Abstract
Type 1 diabetes (T1DM) is characterized by loss of virtually all endogenous insulin secretion. If residual insulin secretion is preserved, this will lead to improved metabolic balance, less acute and late complications, improved quality of life, and, in case of pronounced improvement of residual insulin secretion, complete remission and even cure of the disease.
Immune suppression or immune modulation have been demonstrated as a proof of principle to stop/decrease the destructive process and thereby preserve beta-cell function. Several methods to save residual beta-cell function have been tried for more than three decades with little or no evidence of efficacy. Positive effects have been seen mainly in adult patients but have been minimal or absent in children with diabetes. Furthermore, the safety of these immune interventions and/or their benefit to risk relationships have not been found to justify clinical use.
More specific immune modulation with anti-CD3 monoclonal antibodies has resulted in more encouraging postponement of C-peptide decline, but with frequent and serious adverse effects. Still more promising are the autoantigen therapies, of which glutamic acid decarboxylase (GAD) vaccination has shown significant preservation of residual insulin secretion in 10–18-year-old type 1 diabetes patients with recent onset. Efficacy was most impressive in the subgroup of patients with diabetes of short duration (<3 months). The treatment was simple, well tolerated, and showed no treatment-related adverse events. If these results can be confirmed, there is a realistic hope that GAD vaccination, perhaps in combination with vaccinations with other autoantigens and/or other therapies, will result in remission for some patients. The prospects of cure and prevention of T1DM will become less remote.
Keywords: autoantigen treatment, C-peptide, glutamic acid decarboxylase vaccination, immune intervention, immunomodulation, type 1 diabetes
Introduction
Type 1 diabetes (T1DM) is characterized by lack of insulin. Even though some patients at diagnosis have quite impressive residual beta-cell function,1 the deficiency soon becomes pronounced and gradually complete. While preschool children may have lost almost all beta-cell function within a year after diagnosis, older school children or adolescents may have some insulin secretion for years2,3 In rare cases of T1DM, the beta-cell function drastically improves shortly after diagnosis, glucose metabolism normalizes and no insulin is required for some time, so-called complete remission. This is more often reported in certain countries.4
It is evident that, as long as the patient is in a complete remission, there is no need for insulin replacement therapy. Maintaining a lifestyle that includes physical exercise and a healthy diet is prudent. While patients in remission are asymptomatic, the long-term significance of chemical diabetes or impaired glucose tolerance is not clear.
Partial remission in T1DM is much more common than complete remission. If partial remission is defined as the “honeymoon” phase, with very stable blood glucose, then partial remission probably occurs in most patients with diabetes, at least for a few months;4 more often in older patients. During this period the patient usually has near-normal blood glucose values, no hypoglycemia, no episodes of ketoacidosis, and a very good quality of life. Insulin requirement is low, usually <0.5 U/kg body weight in 24 h, even in a child. This low insulin requirement in combination with near-normal glycemic control implies adequate residual insulin secretion.
Autoimmune diabetes includes not only T1DM but also slowly progressive insulin-dependent diabetes mellitus (SPIDDM)5 and latent autoimmune diabetes in adults (LADA). Latent autoimmune diabetes in adults is similar to and is frequently confused with type 2 diabetes but is distinguished by the presence of autoantibodies, mainly glutamic acid decarboxylase (GAD) autoantibodies (GADA). In contrast to classic type 2 diabetes, LADA patients usually become insulin dependent at much faster rates. Some view LADA as a distinct entity and type of diabetes; others believe that LADA is just a mild variant of T1DM and should be treated as such.
In Asia, especially Japan, characteristics of SPIDDM have been described as follows: autoimmune destruction is more gradual, and insulin secretion relatively preserved at diagnosis.
Also in Japan, a more fulminant process leading to diabetes occurs, which is characterized by very rapid and complete destruction of the beta cells and complete loss of residual insulin secretion, but without clear evidence of autoimmunity.6 Similar cases are also described sporadically in other parts of the world. It should be noted that some 5–10% of newly diagnosed T1DM children have no signs of autoimmunity but have an idiotypic form of insulin-dependent T1DM.
In addition to T1DM, 0.5–1% of children with juvenile-onset diabetes may have different forms of maturity-onset diabetes in the young, some of whom should actually not be treated with insulin.
Residual Insulin Secretion is of Great Importance
By definition, a complete remission of diabetes means that endogenous insulin secretion alone is adequate for maintaining euglycemia. The honeymoon phase represents a partial remission and is characterized by relative ease of treatment with insulin, more stable glycemia, and few or no symptoms. Among patients with established T1D, a small amount of preserved residual insulin secretion is important in contributing to lower HbA1c, less blood glucose fluctuations, and diminished risk of ketoacidosis.7 A rather modest residual insulin secretion, shown as a response to a beta-cell stimulation with serum C-peptide >0.20 pmol/ml, decreases the risk of both serious hypoglycemia and late complications.8 In addition, C-peptide per se may have a physiological function.9 Thus it has been reported that C-peptide influences vascular permeability, decreases leakage in retinal vessels, and has a positive effect on nerve function.
Interventions to Preserve Residual Insulin Secretion
Guidelines for intervention trials in newly diagnosed T1DM patients have been published,10 but long before those guidelines existed, different forms of intervention have been tried. Active insulin treatment begun soon after diagnosis of T1DM was found to prolong the partial remission period. This finding was confirmed and validated by improved residual insulin secretion.2 Intensified treatment seems to improve residual beta-cell function, at least for some time,11 but it may also have long-term positive effects.12 Active insulin treatment has been shown to prevent or postpone diabetes in experimental animals, and studies have indicated that such treatment could prevent diabetes in high-risk individuals.13 Some evidence suggests that administration of insulin, one of the main autoantigens implicated in the pathogenesis of T1D, may itself affect the immune system and may in some way protect the beta cells from the destructive immune process. However, when tried on a larger scale in the Diabetes Prevention Trial, daily subcutaneous insulin administration did not prevent diabetes.14 An oral insulin treatment arm in that trial was associated with a trend toward reduced occurrence of diabetes.15 These findings suggested that further trials of immunomodulation were needed; leading to the establishment of TrialNet and commencement of a new trial with oral insulin. Nasal insulin has been used to modify the immune response and create tolerance, but no effect has been observed.16
In accordance with the idea that beta-cell rest via active insulin treatment might protect the beta cells, agents blocking the insulin secretion have been tested. Diazoxide, primarily an antihypertensive drug, blocks endogenous insulin secretion, leading to beta-cell rest, which seems to prolong the residual beta-cell function in adult T1DM patients.17 However, when this drug was tried in children, it caused adverse events (AEs), and it only postponed the decline of beta-cell function for a limited time. The total C-peptide area under the curve (AUC) remained the same for diazoxide treatment as for placebo.18
Immunotherapies and Beta-Cell Protection
The first immune intervention at diagnosis of T1DM in children and adolescents was plasmapheresis, which started at the end of the 1970s. It showed a positive effect on preservation of residual insulin secretion19 in comparison with controls, but it was not a double-blind randomized trial. The use of cyclosporin has been regarded as the breakthrough and proof of concept, as cyclosporine certainly showed a significant preservation of insulin secretion.20 However, the AEs were too serious to allow clinical use. Since then several other forms of immune intervention have been tried (immunoglobulins,21 azathioprine,22 linomide,23 antithymocyte globulin and prednisone,24 photopheresis,25,26 and antioxidants27) but with too limited effect and/or too serious risks or AEs. Nicotinamide has also failed for prevention of T1DM.28
More specific immunotherapy has also been tried. When antigen is presented to the T cells, one of the important receptors is the CD3 receptor. Monoclonal antibodies against this receptor can be expected to block or at least modulate the immune process. Both North American and French studies using monoclonal anti-CD3 antibodies have shown that it is possible to block the destructive autoimmune process and thereby at least postpone the decline of beta-cell function.29,30 The decline of residual insulin secretion is significantly slowed down, but with the protocols used so far, it appears that the decline of residual insulin secretion is postponed just a year, as the declining C-peptide curve one year after treatment is parallel to that of the placebo group. To determine if the effect can be prolonged with a booster treatment, further studies are ongoing, where the initial treatment is followed by a booster treatment period six months later.
The anti-CD3 treatment is perhaps the most efficacious immune modulation at this time, but it is not specific enough to avoid side effects. A majority of patients experienced some degree of cytokine release syndrome. A number of side effects are seen in most patients, such as nausea, fever, muscle pain, thrombocytopenia with risk of bleedings, leukocytopenia with increasing frequency of infections, and anemia. Of particular note, all treated patients converted to Epstein–Barr virus positivity. This raises concerns about long-term AEs such as lymphomas. As the possible benefit of preserving residual insulin secretion is very important, some safety risks can be accepted. It will be more difficult to justify treatment with therapies that carry substantial risk in children and adolescents. Some young patients hesitate to accept treatment because of the long and intensive treatment. Even adults hesitate to accept a treatment that carries significant risks and burden without evidence that the effect on preservation of insulin secretion is long lasting.
Autologous nonmyeloablative hematopoietic stem-cell transplantation, a more heroic form of immune intervention, has been performed in 15 newly diagnosed T1DM patients aged 14–31 years.31 Five patients were reported insulin-free after more than 21 months, and another 7 were reported insulin-free after more than 6 months. These results have to be weighed against serious adverse events (SAEs) observed in several of the patients and serious potential risks, since this type of treatment has caused acute mortality when used for other autoimmune diseases. In addition, use of such heavy cytostatic treatment (e.g., cyclophosphamide 2 g/m2 body surface) causes substantial risk of late AEs such as secondary cancer. Thus more studies in well-informed adult T1DM patients are needed before this type of treatment can be regarded as ethically and clinically justified, especially in younger patients.
Autoantigen Treatment
From the field of allergen desensitization, it has long been known that exposure of specific amounts of the actual antigen can cause modulation of the immune system, resulting in reduction or prevention of the allergic reaction. This phenomenon appears to be, in part, mediated by increased T-cell regulation.32 Studies of animal models of autoimmune diabetes have shown that treatment with autoantigens may delay or postpone/prevent development of diabetes.
Beside insulin, a number of agents are under development to prevent immune attack of beta cells by modulating the immune system. As a putative shared mechanism, these therapies shift the balance among the CD4+ T cells from the Th1 state (characterized by “attacking” killer T cells) to the Th2 state (characterized by cytokines that inhibit inflammation). This intended Th1–Th2 shift should result in reduced proinflammatory cytokines and increased regulatory T-cells that release inhibitors of inflammation.33
One immodulatory approach is a synthetic peptide sequence of an endogenous heat shock protein 60, Diapep 277 (AndroMeda Biotech, Ness Ziona, Israel). This agent has reached phase-3 trials of adults with new-onset T1DM. Treatment has been associated with significant preservation of insulin secretion and no apparent drug-related AEs.34 However, these results remain to be confirmed in ongoing trials. Studies of children and adolescents with T1DM have shown no effect,35 which could possibly be explained by the more intense autoimmunity typically seen with diabetes onset in the younger ages. Studies with Diapep 277 treatment in LADA patients are ongoing.
Heat shock proteins probably cannot be regarded as pure autoantigens in the diabetes autoimmune process; at least they are not beta-cell specific. Insulin is clearly a beta-cell-specific autoantigen, and as mentioned earlier, it has been administered subcutaneously to prevent T1DM in high-risk individuals without effect. It would also be unclear whether daily insulin given subcutaneously in rather large doses would have effects mediated by immune modulation or simply beta-cell rest. In either case, insulin had no effect in preventing diabetes in high-risk individuals, even though active insulin treatment at diagnosis of T1DM has been shown to preserve residual insulin secretion.10 This seems to be the case for LADA patients36 and for SPIDDM.37 Representing more specific immune intervention, insulin was given orally to prevent T1DM. Efficacy results were negative,14 but unfortunately the inclusion criteria were changed during the ongoing study. Later subanalyses of the results, which maintained the initial inclusion criteria (autoantibodies to insulin >80 nU/ml), revealed that treated individuals developed diabetes at significantly lower rates than individuals in the placebo group.14 New studies have been started by TrialNet to evaluate the original hypothesis. Nasal insulin has also been tried for the prevention of diabetes in high-risk children in a large randomized double-blind study in Finland, but there was no difference between treatment groups.16 However, efficacy in preventing or stopping the autoimmune process may be very sensitive to dose and route of administering insulin and other autoantigens.38
Immunomodulation with Glutamic Acid Decarboxylase
The Physiological Role of Glutamic Acid Decarboxylase
In the central nervous system, gamma-aminobutyric acid (GABA) is one of the important neurotransmitters. It is formed on demand when glutamic acid, or glutamate, is decarboxylated by the enzyme, GAD.
As GABA is an inhibitory neurotransmitter, loss of GAD activity and decrease of GABA synthesis from glutamate can result in loss of GABAergic modulation of signaling, which may lead to hyperactivity and seizures. A reduction of GABA in brain levels has been demonstrated in patients with stiff person syndrome (SPS). This syndrome is a very rare disorder characterized by muscle rigidity and episodic spasms. Anti-GAD antibodies are found in high titers in most SPS patients,39 but patients with SPS and T1DM differ both in the epitope recognition and the isotype pattern of autoantibodies to GAD65.40
Glutamic acid decarboxylase also exists in the pancreas,41 although its functional role in the pancreas is unknown. It has been suggested that GABA regulates hormone release in the pancreas and/or functions as a paracrine signaling molecule for communication between the beta cells and other endocrine cells in the islets. Other studies suggest that GABA, generated by GAD65, may function as a negative regulator of first-phase insulin secretion in response to glucose.42
Rationale for Glutamic Acid Decarboxylase Treatment of Type 1 Diabetes
During our studies with plasmapheresis,19 we discovered a new 64 kD antigen in the serum of children with diabetes.41 Later this antigen was shown to be an isoform of GAD.42 Glucose stimulation of the beta cells leads to the release of GAD.43 The reason why GAD is a major autoantigen in autoimmune diabetes is not known. Antibodies against two isoforms of GAD have been described.44 Autoantibodies to GAD (GADA) may be an early sign of the autoimmune process of diabetes, and GADA has become one of the most important predictive markers of T1DM risk.45,46 In autoimmune diabetes a T-cell response against the beta cells seems to be crucial.47–51 T-cell reactivity to GAD65 peptide is shared with a protein of the Coxsackie virus, which itself has been implicated as an environmental trigger of T1DM.52–54
Glutamic acid decarboxylase vaccination is intended to modulate the immune system and thereby prevent the destruction of beta cells. Studies of nonobese mice with diabetes show that administration of the GAD65 isoform can prevent autoimmune destruction of beta cells.55–65 These findings suggest that GAD65 administration could be used as a preventive treatment for T1DM.
A GAD vaccine (Diamyd®, Diamyd Medical AB, Stockholm, Sweden) with aluminum hydroxide (alum) as adjuvant has been produced and is now being investigated in phase-3 trials. Aluminum salts enhance the presentation of antigens to antigen-presenting cells. Injected GAD65 is processed by antigen-presenting cells to provide peptide fragments recognized by T cells. This results in a Th1/Th2 shift consisting of induction and proliferation of a subset of GAD65-specific regulatory T cells. These specific T cells down-regulate antigen-specific killer T cells that would otherwise attack the beta cells.
A standard animal toxicology program has not revealed any concerns for clinical safety, even at high-dose levels, nor has any toxicity of target organs been observed. Evaluation of the effects of GAD65 in several different animal models of autoimmune disease did not indicate any undesirable effects on the immune system.
In 1995, a skin-prick test was performed in 15 T1DM subjects and healthy controls, and it showed no skin reactions or other AEs. In 1999, administration of recombinant human GAD65 (rhGAD65) was shown to be safe and well tolerated in a phase-1 study in healthy male volunteers. There were no treatment-related AEs or SAEs at any dose level.
Results from Clinical Trials in Latent Autoimmune Diabetes in Adults
Diamyd has been evaluated in 47 LADA subjects. This randomized double-blind placebo-controlled phase-2a study demonstrated efficacy in preventing beta-cell destruction in the 20 μg group.66 Fasting C-peptide levels at 24 weeks were increased compared with placebo (p = .0015) in the 20 μg dose group but not in the other dose groups. In addition, both fasting (p = .0081) and stimulated (p = .0236) C-peptide levels increased from baseline to 24 weeks in the 20 μg dose group. The number of patients was very small, but this result is still encouraging. There were no SAEs during the 6-month study period. A minority of injections resulted in injection-site reactions, which were mild, and most, in particular, “tenderness,” occurred primarily on the day of the injections. These findings support the safety of immunomodulation by GAD65 immunization. The differences in favor of the GAD vaccination remained after a 5-year follow-up in 2008, and there have been very few AEs, and none have been considered to be treatment related.67
Results from a Phase-2 Trial in Type 1 Diabetes
To investigate safety and efficacy of Diamyd in T1DM, a phase-2 clinical trial of 70 recently diagnosed T1DM children and adolescents was conducted.68 The study was a randomized double-blind placebo-controlled multicenter study with the same dose regimen associated with encouraging results in the previous LADA trial.66 The prespecified main study period of 15 months was completed, and the trial was partly unblinded for sponsor and statistician in August 2006, but it was continued blinded for all investigators for another prespecified 15-month follow-up.
During the study period there were four SAEs: three in the placebo group and one in the treated group. None of the SAEs was considered to be treatment related. During the follow-up period, a few additional SAEs were reported both in the Diamyd group and in the placebo group, none of which was considered to be treatment related. The frequency and pattern of reported AEs during the 15-month main study period did not differ significantly between placebo and active treatment groups. Only in two subjects was the AE judged as possibly related to the study drug. Both subjects were in the active treatment group: one mild and one moderate hypoglycemia. In both study groups, mild discomfort was reported at the sites of injection. A neurological examination was performed at study day 1 as well as at month 15. In all cases but two, the results were normal: one patient in the placebo group had a restricted patellar reflex at month 15, and one patient in the active treatment group had a missing Achilles reflex at day 1 (but normal at month 15).
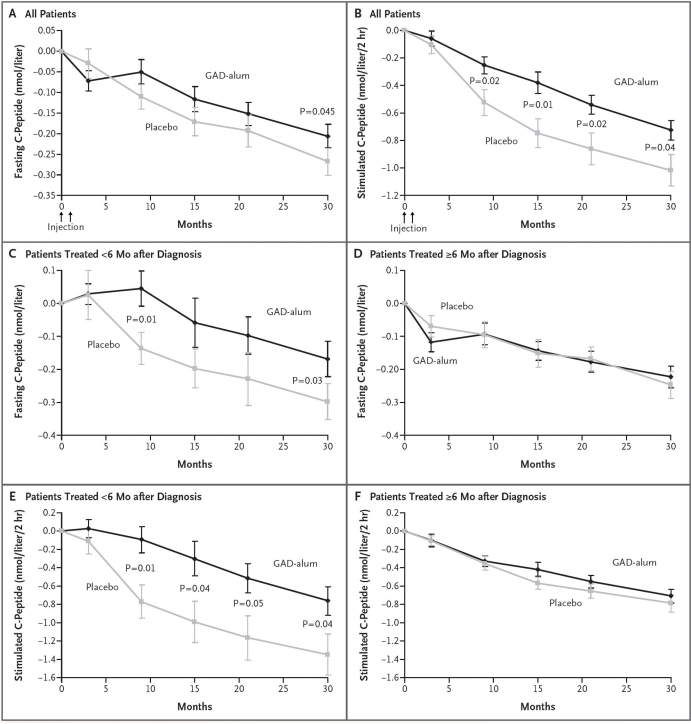
Residual insulin secretion was followed by a mixed meal tolerance test (MMTT) at each follow-up.69 Both groups showed a progressive decline of both fasting and stimulated C-peptide secretion. There was no significant effect of treatment on change in fasting C-peptide at 15 months (prespecified primary endpoint), but there was at 30 months (p = .045) (Figure 1), which was also seen when change in C-peptide/plasma glucose ratio was taken into account (p = .02). Stimulated C-peptide secretion, AUC, decreased significantly less in the Diamyd-treated group than in the placebo group, both after 15 months (p = .01) and 30 months (p = .04). The statistically significant effect of treatment on change in fasting and stimulated C-peptide at month 30 remained after adjusting for differences in duration of diabetes, age, gender, and baseline GADA levels.
Figure 1.
Mean changes from baseline levels of fasting and stimulated C-peptide according to treatment group and time of treatment relative to diagnosis. Mean changes from baseline in (A) fasting and (B) stimulated C-peptide levels are given for all patients included in intention-to-treat analyses in the group receiving the recombinant human 65 kD isoform of GAD in a standard vaccine formulation with alum (GAD alum, 35 patients) and in the group receiving placebo (34 patients). Mean changes from baseline in (C) fasting and (E) stimulated C-peptide levels are also shown for patients treated less than 6 months after receiving the diagnosis of diabetes (11 patients in the GAD alum group and 14 patients in the placebo group). Mean changes from baseline in (D) fasting and (F) stimulated C-peptide levels are shown for those treated 6 months or more after diagnosis (24 patients in the GAD alum group and 20 patients in the placebo group). Stimulated C-peptide level was measured on the basis of AUC in response to the MMTT. The bars indicate standard errors. To convert values for C-peptide to nanograms per milliliter, divide by 0.33. Reproduced from Reference 68 with permission from New England Journal of Medicine.
Insulin requirement in both treatment groups increased gradually, while HbA1c and plasma glucose levels increased. HbA1c did not differ between the groups, but that was not expected, as a low HbA1c was intended for all patients. The significant effect on fasting and stimulated C-peptide secretion (AUC) was only seen in patients with less than 6 months duration at treatment.
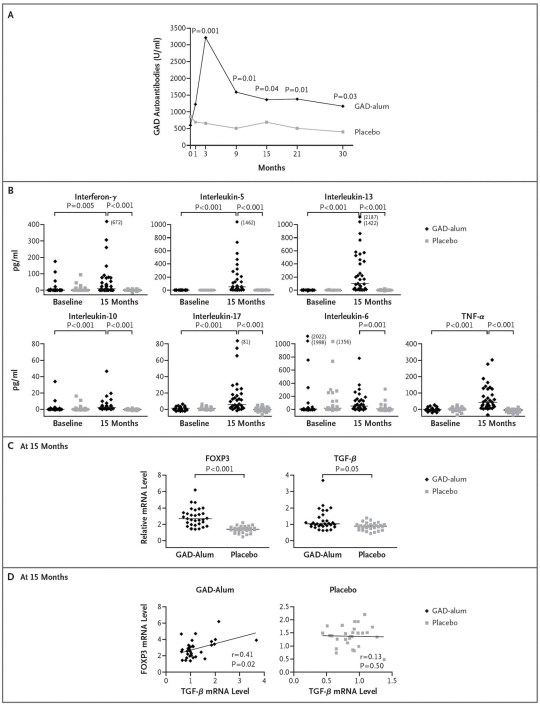
In the group treated with Diamyd, GADA levels increased rapidly, reached a maximum at 3 months, and then decreased but remained significantly higher than in the placebo group (Figure 2).
Figure 2.
Effects on the Immune System. (A) The median titers of autoantibodies against the 65 kD isoform of GAD, from baseline to month 30, in the group receiving recombinant human GAD in a standard vaccine formulation with alum (GAD alum) (35 patients) and in the group receiving placebo (34 patients). (B) The GAD-induced secretion of cytokines in peripheral-blood mononuclear cells at baseline and at 15 months. The outlier values exceed the plotted values and are thus given in parentheses. (C) The GAD-induced messenger RNA levels for the transcription factor forkhead box P3 and transforming growth factor β in peripheral-blood mononuclear cells at 15 months. The levels are a measure of the relative transcription based on the comparative cycle-threshold method. (D) The correlations between relative levels of messenger RNA transcripts of forkhead box P3 and transforming growth factor β in peripheral-blood mononuclear cells at 15 months (Spearman's rank correlation). In (B) and (C), individual data points are shown along with medians, indicated by horizontal lines. P values in (A), (B), and (C) were calculated using the Mann–Whitney U test with Bonferroni correction. In (B) data were available at baseline and at 15 months for 27 patients and 33 patients, respectively, in the GAD alum group and 26 patients and 29 patients, respectively, in the placebo group. In (C) and (D), data were available for 31 patients in the GAD alum group and 29 patients in the placebo group. TNF-α, tumor necrosis factor α; FOXP3, forkhead box P3; TGF-β, transforming growth factor β; mRNA, messenger RNA. Reproduced from Reference 68 with permission from New England Journal of Medicine.
Spontaneous and phytohemagglutinin-induced secretion of all cytokines was similar in samples from children receiving GAD alum and placebo before and 15 months after the first injection. Cytokine secretion of interleukin IL-5, IL-6, IL-10, IL-13, IL-17, IFN-γ, and tumor necrosis factor α, but not of IL-12, in response to in vitro stimulation with GAD65 increased significantly in Diamyd-treated patients from baseline to month 15 (Figure 2).
Ongoing Studies with Glutamic Acid Decarboxylase Vaccination
Two phase-3 trials of Diamyd in T1DM have begun: one in Europe (Johnny Ludvigsson, principal investigator) and one in the United States (Jerry Palmer, principal investigator), both with the same design. In each trial, >300 patients aged 10–20 years with T1DM for at most 3 months, a fasting C-peptide >0.1 pmol/ml, and GADA positivity are randomized in a double-blind controlled trial into three arms. In one arm, the patients are given Diamyd 20 μg subcutaneously at days 1, 30, 90, and 270, the patients in the second arm are given 20 μg GAD65 alum (Diamyd) at days 1 and 30 but then given placebo at days 90 and 270, and the patients allocated to the third arm are given placebo at all four time points. The patients will be followed for 30 months. The primary endpoint is the change from baseline (visit 2) to month 15 (visit 6) in C-peptide (AUCmean 0–120 min) during a MMTT.67 Secondary endpoints, among others, include the following:
HbA1c change between baseline and subsequent visits and
Exogenous insulin dose per kilogram body weight in 24 h, change between baseline and subsequent visits.
Mechanistic studies of both humoral and cell-mediated immunity are ongoing.
In addition to these studies, another intervention trial in newly diagnosed T1DM patients is organized by TrialNet. The primary objective of this trial is to study the effect of GAD vaccination on the immune system, and in this trial the age range is broader (3–45 years). More knowledge is certainly needed to know whether the stimulation of IL-17 or other effects on the immune system in some patients may have a negative rather than a protective effect on the preservation of beta-cell function. Other studies are also planned, e.g., studies combining GAD vaccination with drugs supposed to stimulate beta-cell regeneration/proliferation.
Studies on the use of Diamyd treatment to prevent T1DM in high-risk individuals have been approved and will begin in Europe, and are in discussion in the United States. Positive effects can be expected, but before such studies are done, we can not know if GAD alum treatment will prevent diabetes or whether treatment in some individuals might even have negative effects, e.g., lead to hypersensitivity. Even though it is impossible to completely rule out risks with GAD alum treatment, these risks seem low in relation to the well-known risk of manifest T1D.
Conclusions
Preserved residual insulin secretion is of great importance, as it facilitates the treatment of T1DM and contributes to better metabolic control, better quality of life, and reduced acute and late complications. Immune intervention can protect beta cells from autoimmune destruction and, combined with interventions that improve beta-cell function or number, could lead to complete remission or even cure of the disease.
Effective immune intervention could prevent diabetes in high-risk individuals. Glutamic acid decarboxylase vaccination, as well as treatment with anti-CD3 monoclonal antibodies, has shown encouraging results in preservation of residual insulin secretion in recent-onset T1DM patients. Glutamic acid decarboxylase vaccination was very well tolerated and did not cause treatment-related AEs. Future studies will show if these promising effects can be confirmed. It is plausible that GAD and other immunomodulatory treatments can be improved and/or, in some cases, be combined with each other or with other therapies.70,71 Optimized treatment regimen and/or combination with other treatments may lead to even better results than seen to date. These therapies could result in clinically meaningful preservation of insulin secretion or complete remission. Even the cure and prevention of T1DM seems to be within the scope of possibility.
Acknowledgments
The Linköping Diabetes Immune Intervention Study Group includes Johnny Ludvigsson, M.D., Ph.D., Rosaura Casas, Ph.D., Maria Faresjö, Ph.D., Stina Axelssom, Mikael Cheramy, Maria Hjort, and Mikael Pihl.
Thanks to all patients and parents who have participated in the different trials. I am also grateful to the research nurses, such as Eva Cornell, Iris Franzén, Eva Isacsson, and Ann-Marie Sandström; to a number of skillful people at the laboratory, including Sonja Bergman, Lena Berglert, Gosia Smolinska, and Ingela Johansson; and to and a number of good Ph.D. students. Associate Professor Maria Faresjö has been an important member of the group, first as a Ph.D. student and later as post.doc., with good skills in studies of the immune system.In recent years Rosaura Casas has been the head of our laboratory, which she has led with enthusiasm and great competence.
Abbreviations
- AE
adverse event
- AUC
area under the curve
- GABA
gamma-aminobutyric acid
- GAD
glutamic acid decarboxylase
- GADA
glutamic acid decarboxylase autoantibodies
- IL
interleukin
- LADA
latent autoimmune diabetes in adults
- MMTT
mixed-meal tolerance test
- SAE
serious adverse event
- SPIDDM
slowly progressive insulin dependent diabetes mellitus
- SPS
stiff person syndrome
- T1DM
type 1 diabetes
References
- 1.Nordwall M, Ludvigsson J. Clinical manifestations and beta cell function in Swedish diabetic children have remained unchanged during the last 25 years. Diabetes Metab Res Rev. 2008;24(6):472–479. doi: 10.1002/dmrr.871. [DOI] [PubMed] [Google Scholar]
- 2.Ludvigsson J, Heding LG, Larsson Y, Leander E. C-peptide in juvenile diabetics beyond the postinital remission period. Relation to clinical manifestations at onset of diabetes, remission and diabetic control. Acta Paediatr. Scand. 1977;66(2):177–184. doi: 10.1111/j.1651-2227.1977.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson J, Heding LG. Beta-cell function in children with diabetes. Diabetes. 1978;27(Suppl 1):230–234. doi: 10.2337/diab.27.1.s230. [DOI] [PubMed] [Google Scholar]
- 4.Pozzilli P, Manfrini S, Buzzetti R, Lampeter E, Leeuw ID, Iafusco D, Prisco M, Ionescu-Tirgoviste C, Kolouskovà S, Linn T, Ludvigsson J, Madàcsy L, Mrozikiewicz AS, Mrozikiewicz PM, Podar T, Vavrinec J, Vialettes B, Visalli N, Yilmaz T, Browne PD. IMDIAB Group Glucose evaluation trial for remission (GETREM) in type 1 diabetes: a European multicentre study. Diabetes Res Clin Pract. 2005;68(3):258–264. doi: 10.1016/j.diabres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Maruyama T, Shimada A, Kasuga A, Kanatsuka A, Takei I, Tanaka S, Yokoyama J. Insulin intervention to preserve beta cells in slowly progressive insulin-dependent (type 1) diabetes mellitus. Ann N Y Acad Sci. 2002;958:117–130. doi: 10.1111/j.1749-6632.2002.tb02954.x. [DOI] [PubMed] [Google Scholar]
- 6.Imagawa A, Hanafusa T. Fulminant type 1 diabetes mellitus. Endocr J. 2006;53(5):577–584. doi: 10.1507/endocrj.kr-72. [DOI] [PubMed] [Google Scholar]
- 7.Madsbad S, Alberti KG, Binder C, Burrin JM, Faber OK, Krarup T, Regeur L. Role of residual insulin secretion in protecting against ketoacidosis in insulin-dependent diabetes. Br Med J. 1979;2(6200):1257–1259. doi: 10.1136/bmj.2.6200.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care. 2003;26(3):832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 9.Wahren J, Ekberg K, Jörnvall H. C-peptide is a bioactive peptide. Diabetologia. 2007;50(3):503–509. doi: 10.1007/s00125-006-0559-y. [DOI] [PubMed] [Google Scholar]
- 10.Greenbaum CJ, Harrison LC Immunology of Diabetes Society. Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes. 2003;52(5):1059–1065. doi: 10.2337/diabetes.52.5.1059. [DOI] [PubMed] [Google Scholar]
- 11.Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989;320(9):550–554. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- 12.The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized controlled trial. Ann Intern Med. 1998;128(7):517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Keller RJ, Eisenbarth GS, Jackson RA. Insulin prophylaxis in individuals at high risk of type 1 diabetes. Lancet. 1993;341(8850):927–928. doi: 10.1016/0140-6736(93)91215-8. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 15.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care. 2005;28(5):1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 16.Näntö-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipilä JI, Haavisto L, Siltala M, Tuominen J, Hakalax J, Hyöty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372(9651):1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 17.Björk E, Berne C, Kämpe O, Wibell L, Oskarsson P, Karlsson FA. Diazoxide treatment at onset preserves residual insulin secretion in adults with autoimmune diabetes. Diabetes. 1996;45(10):1427–1430. doi: 10.2337/diab.45.10.1427. [DOI] [PubMed] [Google Scholar]
- 18.Ortqvist E, Björk E, Wallensteen M, Ludvigsson J, Aman J, Johansson C, Forsander G, Lindgren F, Berglund L, Bengtsson M, Berne C, Persson B, Karsson FA. Temporary preservation of beta-cell function by diazoxide treatment in childhood type 1 diabetes. Diabetes Care. 2004;27(9):2191–2197. doi: 10.2337/diacare.27.9.2191. [DOI] [PubMed] [Google Scholar]
- 19.Ludvigsson J, Heding L, Liedén G, Marner B, Lernmark A. Plasmapheresis in the initial treatment of insulin-dependent diabetes mellitus in children. Br Med J (Clin Res Ed) 1983;286(6360):176–178. doi: 10.1136/bmj.286.6360.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupré J, Stiller CR, Gent M, Donner A, von Graffenried B, Heinrichs D, Jenner M, Keown P, Mahon J, Martell R. Clinical trials of cyclosporin in IDDM. Diabetes Care. 1988;11(Suppl 1):37–44. [PubMed] [Google Scholar]
- 21.Heinze E. Immunoglobulins in children with autoimmune diabetes mellitus. Clin Exp Rheumatol. 1996;14(suppl 15):S99–S102. [PubMed] [Google Scholar]
- 22.Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988;319(10):599–604. doi: 10.1056/NEJM198809083191002. [DOI] [PubMed] [Google Scholar]
- 23.Coutant R, Landais P, Rosilio M, Johnsen C, Lahlou N, Cahatelain P, Carel JC, Ludvigsson J, Boitard C, Bougnières PF. Low dose linomide in type I juvenile diabetes of recent onset: a randomised placebo-controlled double blind trial. Diabetologia. 1998;41(9):1040–1046. doi: 10.1007/s001250051028. [DOI] [PubMed] [Google Scholar]
- 24.Eisenbarth GS, Srikanta S, Jackson R, Rabinowe S, Dolinar R, Aoki T, Morris MA. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res. 1985;2(6):271–276. [PubMed] [Google Scholar]
- 25.Ludvigsson J, Samuelsson U, Ernerudh J, Johansson C, Stenhammar L, Berlin G. Photopheresis at onset of type 1 diabetes: a randomised, double blind, placebo controlled trial. Arch Dis Child. 2001;85(2):149–154. doi: 10.1136/adc.85.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faresjö M, Ernerudh J, Berlin G, Garcia J, Ludvigsson J. The immunological effect of photopheresis in children with newly diagnosed type 1 diabetes. Pediatr Res. 2005;58(3):459–466. doi: 10.1203/01.pdr.0000176906.42001.c3. [DOI] [PubMed] [Google Scholar]
- 27.Ludvigsson J, Samuelsson U, Johansson C, Stenhammar L. Treatment with antioxidants at onset of type 1 diabetes in children: a randomized, double-blind placebo-controlled study. Diabetes Metab Res Rev. 2001;17(2):131–136. doi: 10.1002/dmrr.176. [DOI] [PubMed] [Google Scholar]
- 28.Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. 9413. Vol. 363. Lancet: 2004. European Nicotinamide Diabetes Intervention Trial (ENDIT) Group; pp. 925–931. [DOI] [PubMed] [Google Scholar]
- 29.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 30.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1 (Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54(6):1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simões BP, Foss MC, Squiers E, Burt RK. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297(14):1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 32.WHO position paper. Allergen and immunotherapy: therapeutic vaccines for allergic diseases. Allergy. 1998;53:1–42. [PubMed] [Google Scholar]
- 33.Ferrara JL. Cytokines and the regulation of tolerance J Clin Invest. 2000;105(8):1043–1044. doi: 10.1172/JCI9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358(9295):1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 35.Schloot NC, Meierhoff G, Lengyel C, Vándorfi G, Takács J, Panczél P, Barkai L, Madácsy L, Oroszlán T, Kovács P, Sütö G, Battelino T, Hosszufalusi N, Jermendy G. Effect of heat shock protein peptide DiaPep277 on beta-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev. 2007;23:276–285. doi: 10.1002/dmrr.707. [DOI] [PubMed] [Google Scholar]
- 36.Brophy S, Brunt H, Davies H, Mannan S, Williams R. Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Database Syst Rev. 2007;(3) doi: 10.1002/14651858.CD006165.pub2. CD006165. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama T, Tanaka S, Shimada A, Funae O, Kasuga A, Kanatsuka A, Takei I, Yamada S, Harii N, Shimura H, Kobayashi T. Insulin intervention in slowly progressive insulin-dependent (type 1) diabetes mellitus. J Clin Endocrinol Metab. 2008;93(6):2115–2121. doi: 10.1210/jc.2007-2267. [DOI] [PubMed] [Google Scholar]
- 38.Ludvigsson J. Adequate doses of autoantigen administered using the appropriate route may create tolerance and stop autoimmunity. Diabetologia. 2009;52(1):175–176. doi: 10.1007/s00125-008-1211-9. [DOI] [PubMed] [Google Scholar]
- 39.Levy LM, Dalakas MC, Foleter MK. The stiff-person syndrome: an autoimmune disorder affecting neurotransmission of gamma-aminobutyric acid. Ann Intern Med. 1999;131(7):522–530. doi: 10.7326/0003-4819-131-7-199910050-00008. [DOI] [PubMed] [Google Scholar]
- 40.Lohmann T, Hawa M, Leslie RD, Lane R, Picard J, Londei M. Immune reactivity to glutamic acid decarboxylase 65 in stiffman syndrome and type 1 diabetes mellitus. Lancet. 2000;356(9223):31–35. doi: 10.1016/S0140-6736(00)02431-4. [DOI] [PubMed] [Google Scholar]
- 41.Okada Y, Taniguchi H, Schimada C. High concentration of GABA and high glutamate decarboxylase activity in rat pancreatic islets and human insulinoma. Science. 1976;194(4265):620–622. doi: 10.1126/science.185693. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Kanaani J, Menard-Rose V, Ma YH, Chang PY, Hanahan D, Tobin A, Grodsky G, Baekkeskov S. Increased expression of GAD65 and GABA in pancreatic beta-cells impairs first-phase insulin secretion. Am J Physiol Endocrinol Metab. 2000;279(3):E684–E694. doi: 10.1152/ajpendo.2000.279.3.E684. [DOI] [PubMed] [Google Scholar]
- 43.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman DL, Erlander MG, Clare-Salzler M, Atkinson MA, Maclaren NK, Tobin AJ. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992;89(1):283–292. doi: 10.1172/JCI115573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmet P. Antibodies to glutamic acid decarboxylase in the prediction of insulin dependency. Diabetes Res Clin Pract. 1996;34(Suppl):S125–S131. doi: 10.1016/s0168-8227(96)90019-4. [DOI] [PubMed] [Google Scholar]
- 46.Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes. 1997;46(11):1701–1710. doi: 10.2337/diab.46.11.1701. [DOI] [PubMed] [Google Scholar]
- 47.Bach JF. Autoimmunity and type I diabetes. Trends Endocrinol Metab. 1997;8(2):71–74. doi: 10.1016/s1043-2760(96)00271-8. [DOI] [PubMed] [Google Scholar]
- 48.Bach JF. New concepts of the etiopathogenesis and treatment of insulin-dependent diabetes mellitus. Clin Rev Allergy Immunol. 2000;19(3):217–225. doi: 10.1385/CRIAI:19:3:217. [DOI] [PubMed] [Google Scholar]
- 49.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 50.Lernmark A. Controlling the controls: GAD65 autoreactive T cells in type 1 diabetes. J Clin Invest. 2002;109(7):869–870. doi: 10.1172/JCI15381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roep BO. The role of T-cells in the pathogenesis of type 1 diabetes: from cause to cure. Diabetologia. 2003;46(3):305–321. doi: 10.1007/s00125-003-1089-5. [DOI] [PubMed] [Google Scholar]
- 52.Schloot NC, Roep BO, Wegmann DR, Yu L, Wang TB, Eisenbarth GS. T-cell reactivity to GAD65 peptide sequences shared with coxsackie virus protein in recent-onset IDDM, post-onset IDDM patients and control subjects. Diabetologia. 1997;40(3):332–338. doi: 10.1007/s001250050683. [DOI] [PubMed] [Google Scholar]
- 53.Karlsson MG, Ludvigsson J. Peptide from glutamic acid decarboxylase similar to Coxsackie B virus stimulates IFN-gamma mRNA expression in Th1-like lymphocytes from children with recent-onset insulin-dependent diabetes mellitus. Acta Diabetol. 1998;35(3):137–144. doi: 10.1007/s005920050118. [DOI] [PubMed] [Google Scholar]
- 54.Skarsvik S, Puranen J, Honkanen J, Roivainen M, Ilonen J, Holmberg H, Ludvigsson J, Vaarala O. Decreased in vitro type 1 immune response against Coxsackie virus B4 in children with type 1 diabetes. Diabetes. 2006;55(4):996–1003. doi: 10.2337/diabetes.55.04.06.db05-0630. [DOI] [PubMed] [Google Scholar]
- 55.Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366(6450):69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 57.Petersen JS, Karlsen AE, Markholst H, Worsaae A, Dyrberg T, Michelsen B. Neonatal tolerization with glutamic acid decarboxylase but not with bovine serum albumin delays the onset of diabetes in NOD mice. Diabetes. 1994;43(12):1478–1484. doi: 10.2337/diab.43.12.1478. [DOI] [PubMed] [Google Scholar]
- 58.Pleau JM, Fernandez-Saravia F, Esling A, Homo-Delarche F, Dardenne M. Prevention of autoimmune diabetes in nonobese diabetic female mice by treatment with recombinant glutamic acid decarboxylase (GAD 65) Clin Immunol Immunopathol. 1995;76(1 Pt 1):90–95. doi: 10.1006/clin.1995.1092. [DOI] [PubMed] [Google Scholar]
- 59.Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, Kaufman DL. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183(4):1561–1567. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian J, Clare-Salzler M, Herschenfeld A, Middleton B, Newman D, Mueller R, Arita S, Evans C, Atkinson MA, Mullen Y, Sarvetnick N, Tobin AJ, Lehmann PV, Kaufman DL. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat Med. 1996;2(12):1348–1353. doi: 10.1038/nm1296-1348. [DOI] [PubMed] [Google Scholar]
- 61.Plesner A, Worsaae A, Dyrberg T, Gotfredsen C, Michelsen BK, Petersen JS. Immunization of diabetes-prone or non-diabetes-prone mice with GAD65 does not induce diabetes or islet cell pathology. J Autoimmun. 1998;11(4):335–341. doi: 10.1006/jaut.1998.0206. [DOI] [PubMed] [Google Scholar]
- 62.Tisch R, Liblau RS, Yang XD, Liblau P, McDevitt HO. Induction of GAD65-specific regulatory T-cells inhibits ongoing autoimmune diabetes in nonobese diabetic mice. Diabetes. 1998;47(6):894–899. doi: 10.2337/diabetes.47.6.894. [DOI] [PubMed] [Google Scholar]
- 63.Yoon JW, Yoon CS, Lim HW, Huang QQ, Kang Y, Pyun KH, Hirasawa K, Sherwin RS, Jun HS. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in beta cells. Science. 1999;284(5417):1183–1187. doi: 10.1126/science.284.5417.1183. [DOI] [PubMed] [Google Scholar]
- 64.Tisch R, Wang B, Weaver DJ, Liu B, Bui T, Arthos J, Serreze DV. Antigen-specific mediated suppression of beta cell autoimmunity by plasmid DNA vaccination. J Immunol. 2001;166(3):2122–2132. doi: 10.4049/jimmunol.166.3.2122. [DOI] [PubMed] [Google Scholar]
- 65.Jun HS, Chung YH, Han J, Kim A, Yoo SS, Sherwin RS, Yoon JW. Prevention of autoimmune diabetes by immunogene therapy using recombinant vaccinia virus expressing glutamic acid decarboxylase. Diabetologia. 2002;45(5):668–676. doi: 10.1007/s00125-002-0806-9. [DOI] [PubMed] [Google Scholar]
- 66.Agardh C-D, Corrado MC, Lethagen ÅL, Lynch K, Leslie RD, Palmer M, Harris AR, Robertson J, Lernmark Å. Clinical evidence for safety, efficacy and immunomodulation by a novel therapeutic intended for treatment of autoimmune diabetes. Diabetes. 2004;53(suppl 2):272. [Google Scholar]
- 67.Agardh C-D, Lynch K, Palmér M, Link K, Lernmark Å. GAD65 vaccination significantly reduces insulin dependence at five years follow-up in a dose escalating study in adult-onset autoimmune diabetes patients. Diabetologia. 2008;51(suppl 1):S230. doi: 10.1007/s00125-009-1371-2. [DOI] [PubMed] [Google Scholar]
- 68.Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359(18):1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 69.Type 1 Diabetes Trial Net Research Group, European C-Peptide Trial Study Group. Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, Pozzilli P, Lachin JM, Kolb H. The mixed meal tolerance test versus the glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31(10):1966–1971. doi: 10.2337/dc07-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 71.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16(6):261–266. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]