Abstract
Background
Microneedle-mediated drug delivery is a promising method for transdermal delivery of insulin, incretin mimetics, and other protein-based pharmacologic agents for treatment of diabetes mellitus. One factor that has limited clinical application of conventional microneedle technology is the poor fracture behavior of microneedles that are created using conventional materials and methods. In this study polymer microneedles for transdermal delivery were created using a two-photon polymerization (2PP) microfabrication and subsequent polydimethylsiloxane (PDMS) micromolding process.
Methods
Solid microneedle arrays, fabricated by means of 2PP, were used to create negative molds from PDMS. Using these molds microneedle arrays were subsequently prepared by molding eShell 200, a photo-reactive acrylate-based polymer that exhibits water and perspiration resistance.
Results
The eShell 200 microneedle array demonstrated suitable compressive strength for use in transdermal drug delivery applications. Human epidermal keratinocyte viability on the eShell 200 polymer surfaces was similar to that on polystyrene control surfaces. In vitro studies demonstrated that eShell 200 microneedle arrays fabricated using the 2PP microfabrication and PDMS micromolding process technique successfully penetrated human stratum corneum and epidermis.
Conclusions
Our results suggest that a 2PP microfabrication and subsequent PDMS micromolding process may be used to create microneedle structures with appropriate structural, mechanical, and biological properties for transdermal drug delivery of insulin and other protein-based pharmacologic agents for treatment of diabetes mellitus.
Keywords: microneedle, photopolymer, transdermal drug delivery
Introduction
The article presents a new material, eShell 200, that may be used in microneedle fabrication. There is a need for microneedles with improved mechanical properties to deliver insulin and other peptide drugs. The article also examines the mechanical properties of eShell 200 microneedles. Results suggest that eShell 200 may be an appropriate material for use in microneedle fabrication.
Insulin is a hormone produced by the pancreas that promotes the storage of glucose in the liver and in other locations within the body.1 The term “diabetes mellitus” includes several metabolic disorders that result from deficiencies in insulin action or insulin secretion. Damage to the eyes, kidneys, nerves, and blood vessels may occur as a result of elevated blood glucose levels (hyperglycemia).2 Several techniques for administering insulin to diabetes patients have been considered over the past century. Insulin and other protein-based pharmacologic agents cannot be delivered orally since they can be metabolized in the liver or degraded by proteolytic enzymes (e.g., pepsin) in the gastrointestinal tract.3 Insulin also cannot be delivered using conventional transdermal patches due to its high molecular weight.2 Insulin is currently delivered by means of subcutaneous injection; however, this technique has numerous shortcomings, including pain and trauma at the injection site.4
Microneedles, miniaturized needles with at least one dimension under 1 mm in length, may be used to create pores in the stratum corneum layer of the skin and enable diffusion of pharmacologic agents.2,5 The delivery of insulin using microneedles has several benefits over conventional subcutaneous delivery processes.1 For example, no specialized medical training or supervision is required for use of these devices. Pain to the patient and trauma at the injection site is minimized since these devices do not interact with nerve endings located within the dermis layer of the skin.6,7 Microneedle delivery and subcutaneous injection of insulin have been shown to reduce blood glucose levels with similar efficiency.8
A variety of microfabrication techniques have been previously used for fabrication of microneedles, including wet etching, dry etching, two-photon polymerization (2PP), electroplating, and micromolding.9–16 For example, Wilke and colleagues12 fabricated microneedles by anisotropic wet etching of a silicon substrate; the needle density that may be obtained using this technique is limited since only one row of needles may be fabricated per chip. Kobayashi and Suzuki9 and McAllister and associates15 fabricated electroplated shell structures; these structures demonstrate thin microneedle walls. Chun and coworkers10 demonstrated fabrication of microcapillaries using deep reactive ion etching; however, the needle density was limited by isotropy of the etch step. Griss and Stemme11 and Gardeniers and colleagues13 fabricated single-crystal silicon microneedle arrays. In these studies needle openings were positioned far from the tip, which necessitated deeper insertion within the skin. Despite these efforts microneedles prepared using conventional microfabrication processes have not been employed in widespread clinical use.
One factor that has limited clinical application of conventional microneedle technology is the poor fracture behavior of microneedles that are prepared using conventional materials and methods. Microneedles undergo mechanical failure by a variety of methods, including tip buckling, tip fracture, and base fracture.16,17 Since many biomedical microdevice fabrication technologies originated in the semiconductor industry, silicon is widely used for fabricating microneedles. However, silicon is extremely brittle, and silicon microneedles are prone to catastrophic failure; for example, stress concentration regions at the microneedle tip are susceptible to fracture. Fracture of silicon microneedles during in vivo studies has been reported.18 Metal microneedles produced by electroplating possess thin walls and exhibit poor mechanical properties. These devices are prone to buckling of the tip during insertion; failure of metal microneedles has been reported at less than 1 N compressive force.17 Microneedles that are attached to a substrate (e.g., Ormocer® microneedles on a glass substrate) are susceptible to detachment from the substrate and retention in the skin.16 The development of novel materials and fabrication methods for producing microneedles with appropriate mechanical properties for transdermal delivery will enable wider clinical use of this technology.
In this study microneedles containing eShell 200 polymer were created using a 2PP microfabrication and subsequent polydimethylsiloxane (PDMS) micromolding process. LaFratta and associates19 and Li and Fourkas20 demonstrated that a combination of photopolymerization and micromolding may be used to produce three-dimensional microstructures. eShell 200 is a photo-reactive acrylate-based polymer that is suitable for rapid prototyping of functional medical parts.21 It is a rigid, durable polymer produced by Envisiontec (Ferndale, MI) for use in medical applications, including fabrication of thin-walled hearing aid shells. Due to the fact that it exhibits high cross linkage, eShell 200 polymer is suitable for use in environments containing high humidity (e.g., water or perspiration). It exhibits hardness of 83 Shore (D2240 test method), tensile strength of 57.8 MPa (D638M test method), flexural strength of 2300 MPa (D790M test method), Young's modulus of 2400 GPa (D638M test method), elongation at yield of 3.2% (D638M test method), dielectric strength of 14.6 (D149-97a test method), and glass transition temperature of 109 °C (E1545-00 test method).21
The structural, mechanical, and biological properties of eShell 200 microneedles created using a 2PP micro-fabrication and subsequent PDMS micromolding process were examined by scanning electron microscopy, energy dispersive x-ray spectroscopy, compressive force failure testing, and MTT cell viability assay. We anticipate that microneedles fabricated using this high-throughput technique may be used for delivery of insulin and other protein-based pharmacologic agents.
Experimental Procedure
A computer-aided design (CAD) program (DeskArtes Oy, Espoo, Finland) was used to prepare an STL format file for fabrication of the microneedle array master structure. The master structure was a 5 × 5 array of 25 identical solid microneedles (needle height = 500 µm; needle base diameter = 150 µm; needle center to needle center distance = 500 µm).
The 2PP technique was used to create a microneedle master.14,16,22 This process involves nonlinear light absorption, igniting chemical reactions and material hardening within well-defined, highly localized volumes. The 2PP process involves spatial and temporal overlap of photons in order to bring about chemical reactions, which lead to photopolymerization and material hardening within well-defined, highly localized volumes. A titanium: sapphire laser (Kapteyn-Murnane, Boulder, CO) was used to obtain femtosecond laser pulses (60 fs; 94 MHz; <450 mW; 780 nm), which were focused using a 10× plan achromat microscope objective. Nonlinear absorption of laser pulses cleaved chemical bonds of photoinitiator molecules located in a small focal volume within the polymer resin. The radicalized photoinitiator molecules created radicalized polymolecules through interaction with the monomers; reactions were terminated when radicalized polymolecules interacted with other radicalized polymolecules. By moving the laser focus in three dimensions within the photopolymer, material was polymerized along the laser trace. A combination of three C-843 linear translational stages (Physik Instrumente, Karlsruhe, Germany) and a galvo scanner (Scanlab AG, Puchheim, Germany) was utilized in order to alter the laser focus position in three dimensions.
The original microneedle array was fabricated by 2PP of SR 259 polymer (Sartomer, Paris, France) containing 2 wt % Irgacure 369 photoinitiator (Ciba Specialty Chemicals, Basel, Switzerland) on a glass cover slip. SR 259 is a polyethylene glycol (200) diacrylate that exhibits low volatility, a refractive index of 1.4639, viscosity of 25 cps at 25 °C, surface tension of 41.3 dynes/cm, and a molecular weight of 302.23 The Irgacure® 369 photoinitiator exhibits an absorption peak at ∼λ = 320 nm. The microneedle array was subsequently sputter coated with gold (coating time = 245 s; coating current = 10 mA) to improve separation of the master structure from the mold. The glass cover slip containing the microneedle array was then fixed to a glass microscope slide using double-sided tape.
A negative mold of the microneedle array was fabricated using PDMS. Sylgard® 184 silicone elastomer and hardening agent (Dow Corning, Midland, MI) were prepared according to manufacturer instructions. The liquid mixture was subsequently degassed under vacuum. After degassing, a 20 mm diameter open center aluminum crimp seal (Sigma Aldrich, St. Louis, MO) was placed on the glass with the microneedle array located in the center of the ring. The unpolymerized silicone elastomer was poured over the microneedle array until the aluminum ring was completely filled. The glass slide with microneedle array, aluminum ring, and silicone elastomer were placed under 100 mbar vacuum in order to remove residual gas voids. The sample, consisting of a glass slide, microneedle array, aluminum seal, and silicone, was subsequently placed on a hot plate for PDMS cross linking. The hot plate temperature was increased from 25 to 100 °C over 30 min and then maintained at 100 °C for 30 min. Once the polymerized mold had cooled, it was mounted onto a C843 linear translational stage (Physik Instrumente, Karlsruhe, Germany) in order to separate the mold from the master structure. The glass slide was held in place against the table using two metal restraining bars, while the aluminum seal containing the PDMS mold was vertically moved by the stage. A second PDMS mold (depth = 2 mm; diameter = 1 cm) was used for molding the substrate.
Approximately 50 µl of eShell 200 polymer (Envisiontec, Gladbeck, Germany) was placed on the surface of the PDMS mold over the microneedle array. The mold containing eShell 200 polymer was subsequently degassed under vacuum in order to allow the eShell 200 polymer to completely fill the mold. The cylindrical substrate mold was then filled with eShell 200 polymer, and the microneedle mold was placed on top of it. The two molds were placed in contact, with the substrate mold facing up and the microneedle mold facing down so that the two surfaces with eShell 200 were in contact. The molds were then exposed for 2 min to an ultraviolet curing lamp, which provided visible and ultraviolet light emission (Thorlabs, Newton, NJ). The molds were subsequently inverted; the substrate side was exposed to ultraviolet light for 1 min. After ultraviolet curing, the molds and polymerized microneedle arrays were separated by hand.
Images of the eShell 200 microneedles were obtained using a S3200 scanning electron microscope (Hitachi, Tokyo, Japan), which was equipped with a Robinson back-scattered electron detector. Energy dispersive x-ray spectroscopy was performed to determine the chemical composition of the microneedles. Compression testing of the eShell 200 microneedle arrays was performed using an Electroforce 3100 system (Bose, Eden Prairie, MN). The eShell 200 microneedle arrays were placed directly on the load cell surface. Axial loading was applied to the microneedle array (displacement rate = 10 µm/s) until a force of 10 N was achieved. Compression testing of microneedles against hard surfaces such as polytetrafluoroethylene has been used to examine the mechanical properties of microneedle arrays.14,16,17
Human epidermal keratinocyte viability on polymerized eShell 200 material was examined using the MTT assay.24 The MTT assay is based on reduction of a yellow tetrazolium salt (3-[4,5-dimethylthiazol-2-yl] 2,5-diphenyl tetrazolium bromide) to a purple formazan dye by mitochondrial succinic dehydrogenase. The MTT assay uses mitochondria metabolism as a measure of cell viability. Cylindrical pellets of eShell 200 (n = 3) were created by exposure of the polymer in PDMS molds (diameter = 6 mm; depth = 1 mm) using the same curing lamp and exposure time that were used to fabricate the microneedle arrays. Prior to conducting the MTT assay, the pellets were sterilized by ultraviolet B (UVB) radiation for 3 h, with both sides of the pellets receiving equal UVB light exposure. Cryopreserved human epidermal keratinocytes (Lonza, Walkersville, MD) were passed twice to initiate propagation, and 285,000 human epidermal keratinocytes were seeded in 10 ml of keratinocyte growth medium-2 (KGM-2), consisting of serum-free keratinocyte basal media, which is supplemented with bovine pituitary extract, epinephrine, GA-1000 (gentamicin-amphotericin), human epidermal growth factor, hydrocortisone, insulin, and transferrin (Lonza, Basel, Switzerland). The seeded human epidermal keratinocytes were then cultured at 37 °C and 5% CO2 until 80% confluency was obtained. The cells were then transferred to 96 well plates, in which pellets were placed at the bottom of the wells. The pellets and polystyrene culture wells were washed with 1 ml of KGM-2 prior to seeding. The pellets were held to the bottom of the culture wells by applying a drop of Akwa Tears® (Akorn, Buffalo Grove, IL) to the bottom of the wells prior to pellet placement. The human epidermal keratinocytes were allowed to proliferate for 24 h before the MTT assay was performed. Human epidermal keratinocyte viability was determined by measuring absorbance at λ = 550 nm using a Multiskan RC ELISA plate reader (Labsystems, Helsinki, Finland). Cell viability on the eShell 200 polymer pellets was normalized to surface area and compared with growth on the surface of polystyrene wells with no pellets.
Human abdominal skin obtained from a surgical abdominoplasty was used to assess skin penetration of the microneedles. The use of this tissue received institutional approval according to the Declaration of Helsinki of the World Health Association. The skin was utilized within 24 h of removal. Subdermal fat was mechanically removed via dermatome to achieve uniform layers of full thickness skin. Stratum corneum and epidermal layers were obtained from full thickness skin by a heat separation method. The skin was immersed in distilled water at 60 ± 1 °C for 1 min. Then the stratum corneum and epidermis was gently peeled from the dermis using forceps. Isolated stratum corneum and epidermal layers were dried in a desiccator at ∼30% relative humidity, wrapped in aluminium foil, and stored at −20 ± 1 °C until use. The integrity of the barrier was visually inspected to ensure that the stratum corneum and epidermal layers were unaltered during this process. This method of skin preparation has previously been used to examine microneedle-mediated drug delivery and other transdermal drug delivery techniques.25–28 Microneedles were inserted into the tissue using an electronic texture analyzer (Acquati, Arese, Italy). An eShell 200 microneedle array was placed on the stratum corneum and epidermis sample. The eShell 200 microneedle array was held in place using a polystyrene support and was compressed against the sample using a stainless steel probe (surface area = 1.13 cm2). The probe was moved toward the sample at a rate of 300 mm/min until a maximum load of 4 N was obtained. The microneedles were held in place for 3 s and then removed. Similar protocols for examining microneedle insertion into human stratum corneum and epidermis have been reported in the literature.26,29–31 Optical microscopy was used to examine the microneedle–skin interaction.
Results
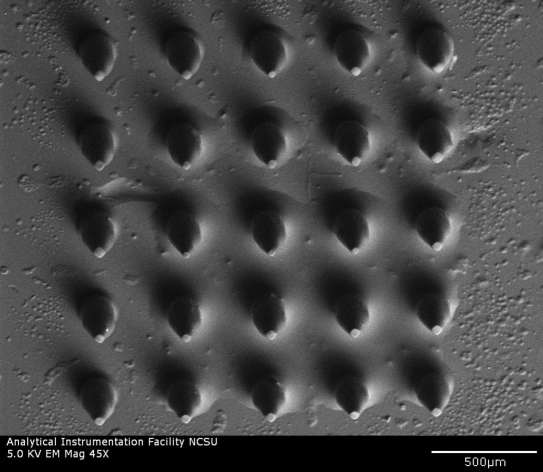
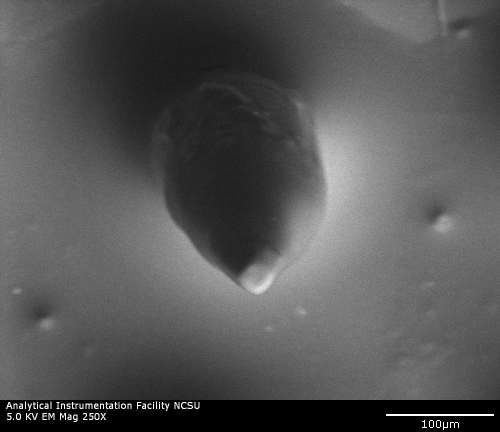
Scanning electron microscopy was used to examine microscale features of microneedles fabricated using the 2PP microfabrication and subsequent PDMS micromolding process. A scanning electron micrograph of a microneedle array is shown in Figure 1a, and an individual microneedle is shown in Figure 1b. The microneedles exhibited length values of 500 µm and base diameter values of 150 µm; good microneedle-to-microneedle uniformity was observed in the microneedle array. The microneedles exhibited tip angle values of 45°. In some cases microneedles in the microneedle arrays demonstrated slight deviations from the dimensions that were specified in the stereolithography CAD file. For example, the tips of some microneedles in the microneedle arrays were slightly truncated. In other cases sharper needle tips were observed. Iteration of processing parameters, including molding materials and mold fabrication parameters, may enable the development of microneedle arrays containing microneedles with more uniform tip diameters. In general, microscale features in the microneedles were consistent with the dimensions specified in the stereolithography CAD file. Energy-dispersive x-ray spectroscopy studies of the microneedle arrays indicated that eShell 200 is composed of 78% carbon, 20% oxygen, and 2% titanium. No trace amount of other elements, including those with known toxicity, was noted. Carbon and oxygen are common components of organic molecules, and titanium exhibits excellent compatibility with human tissues.32
Figure 1a.
Scanning electron micrograph of an eShell 200 microneedle array created using the 2PP microfabrication and subsequent PDMS micromolding process.
Figure 1b.
Scanning electron micrograph of an eShell 200 individual microneedle created using the 2PP microfabrication and subsequent PDMS micromolding process.
Cell growth on the polystyrene control surfaces and the Envisiontec eShell 200 polymer surfaces was examined using an inverted microscope. The Envisiontec eShell 200 polymer surfaces were shown to support human epidermal keratinocyte growth, and 24 h MTT assays indicated that human epidermal keratinocyte growth on the eShell 200 surfaces was similar to that on polystyrene control surfaces (>95%). In addition, these growth values were not significantly different (p < .05). These results suggest that Envisiontec eShell 200 polymer processed using micromolding does not decrease cell viability or cell growth.
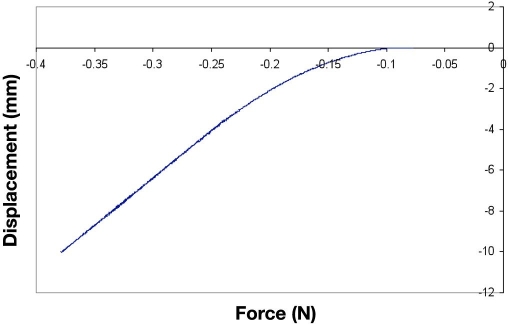
A plot of force versus displacement for the eShell 200 microneedle array obtained during compression testing is shown in Figure 2. None of the 25 eShell 200 microneedles in the microneedle array fractured during axial compression. The 5 × 5 eShell 200 microneedle arrays were able to withstand an axial load of 10 N without fracture, which corresponds to an axial load of 0.4 N per needle. The axial load applied to the Envisiontec eShell 200 microneedle array in this study was more than seven times greater than the force for microneedle insertion into skin that was previously described by Davis and coworkers17 (1.29 N); their study involved microneedles with similar tip diameters. The force versus displacement curve was linear at axial loads between 4 and 10 N, indicating that the microneedle array exhibited elastic deformation and not plastic deformation over these values. These results suggest that eShell 200 polymer microneedles fabricated using the PDMS micromolding process are able to penetrate through human skin without fracture.
Figure 2.
Characteristic plot of force versus displacement for eShell 200 microneedle array obtained during axial compression testing.
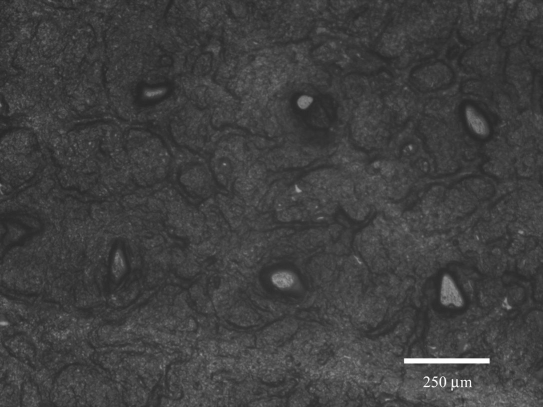
The eShell 200 microneedle arrays were successfully able to create pores in human stratum corneum and epidermis. An image of the pores created by microneedle insertion and removal is shown in Figure 3. Approximately 58 μm diameter pores were observed in the human stratum corneum and epidermis. The pores are irregular in shape and smaller than the microneedle diameter. These features were attributed to anisotropic tensile forces caused by the collagen and elastic fibers in the skin. These forces can contract the skin and thereby reduce the pore size in the stratum corneum. By adjusting the focal height of the microscope, we were able to verify that the needles created pores that completely passed through the stratum corneum layer and entered the deeper layers of the epidermis. The pores became smaller in size but remained open after removal of the microneedle arrays. These open pores may serve as conduits for transport of pharmacologic agents after microneedle removal. Park and colleagues26 reported similar results in a poly(L-glycolic acid) microneedle skin penetration study using the human stratum corneum and epidermis model.
Figure 3.
Optical micrograph of human stratum corneum and epidermis sample after eShell 200 microneedle array insertion and removal.
Conclusions
Polymer microneedles for transdermal delivery of insulin or other protein-based pharmacologic agents were created using a combination of 2PP microfabrication and PDMS micromolding. This approach generally provides high-fidelity reproduction of microscale features; however, some improvement in reproduction of small scale features (e.g., microneedle tips) may be possible. The eShell 200 microneedle array demonstrated suitable compressive strength for use in transdermal drug delivery applications. Human epidermal keratinocyte viability on the eShell 200 polymer surfaces was similar to that on polystyrene control surfaces. In vitro studies demonstrated that microneedle arrays fabricated from eShell 200 successfully produced pores in stratum corneum and epidermis. The microscale pores produced by microneedle–skin interaction may be used to facilitate the diffusion of pharmacologic agents through the skin. Our results suggest that a combination of micromolding and 2PP microfabrication represents a high-throughput approach that may be used to create microneedle structures with appropriate structural, mechanical, and biological properties for transdermal drug delivery of insulin and other protein-based pharmacologic agents. In vitro and in vivo studies are underway to examine whether microneedles prepared using this approach exhibit suitable biological properties for insulin delivery. In vitro permeation studies of various pharmacologic agents through animal and human skin models are also being investigated.25,27,28 Several medical uses for these devices are anticipated. For example, eShell 200 microneedles may be utilized to deliver incretin mimetics and other novel protein-based pharmacologic agents for treatment of diabetes, which cannot be administered in oral form because they may be metabolized in the gastrointestinal tract or in the liver before reaching systemic circulation.
Acknowledgments
The authors thank K. Evaul and A. O. Inman (Center for Chemical Toxicology Research and Pharmacokinetics, North Carolina State University) for their assistance with the MTT assays.
Abbreviations
- 2PP
two-photon polymerization
- CAD
computer-aided design
- KGM-2
keratinocyte growth medium-2
- PDMS
poly-dimethylsiloxane
- UVB
ultraviolet B
References
- 1.Gerstel MS, Place VA. Drug delivery device. U.S. Patent 3,964,482. 1976 Jun 22; [Google Scholar]
- 2.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 3.Gowthamarajan K, Kulkarni GT. Oral insulin—fact or fiction? Possibilities of achieving oral delivery for insulin. Resonance. 2003;8(5):38–46. [Google Scholar]
- 4.Nordquist L, Roxhed N, Griss P, Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration. Pharm Res. 2007;24(7):1381–1388. doi: 10.1007/s11095-007-9256-x. [DOI] [PubMed] [Google Scholar]
- 5.Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006;13(3):175–187. doi: 10.1080/10717540500455975. [DOI] [PubMed] [Google Scholar]
- 6.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24(7):585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92(2):502–504. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 8.Martano W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K, Suzuki H. A sampling mechanism employing the phase transition of a gel and its application to a micro analysis system imitating a mosquito. Sens. Actuators B. 2001;80(1):1–8. [Google Scholar]
- 10.Chun K, Hashiguchi G, Toshiyoshi H, Fujita H. Fabrication of array of hollow microcapillaries used for injection of genetic materials into animal/plant cells. Jpn J Appl Phys. 1999;38(3A):L279–L281. [Google Scholar]
- 11.Griss P, Stemme G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J Microelectromech Syst. 2003;12(3):296–301. [Google Scholar]
- 12.Wilke N, Mulcahy A, Ye SR, Morrissey A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron J. 2005;36(7):650–656. [Google Scholar]
- 13.Gardeniers HJGE, Luttge R, Berenschot EJW, De Boer MJ, Yeshurun SY, Hefetz M, Van't Oever R, van den Berg A. Silicon micromachined hollow microneedles for transdermal liquid. J Microelectromech Syst. 2003;12(6):855–862. [Google Scholar]
- 14.Doraiswamy A, Jin C, Narayan RJ, Mageswaran P, Mente P, Modi R, Auyeung R, Chrisey DB, Ovsianikov A, Chichkov B. Two photon induced polymerization of organic-inorganic hybrid biomaterials for microstructured medical devices. Acta Biomater. 2006;2(3):267–275. doi: 10.1016/j.actbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 15.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100(24):13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ovsianikov A, Chichkov B, Mente P, Monteiro-Riviere NA, Doraiswamy A, Narayan RJ. Two photon polymerization of polymer-ceramic hybrid materials for transdermal drug delivery. Int J Appl Ceramic Technol. 2007;4(1):22–29. [Google Scholar]
- 17.Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of microneedles into skmeasurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37(8):1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Griss P, Tolvanen-Laakso HK, Meriläinen P, Stemme G. Characterization of micromachined spiked biopotential electrodes. IEEE Trans Biomed Eng. 2002;49(6):597–604. doi: 10.1109/TBME.2002.1001974. [DOI] [PubMed] [Google Scholar]
- 19.LaFratta CN, Li L, Fourkas JT. Soft-lithographic replication of 3D microstructures with closed loops. Proc Natl Acad Sci USA. 2006;103(23):8589–8594. doi: 10.1073/pnas.0603247103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Fourkas JT. Multiphoton polymerization. Mater Today. 2007;10(6):30–37. [Google Scholar]
- 21. envisionTEC GmbH. envisionTEC e-Shell 200 Series Technical Data. http://www.envisiontec.com/fileadmin/pdf/MatSheet_eShell200_en_s.pdf. Accessed November 5, 2008.
- 22.Serbin J, Egbert A, Ostendorf A, Chichkov BN, Houbertz R, Domann G, Schulz J, Cronauer C, Fröhlich L, Popall M. Femtosecond laser-induced two-photon polymerization of inorganic-organic hybrid materials for applications in photonics. Opt Lett. 2003;28(5):301–303. doi: 10.1364/ol.28.000301. [DOI] [PubMed] [Google Scholar]
- 23.Sartomer Company, Inc. Product bulletSR-259. http://www.sartomer.com/wpapers/2036.pdf. Accessed November 5, 2008.
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Metth. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Minghetti P, Cilurzo F, Casiraghi A, Montanari L, Santoro A. Development of patches for the controlled release of dehydroepiandrosterone. Drug Dev Ind Pharm. 2001;27(7):711–717. doi: 10.1081/ddc-100107328. [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Minghetti P, Cilurzo F, Casiraghi A, Montanari L, Fini A. Ex vivo study of transdermal permeation of four diclofenac salts from different vehicles. J Pharm Sci. 2007;96(4):814–823. doi: 10.1002/jps.20770. [DOI] [PubMed] [Google Scholar]
- 28.Cilurzo F, Minghetti P, Pagani S, Casiraghi A, Montanari L. Design and characterization of and adhesive matrix based on a poly(ethyl acrylate, methyl methacrylate) AAPS PharmSciTech. 2008;9(3):748–754. doi: 10.1208/s12249-008-9102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stumpp OF, Welch AJ, Gill HS, Prausnitz MR. Jacques SL, Roach WP, editors. OCT analysis of microneedle and Er:YAG surface ablation for enhanced transdermal delivery of hyperosmotic agents for optical skin clearing. 2004;5319:121–129. Laseri with tissue and cells XV. Proc SPIE. [Google Scholar]
- 30.Kumar R, Philip A. Modified transdermal technologies: breaking the barriers of drug permeation via the skin. Tropical J Pharm Res. 2007;6(1):633–644. [Google Scholar]
- 31.Herndon TO, Gonzalez S, Gowrishankar TR, Anderson RR, Weaver JC. Transdermal microconduits by microscission for drug delivery and sample acquisition. BMC Med. 2004;2:12. doi: 10.1186/1741-7015-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn SD, Sridharan K, Hao Z, Muir P, Suresh M, Singh A, Raj SV. Biocompatibility of uncoated and diamond-like carbon coated Ti-20%Hf alloy. Mater Sci Technol. 2008;24(5):575–578. [Google Scholar]