NovoLet® pen (Novo Nordisk A/S, Copenhagen, Denmark) (p < .0001) regarding confidence in delivering correct dose (63% versus 9%), ease of dose setting (71% versus 8%), discreetness in public (39% versus 9%), handling (54% versus 12%), and ease of use (73% versus 8%), as well as overall preference for continuing use (77% versus 12%).
In the current study, FlexPen was rated the most intuitive pen by the intuitiveness group. Similarly, in a randomized crossover study of 61 patients with type 2 diabetes, the NovoMix® 30 (biphasic insulin aspart) FlexPen (Novo Nordisk A/S, Copenhagen, Denmark) was found to be significantly more intuitive (p < .05), simpler to use (p < .01), and easier to learn (p < .01) than the HumaPen® Luxura™ with insulin lispro (Eli Lilly and Company, Indianapolis, IN).26 Mean total instruction time (including cartridge insertion for HumaPen Luxura) was also significantly shorter for the FlexPen (43 s versus 1 min and 7 s, p = .0004).26 Another randomized crossover study comparing the NovoMix 30 FlexPen with the Humalog® Mix25™ (insulin lispro mix) pen found a significantly higher preference for FlexPen (74.6% versus 14.3%, p < .001) among the 133 participants with type 2 diabetes.27 In addition, patients expressed greater confidence in FlexPen versus the Humalog pen in setting (36% versus 8.5%, p < .001) and injecting the correct dose every time (40% versus 9%, p < .001), managing daily injections (48% versus 7%, p < .001), and controlling blood sugar level (35% versus 15%, p < .005).27
Insulin pens that are simple to learn to use are more likely to give patients the confidence to self-inject and therefore lead to better adherence and improved glycemic control, leading to decreased health care costs.16 Consequently it is reassuring to observe in our study that patients found FlexPen easy to learn.
Preference for FlexPen is also evident in studies involving the conventional vial and syringe.19,22 For example, 74% of 108 patients with type 1 or 2 diabetes preferred to continue using FlexPen versus 20% who preferred to continue with the vial and syringe in a crossover study by Korytkowski and colleagues.19 The majority of patients found FlexPen easier overall to use (74% versus 21%), more discreet to use in public than the vial and syringe (85% versus 9%), and were more confident in using FlexPen in general (82% versus 11%). Patients were more confident in using FlexPen compared with the vial and syringe to deliver an accurate dose (73% versus 19%) and the required dose (82% versus 11%) and also to self-maintain glycemic control (61% versus 16%).19 This statistically significant improvement in glycemic control attests to patient confidence in FlexPen to deliver accurate doses.
Although the current investigation did not measure the accuracy of dose delivery, it did record patients' confidence in each pen to deliver full doses. Ultimately more patients trusted FlexPen to deliver doses accurately. Several studies corroborate this patient confidence in FlexPen by demonstrating its greater dosing accuracy compared with other insulin pens.23,24,28,29 In a study comparing FlexPen with OptiClik, FlexPen delivered doses that were significantly more accurate at 10 U (p < .0001) and 30 U (p < .0001), and all FlexPen doses were delivered within the specified range.29 OptiClik, on the other hand, underdosed for 17.1% of doses at 10 U and for 28.9% of doses at 30 U. These findings were corroborated in a study by Nayak and Clement,28 where, for a 10 U dose, 99.47% of doses delivered by FlexPen were accurate compared with 90.53% of doses delivered by OptiClik. For a 30 U dose, 100% versus 87.5% of doses delivered by FlexPen and OptiClik, respectively, were accurate.28 In another study, FlexPen proved to be more accurate in injecting 5, 10, and 30 U doses than SoloSTAR® (Sanofi-Aventis, Paris, France), with all FlexPen doses within the specified limits for 5 and 30 U doses and 1.3% of doses outside the specified limits for the 10 U dose.23 In contrast, SoloSTAR dosed outside the specified limits for all three doses, underdosing 1.6%, 29.3%, and 33.3% of the 5, 10, and 30 U doses, respectively. Similarly, in a head-to-head comparison of four insulin pens (FlexPen, OptiClik, SoloSTAR, and HumaPen Luxura), FlexPen exhibited significantly superior dosing accuracy versus OptiClik and SoloSTAR.24
Insulin pens in general offer a convenient, accurate, preferred alternative to the vial and syringe, improving quality of life among patients.8,19–22 As many studies depict, patients are more compliant with their treatment when using insulin pens rather than vial and syringe.16,18–21 Those pens that instill patient confidence based on ease of learning and superior handling may improve compliance to intensive insulin therapy.
Conclusions
In this investigation the FlexPen was found to be faster to teach, simpler to use, and more trusted by patients compared with the OptiClik pen. Mean injection time was significantly shorter for the FlexPen than for OptiClik, with or without instruction. These results have been corroborated in several other studies comparing FlexPen with other insulin pens or the conventional vial and syringe. This study highlights not only how easy it is for patients to both use and learn to use the FlexPen, but also the ease with which health care providers can teach patients to use it. These findings also demonstrate the importance of instructing patients on the correct use of insulin injection devices so patients feel confident in performing injections and, ultimately, in their ability to self-manage their diabetes.
Acknowledgments
The authors thank Stephanie Finucane and Catherine Jones (Watermeadow Medical, Witney, UK) for their assistance with the preparation of this manuscript, which was supported by Novo Nordisk A/S.
Abbreviations
- CI
confidence interval
- HAQ
handling and acceptance questionnaire
- IFD
important features of the devices
- IG
intuitiveness and device understanding
- LQ
learning to use questionnaire
- OAD
oral antidiabetes drug
References
- 1.The Diabetes Control and Complications Trial Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45(10):1289–1298. [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 5.IDF Clinical Guidelines Task Force. Brussels: International Diabetes Federation; 2005. Global guideline for type 2 diabetes. http://www.idf.org/home/index.cfm?node=1457. Accessed December 8, 2008. [Google Scholar]
- 6.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 7.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–B29. [PubMed] [Google Scholar]
- 8.Hörnquist JO, Wikby A, Andersson PO, Dufva AM. Insulin-pen treatment, quality of life and metabolic control: retrospective intra-group evaluations. Diabetes Res Clin Pract. 1990;10(3):221–230. doi: 10.1016/0168-8227(90)90065-2. [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.Epidemiology of Diabetes Interventions and Complications Research Group. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peyrot M, Rubin RR, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR, Landgraf R, Kleinebreil L. International DAWN Advisory Panel. Resistance to insulin therapy among patients and providers results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28(11):2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 12.Skovlund SE, Peyrot M. the DAWN International Advisory Panel. The Diabetes Attitudes, Wishes, and Needs (DAWN) program: a new approach to improving outcomes of diabetes care. Diabetes Spectrum. 2005;18(3):136–142. [Google Scholar]
- 13.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. 2005;22(10):1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 14.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 15.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 16.Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28(10):1712–1725. doi: 10.1016/j.clinthera.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Cobden D, Lee WC, Balu S, Joshi AV, Pashos CL. Health outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitus. Pharmacotherapy. 2007;27(7):948–962. doi: 10.1592/phco.27.7.948. [DOI] [PubMed] [Google Scholar]
- 18.Korytkowski M, Niskanen L, Asakura T. FlexPen: addressing issues of confidence and convenience in insulin delivery. Clin Ther. 2005;27(Suppl B):S89–S100. doi: 10.1016/j.clinthera.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Korytkowski M, Bell D, Jacobsen C, Suwannasari R. FlexPen Study Team. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 20.Graff MR, McClanahan MA. Assessment by patients with diabetes mellitus of two insulin pen delivery systems versus a vial and syringe. Clin Ther. 1998;20(3):486–496. doi: 10.1016/s0149-2918(98)80058-1. [DOI] [PubMed] [Google Scholar]
- 21.Coscelli C, Lostia S, Lunetta M, Nosari I, Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28(3):173–177. doi: 10.1016/0168-8227(95)01092-r. [DOI] [PubMed] [Google Scholar]
- 22.Rubin RR, Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care. 2004;27(10):2495–2497. doi: 10.2337/diacare.27.10.2495. [DOI] [PubMed] [Google Scholar]
- 23.Asakura T, Seino H, Kageyama M, Yohkoh N. Dosing accuracy of two insulin pre-filled pens. Curr Med Res Opin. 2008;24(5):1429–1434. doi: 10.1185/030079908x297394. [DOI] [PubMed] [Google Scholar]
- 24.Hänel H, Weise A, Sun W, Pfützner JW, Thomé N, Pfützner A. Differences in dose accuracy of insulin pens. J Diabetes Sci Technol. 2008;2(3):478–481. doi: 10.1177/193229680800200318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyer M. Comparison of metabolic control, safety, handling and patient acceptance of insulin delivery devices in patients with type 1 and type 2 diabetes. J Clin Res. 2005;8:1–7. [Google Scholar]
- 26.Reimer T, Hohberg C, Pfützner AH, Jørgensen C, Jensen KH, Pfützner A. Intuitiveness, instruction time, and patient acceptance of a prefilled insulin delivery device and a reusable insulin delivery device in a randomized, open-label, crossover handling study in patients with type 2 diabetes. Clin Ther. 2008;30(12):2252–2262. doi: 10.1016/j.clinthera.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Niskanen L, Jensen LE, Råstam J, Nygaard-Pedersen L, Erichsen K, Vora JP. Randomized, multinational, open-label, 2-period, crossover comparison of biphasic insulin aspart 30 and biphasic insulin lispro 25 and pen devices in adult patients with type 2 diabetes mellitus. Clin Ther. 2004;26(4):531–540. doi: 10.1016/s0149-2918(04)90055-0. [DOI] [PubMed] [Google Scholar]
- 28.Nayak B, Clement S. Insulin pen delivery systems: comparison of dosage accuracy of OptiClik and FlexPen insulin pens. Diabetes. 2007;56(Suppl. 1):A533. [Google Scholar]
- 29.Asakura T. Comparison of the dosing accuracy of two insulin injection devices. J Clin Res. 2005;8:33–40. [Google Scholar]